Engineering
Vol.4 No.11(2012), Article ID:24524,4 pages DOI:10.4236/eng.2012.411093
Effect of Temperature and the Organic Phase Content on the Stripping of Mn(II) from a D2EPHA-KEROSENE Pseudo-Emulsion Using a Sulfuric Acid Aqueous Solution
Centro de Investigación y de Estudios Avanzados del IPN, Unidad Saltillo, Ramos Arizpe, México
Email: jaime.rojas@cinvestav.edu.mx
Received August 31, 2012; revised September 31, 2012; accepted October 10, 2012
Keywords: Manganese Solvent Extraction; Manganese Stripping; Manganese; Pyrolusite
ABSTRACT
There are few studies oriented to analyze the interaction between the variables of the stripping process of actual leaching solutions. The aim of this work was to study the effect of temperature and the percent of organic phase in the stripping pseudo-emulsion (D2EPHA and kerosene) on the manganese stripping process. Other studied variables were the acid concentration (H2SO4) in the aqueous phase, the percent of the organic phase in the pseudo-emulsion and the number of stripping stages. This information may be of interest to countries with radical temperature variations. It was discovered that in the range of 10˚C to 50˚C, temperature improves the depletion of manganese from the D2EPHA. Additionally, the percent of the organic phase should be less than 90% by volume of the pseudo-emulsion to favour the manganese exchange. Moreover, the H2SO4 concentration in the aqueous phase should be less than 1 M to avoid chemical instability of the organic phase.
1. Introduction
In the last few years, the world demand for manganese has increased due to steel production, primarily in China. The steelmaking industry consumes between 85% and 90% of the world’s manganese production [1]. The electronic industry also consumes a large quantity of manganese (Mn) in the form of electrolyticand chemicalgrade of manganese dioxide (EMD and CMD), for producing disposable and rechargeable batteries. Currently, the growing market of mobile communications has significantly increased the demand for batteries and consequently, for EMDs.
This growing demand for manganese makes low-grade ore processing an important alterative, with hydrometallurgy being the more profitable approach for this type of processing. One of the more recently developed processes for leaching low-grade manganese ore is reductive leaching in a stirred reactor, using SO2 gas [2], followed by solvent extraction, stripping and electrowinning.
The elimination of manganese impurities from leach liquors of other metals is the most common objective of solvent extraction studies of manganese, but there are few studies on solvent extraction and stripping to concentrate the manganese that is present in the leachate obtained by SO2 leaching of low-grade pyrolusite ores [3-5]. Stripping manganese from solvent-extractant solutions is considered a simple process and maybe this is the explanation why there are few studies that analyse the interaction of the process variables to optimise the stripping process. The scope of this work was to study the effect of temperature, acid concentration (H2SO4) in the aqueous phase, the percent of the organic phase in the pseudo-emulsion and the number of stripping stages on the extractant depletion. This paper may be of interest to operators of manganese stripping processes in plants where a dynamic range of operating temperatures exists.
2. Background
Solvent extraction is used to separate a component from a homogeneous solution by adding a second solvent that is immiscible in the first solvent and creates a solute distribution between the two phases. Extractant di-2 ethyl hexyl phosphoric acid (D2EHPA) dissolved in kerosene is the organic solution most frequently used in manganese extraction from an aqueous leach liquor [6]. Once the organic phase is manganese-enriched from the solvent extraction process, the next stage involves reversing the reaction to obtain a concentrated and acidified aqueous solution of manganese (stripping). The final concentrated manganese solution helps to increase the efficiency of the subsequent electrowinning process. Because stripping is the inverse process of solvent extraction, and according to Sato and Nakamura [6], extraction with D2EHPA is governed by a cationic mechanism, the stripping process may be described by Equation (1).
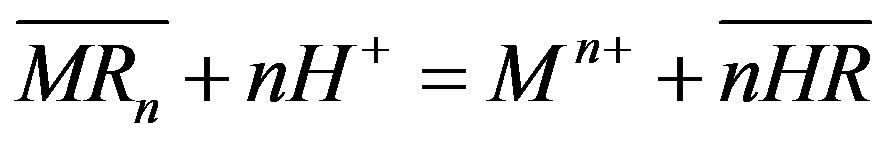 (1)
(1)
where M is the metal, HR is the D2EHPA in the organic phase and MR is the metal-organic complex in the organic solution. It should be noted that after stripping the manganese, the organic (solvent and extractant) and the aqueous phases are recycled to achieve both economic and environmental targets [7]. Several studies also report using a sulphuric acid solution to separate metals from the loaded organic phase [8-10]. Hossain et al. [11] performed manganese stripping in a batch process (H2SO4, shaking speed of 300 rpm and temperature of 25˚C, for a period of 2 h), founding that 100% of Mn was stripped using a 0.4 M H2SO4 solution in a single stage process, and higher concentrations did not provide any additional benefit. Agatzini-Leonardou [12] studied the strippability of Mn(II) from Cyanex 272 into an aqueous solution containing 0.76 M sulphuric acid, reaching manganese recoveries of 99% with diluted sulphuric acid and two stripping stages.
In other studies conducted by Saeed et al. [13] using liquid membrane extraction, the authors analysed the effect of the concentration of H2SO4 and FeSO4 in the stripping solution using concentrations from 0.25 M to 0.75 M and 1.58 × 10−3 mol/dm3 to 11.1 × 10−3 mol/dm3, respectively, maintaining a feed concentration of 1.82 × 10−3 mol/dm3 manganese ions in a 1 M H2SO4 solution. The authors conclude that by increasing the H2SO4 concentration from 0.25 M to 0.50 M, the extraction of Mn(II) ions also increases. Beyond this concentration of H2SO4, the extraction of Mn(II) ions decreases. Additionally, as the concentration of ferrous ions in the stripping phase increases gradually, the dissociation of the (C2H4OH)3NHMnO4 complex by a redox reaction at the membrane interface is enhanced, and Mn(II) ions are released into the stripping phase.
Cheng [14] conducted another study to improve the nickel, cobalt and manganese stripping kinetics, adding TBP (tri-n-butyl-phosphate) in a V10 (tertiary carboxylic acid)/L63 (an α-hydroxyoxime) system. Stripping tests were conducted using the loaded Versatic 10/LIX63/TBP system containing 2.25 g/L Ni, 0.112 g/L Co and 0.033 g/L Mn. Additionally, a synthetic spent-nickel electrolyte containing 6 g/L Ni and 7 g/L sulphuric acid was added to the system at an organic:aqueous (O:A) ratio of 1:1 at 40˚C to mimic an operation with a strip solution containing 60 g/L nickel and 70 g/L sulphuric acid at an O:A ratio of 10:1. The authors observed rapid stripping kinetics for the three metals. After 2 min of mixing, the stripping efficiencies of nickel, cobalt and manganese were 92%, 98% and 99%, respectively.
3. Experimental Procedure
3.1. Leaching and Solvent Extraction
Manganese dissolution was conducted by reductive leaching of the pyrolusite mineral (d10 = 38 µm) using SO2 gas. The elemental analysis of the ore was conducted using X-ray fluorescence (%, w/w): Mn (18.80), Fe (12.81), Si (23.83), Al (1.18), Ba (1.11), Ca (0.59) and O (42). Based on this analysis and on the stoichiometry of the oxidised species, a mineralogical composition was estimated (%, w/w): MnO2 (28.78), Fe2O3 (17.72), SiO2 (49.33), Al2O3 (2.15), BaO (1.19) and CaO (0.79). Notably, these results are in qualitative agreement with the results obtained using X-ray diffraction. The leaching process was performed in a 2 L reactor operated in batch mode. The reactor was properly equipped to measure and control the temperature, pH, mixing rate and SO2 gas flow fed to the reactor. The leaching process was performed at 60˚C and a stir speed of 800 rpm for 120 minutes in 1600 mL of pulp and 0.096 mL/s of SO2 gas. The process yielded 90% Mn and 0.17% Fe, which was present as a solute in the leach liquor. The insufficient Fe dissolution likely occurred because most of the Fe was occluded into quartz particles. Thus, the manganese concentration in the leach liquor was close to 0.085 M. After the leaching, the liquor was separated from the solids by filtration.
Considering that the selectivity of the extractants for the ions of interest is Fe(II), Fe(III) > Mn(II), it was necessary to eliminate the Fe impurities from the leach solution prior to the extraction stage. To precipitate the Fe impurities, the pH was increased to 7.2 with 5 M NH4OH, carefully avoiding the co-precipitation of Mn(II), which occurs when the pH is above 8.2. Initially, the leaching solution contained 5520 ppm of Mn and 85.04 ppm of Fe. After the precipitation of Fe(OH)2, the iron content dropped to approximately 0.03 ppm, and only a relatively small fraction (7.34%) of Mn(II) precipitated with the iron. Table 1 shows the chemical analysis of the purified leached solution, which includes low contents of other metals.
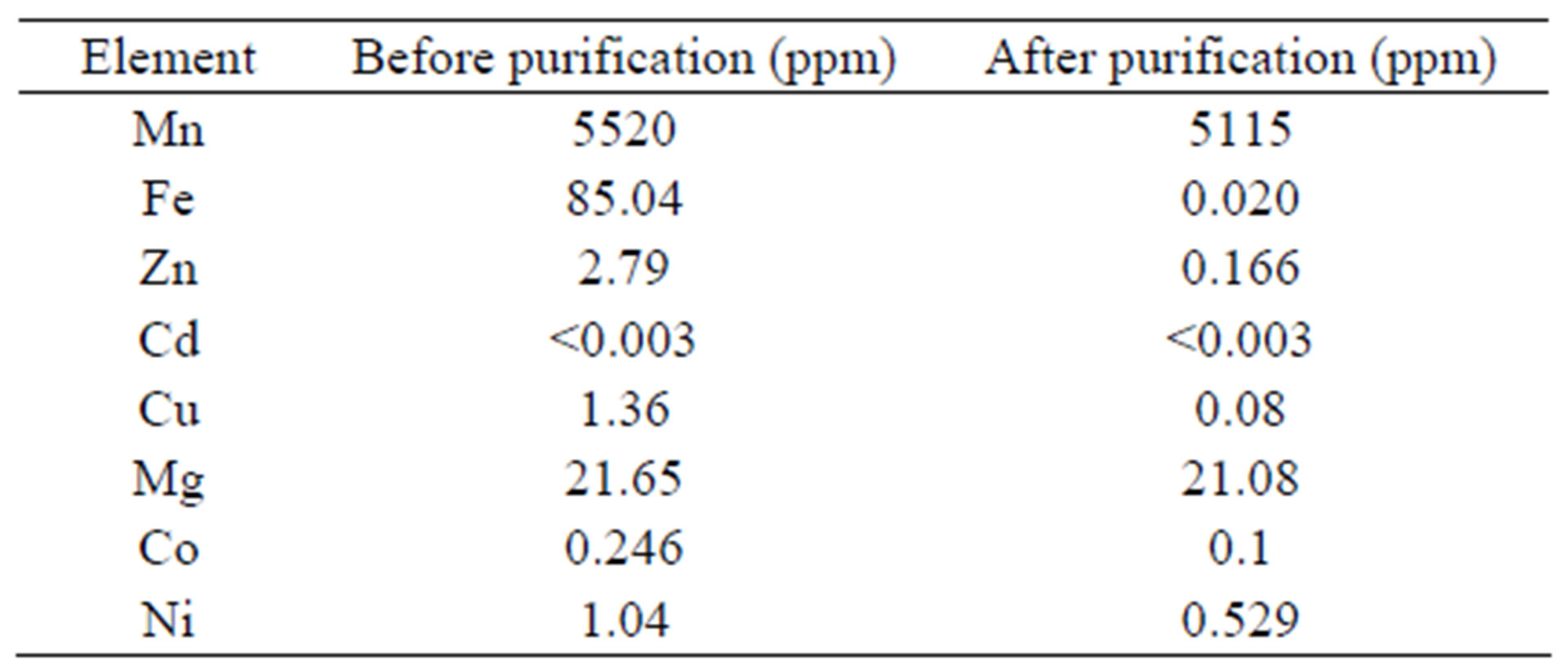
Table 1. Chemical analysis of the leach liquor before and after the precipitation of Fe(OH)2.
For the solvent extraction, the organic solution was prepared by diluting the extractant in kerosene with a D2EHPA:kerosene proportion of 1:9 by volume. The organic and aqueous (leaching purified liquor) solutions were mixed in a 2 L reactor for 5 minutes, keeping a ratio O:A of 2. It was used five minutes to assure a good metal exchange, even when it has been reported that a mixing time of one minute is sufficient for this type of solvent extractions [15]. After each extraction stage, the mixture was left to stand for 15 minutes, permitting the complete separation of the phases, to adjust the pH of the aqueous solution. Notably, this procedure was conducted five times (i.e., five extraction stages). Before and after each extraction stage, the pH of the aqueous phase was measured and eventually adjusted between 8 and 8.5 with a 1 M NH4OH solution. After the five extraction stages, the organic phase passed to the stripping process, and a sample of the aqueous solution was withdrawn for chemical analysis in order to estimate the manganese recovered by the solvent extraction procedure.
3.2. Stripping
The stripping tests were performed in a 125 mL separation funnel, varying the organic: aqueous phase ratio in each experiment. Additionally, different concentrations of H2SO4 in the aqueous phase were used during the stripping tests. Similar to the solvent extraction tests, the pseudo-emulsions were mixed vigorously for 1 minute and left to stand for 20 minutes, following the complete separation of the phases. After each stripping test, a sample of the aqueous solution was withdrawn for chemical analysis (atomic absorption spectroscopy) to estimate the recovery of manganese. The experimental stripping conditions are listed in Table 2.
4. Results and Discussion
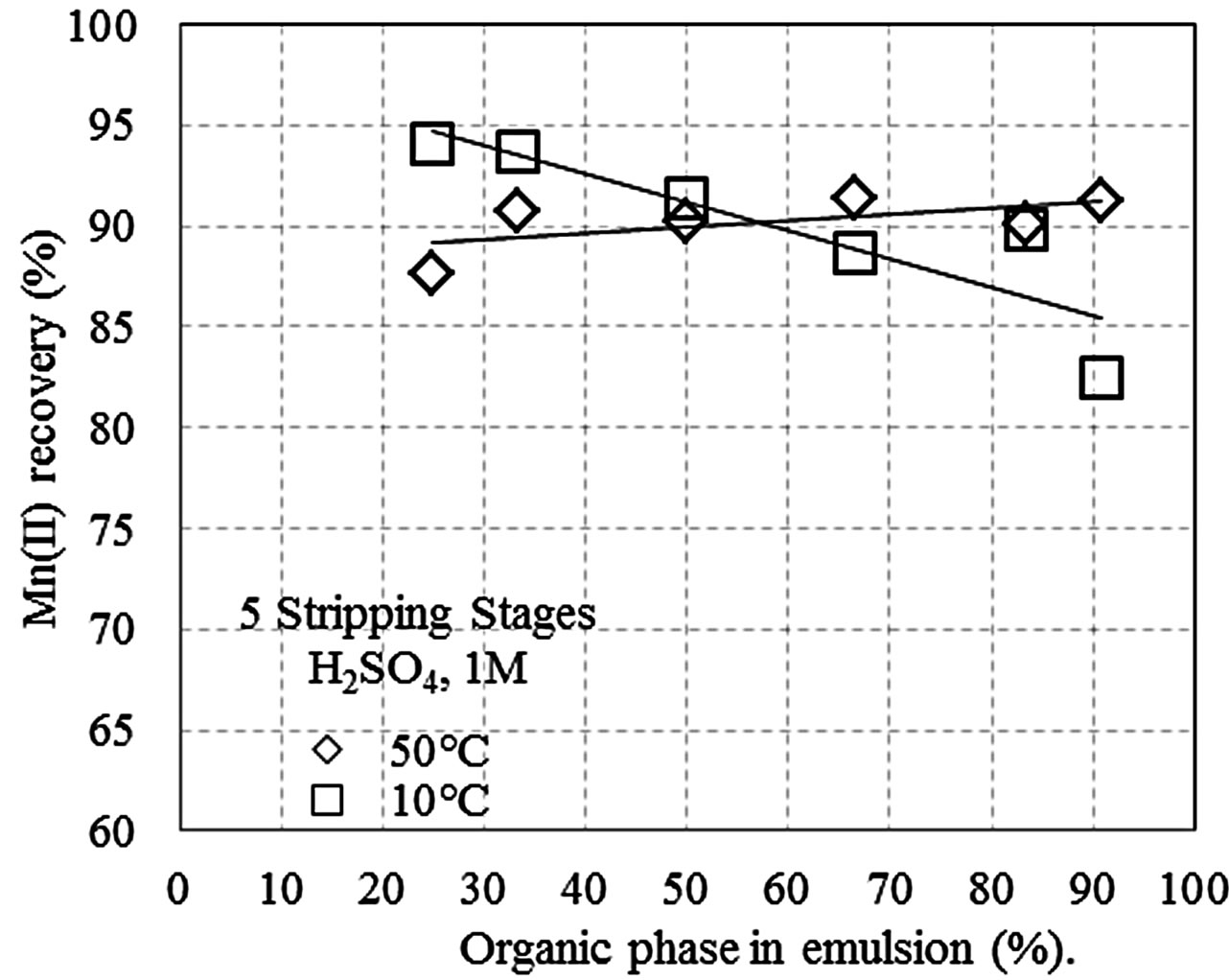
Figures 1(a)-(c) shows the manganese recovery as a function of the percent of the organic phase that is present in the pseudo-emulsion with the aqueous solution, at different H2SO4 concentrations (1, 3, and 5 M). The tests were conducted with five stripping stages at 10˚C and 50˚C, and recoveries between 82% and 92% were obtained. Additionally, at 50˚C manganese recovery increased with the percentage of the organic phase, while
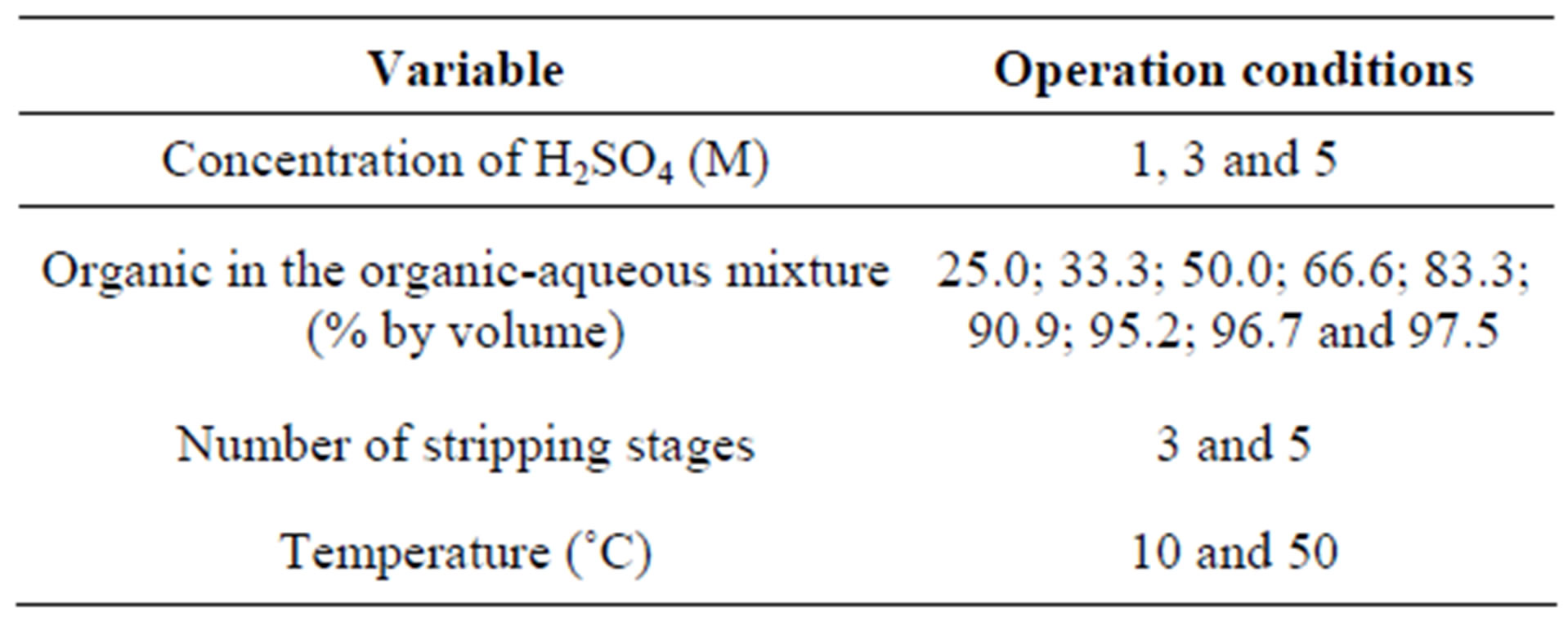
Table 2. Experimental conditions of the stripping tests.
 (a)
(a)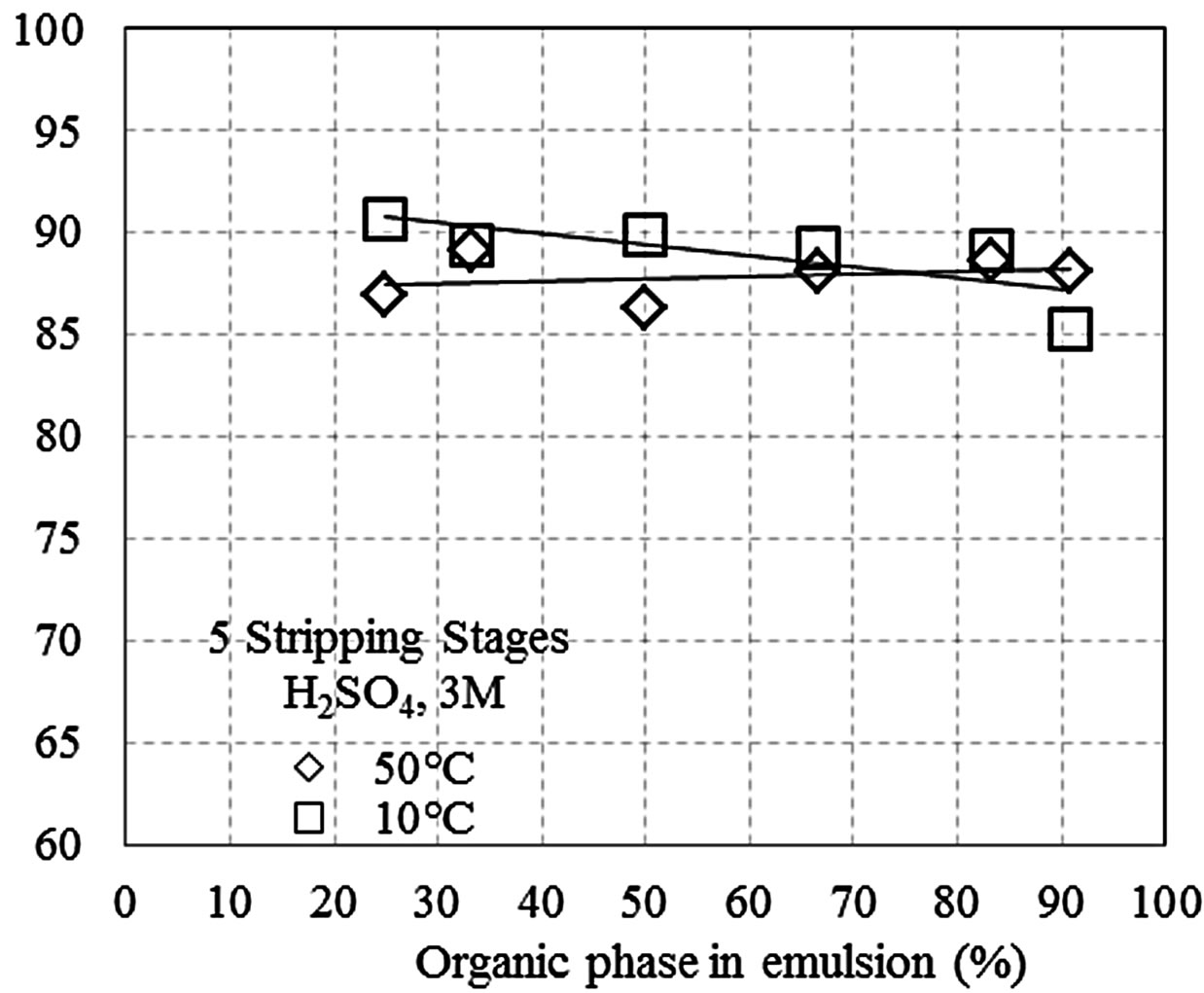 (b)
(b)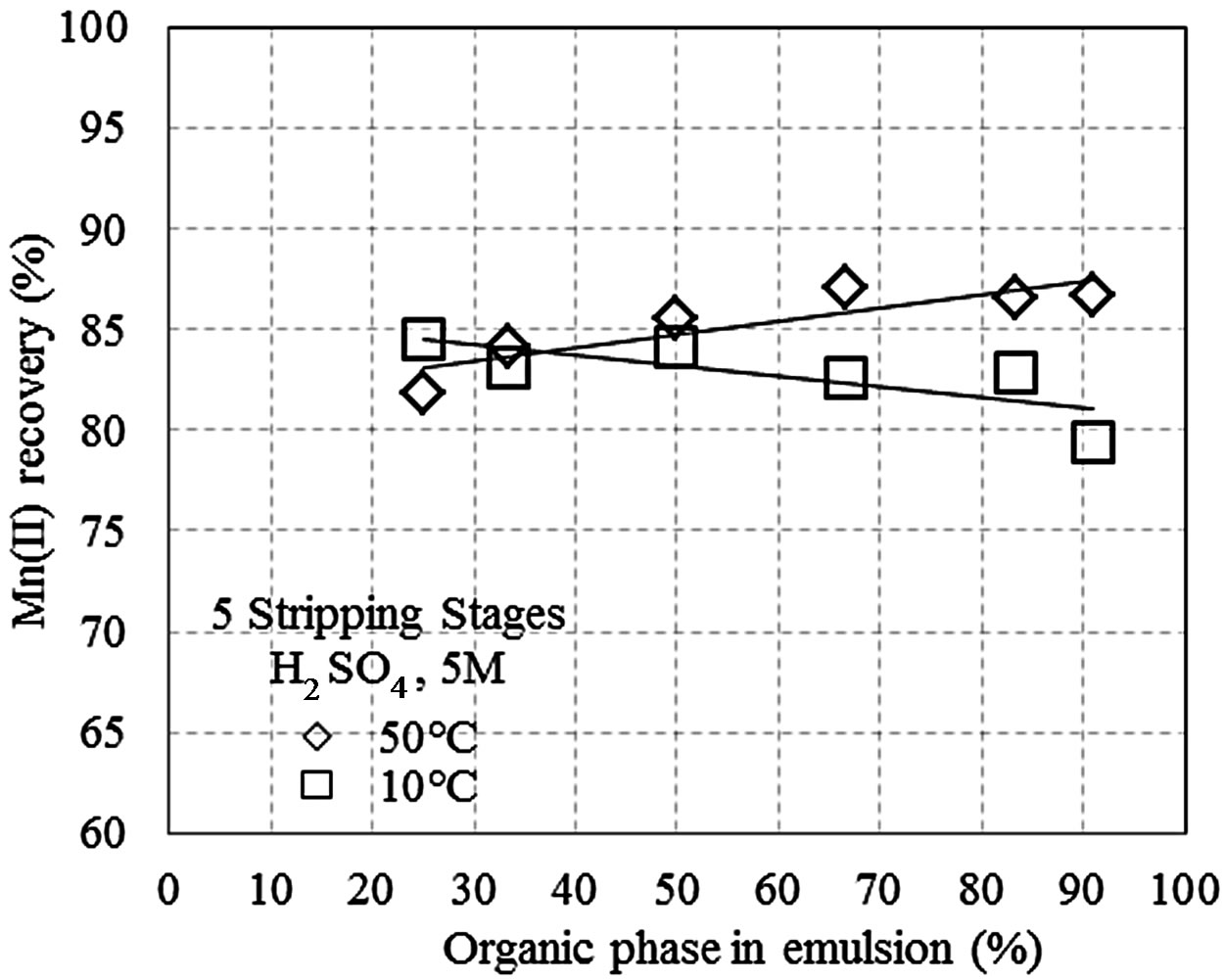 (c)
(c)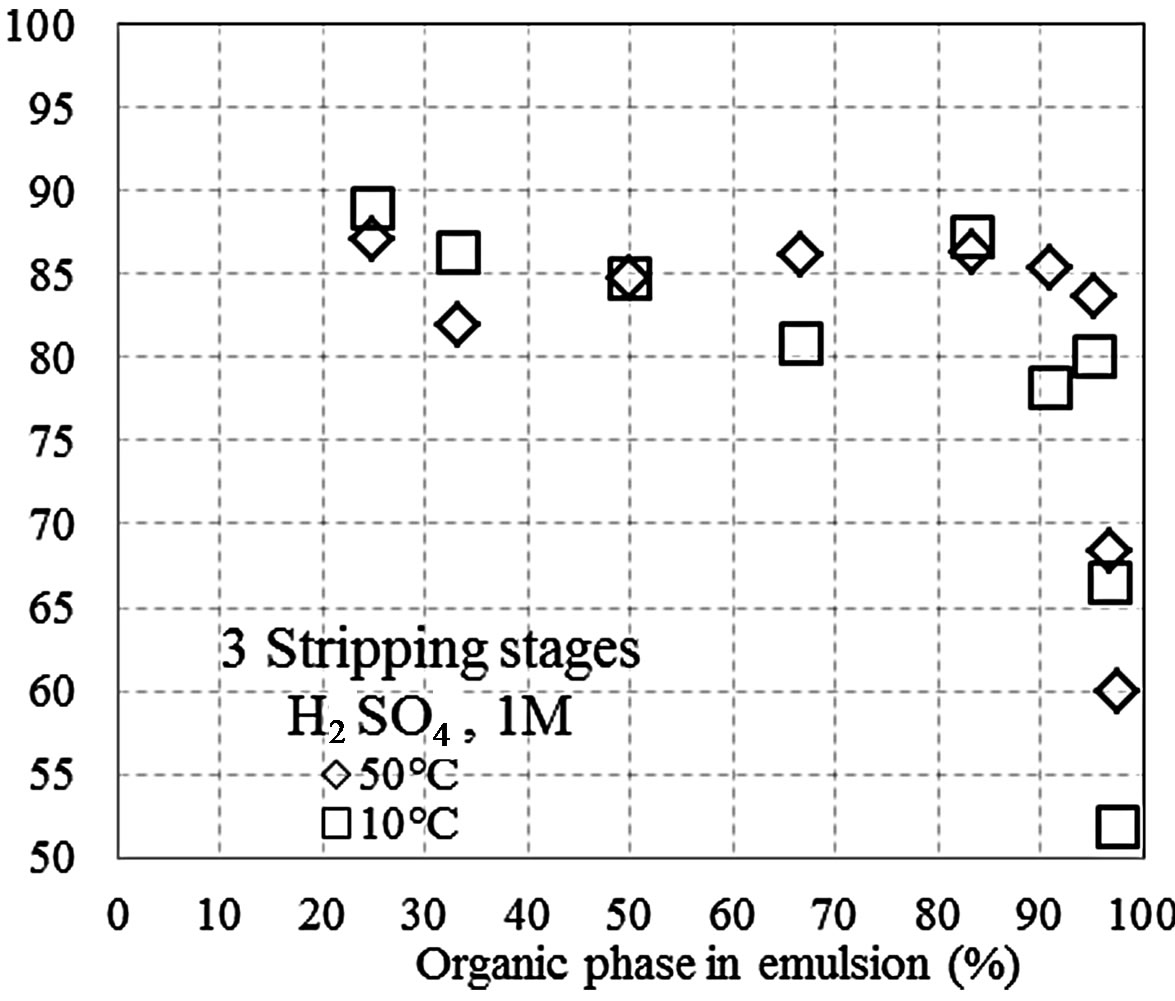 (d)
(d)
Figure 1. Recovery of Mn(II) as a function of the percent of the organic phase in the pseudo-emulsion and the temperature, when five stripping stages are performed. (a) Aqueous solution with H2SO4 1 M; (b) Aqueous solution with H2SO4 3 M; (c) Aqueous solution with H2SO4 5 M; (d) Three stripping stages and aqueous solution with H2SO4 1 M.
at 10˚C the recovery decreased when the organics in the pseudo-emulsion increased. This result is likely due to the organic phase being less viscous at 50˚C, which improves the pseudo-emulsion droplet formation and increases the contact surface area, thus increasing the migration of manganese from the organic phase to the aqueous phase. In general, after the stripping, the aqueous solutions reach 40 g/m3 of Mn(II).
Comparing the results in Figures 1(a) and (b), a little advantage of using solutions acidified at 1 M is noticed. This result is possibly due to the low viscosity of the system under these experimental conditions, which enhances contact between the organic and the aqueous phases. It is also possible that under highly acidic conditions, the organic phase becomes chemically instable. This is suggested by the colour change that occurs when the H2SO4 concentration is greater than 1 M. Table 3 shows the recovery of the impurities in a typical test of stripping (50% of organic phase, aqueous solution 1 M H2SO4, 25˚C and 3 stripping stages).
Figure 1(d) shows the results of the tests conducted with 3 stripping stages and a H2SO4 1 M solution. Below an organic phase percentage of 90%, the results were similar to those obtained in tests using 5 stripping stages. Above this percentage, several changes were observed. The aim of these tests was to determine the maximum content of the organic phase needed to maintain the efficiency of the stripping process. At both 10˚C and 50˚C, above 90% of the organic phase in the pseudo-emulsion, the manganese recovery decreases drastically, indicating that a high percent of organic phase in the pseudo-emulsion affects the interaction between both phases, enhancing the mass transfer and the manganese recovery. Comparing Figures 1(a) and (d), the number of stripping stages does not significantly affect the recovery, suggesting that 3 stages are sufficient for good recovery.
Additionally, the interval of the studied temperatures was selected because en some places the atmospheric temperature can oscillate between –10˚C and 45˚C, and consequently, the temperature of the fluids in the extraction processes may be close to the studied range. Also, it is observed that it is possible to predict the manganese recovery as a function of the percentage of the organic phase and the temperature.
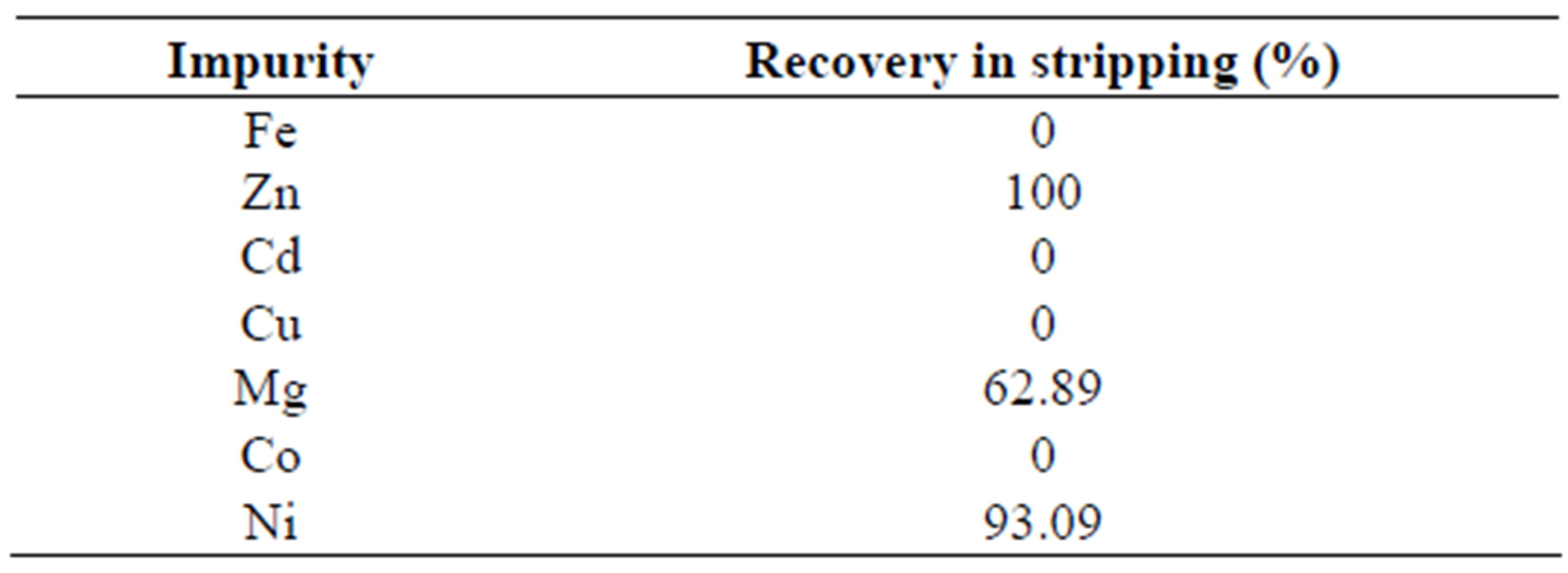
Table 3. Recovery of impurities in the stripping stage. Organic phase in pseudo-emulsion 50%, aqueous phase 1 M H2SO4, 25˚C and 3 stripping stages.
5. Conclusions
Manganese complexed with D2EHPA dissolved in kerosene was extracted using an aqueous solution of sulphuric acid. The process variables that were evaluated included the operating temperature (10˚C and 50˚C), the acid concentration (1, 3 and 5 M) in the aqueous phase and the number of extraction stages (3 and 5).
It was discovered that temperature (between 10˚C and 50˚C) improves the depletion of manganese from the D2EPHA.
The percentage of the organic phase should be less than 90% of the pseudo-emulsion to favour manganese exchange.
The concentration of H2SO4 in the aqueous phase should be less than 1 M to avoid chemical instability of the organic phase.
Considering the environmental and economic impact, it is suggested to work under the following operation conditions: ambient temperatures, 3 stripping stages, pseudo-emulsions that contain less than 90% of organic phase and aqueous solutions with a maximal H2SO4 concentration (1 M).
6. Acknowledgements
The authors are grateful to CONACYT (Mexico) and to Huajicari S.A. de C.V. for the providing the funding for this research.
REFERENCES
- L. A. Corathers, “US Geological Survey (USGS) Minerals Yearbook: Manganese-2005,” 2007. http://minerals.usgs.gov
- W. Zhang and C. Y. Cheng, “Manganese Metallurgy Review: Part I: Leaching of Ores/Secondary Materials and Recovery of Electrolytic/Chemical Manganese Dioxide,” Hydrometallurgy, Vol. 89, No. 3-4, 2007, pp. 137-159. doi:10.1016/j.hydromet.2007.08.010
- N. Mulaudzi and T. Mahlangu, “Oxidative Precipitation of Mn(II) from Cobalt Leach Solutions Using Dilute SO2/ Air Gas Mixture,” The Journal of Southern African Institute of Mining and Metallurgy, Vol. 109, 2009, pp. 375- 381.
- J. Van Rooyen, S. Archer and M. Fox, “Manganese Removal from Cobalt Solutions with Dilute Sulphur Dioxide Gas Mixtures,” The Fourth Southern African Conference on Base Metals, The Southern Africa Institute of Mining and Metallurgy, 2009, pp. 365-376.
- V. Menard and G. Demopuolos, “Gas Transfer Kinetics and Redox Potential Considerations in Oxidative Precipitation of Manganese from an Industrial Zinc Sulphate Soltion with SO2/O2,” Hydrometallurgy, Vol. 89, No. 3-4, 2007, pp. 357-368. doi:10.1016/j.hydromet.2007.03.014
- T. Sato and T. Nakamura, “Solvent Extraction of Divalent Metals from Sulfuric Acid Solutions by Dialkylphosphoric Acid,” Journal of the Mining and Metallurgical Institute Japan, Vol. 101, No. 1167, 1985, pp. 309-312.
- J. Rydberg, M. Cox and C. Musikas, “Solvent Extraction Principles and Practice,” CRC Rress, 2004, p. 480. doi:10.1201/9780203021460
- D. Mohapatra, K. Hong-In, C. W. Nam and K. H. Park, “Liquid-Liquid Extraction of Aluminium(III) from Mixed Sulphate Solutions Using Sodium Salts of Cyanex 272 and D2EHPA,” Separation and Purification Technology, Vol. 56, No. 3, 2007, pp. 311-318. doi:10.1016/j.seppur.2007.02.017
- X. P. Huo, W. Qin, X. W. Sun and Y. Y. Dai, “Recovery of Chromium (III) by Solvent Extraction with D2EHPA,” Journal of Chemical Engineering of Chinese Universities, Vol. 21, No. 5, 2007, pp. 849-852.
- Z. G. Arroyo, M. Stambouli, D. Pareau, A. Buch, G. Durand and M. A. Rodriguez, “Thiosubstituted Organophosphorus Acids as Selective Extractants for Ag(I) from Acidic Thiourea Solutions,” Solvent Extraction and Ion Exchange, Vol. 26, No. 2, 2008, pp. 128-144. doi:10.1080/07366290801904855
- M. R. Hossain, S. Nash, G. Rose and S. Alam, “Cobalt Loaded D2EHPA for Selective Separation of Manganese from Cobalt Electrolyte Solution,” Hydrometallurgy, Vol. 107, No. 3-4, 2011, pp. 137-140. doi:10.1016/j.hydromet.2011.02.011
- S. Agatzini-Leonardou, P. E. Tsakiridis, P. Oustadakis, T. Karidakis and A. Katsiapi, “Hydrometallurgical Process for the Separation and Recovery of Nickel from Sulphate Heap Leach Liquor of Nickeliferrous Laterite Ores,” Minerals Engineering, Vol. 22, No. 14, 2009, pp. 1181-1192. doi:10.1016/j.mineng.2009.06.006
- S. ur Rehman, G. Akhtar, M. A. Chaudry, N. B. Najeebullah and N. Ali, “Mn (VII) Ions Transport by Triethanolamine Cyclohexanone Based Supported Liquid Membrane and Recovery of Mn (II) Ions from Discharged Zinc Carbon Dry Battery Cell,” Journal of Membrane Science, Vol. 366, No. 1-2, 2011, pp. 125-131. doi:10.1016/j.memsci.2010.09.049
- C. Y. Cheng, G. Boddy, W. Zhang, M. Godfrey, D. J. Robinson, Y. Pranolo, Z. Zhu and W. Wang, “Recovery of Nickel and Cobalt from Laterite Leach Solutions Using Direct Solvent Extraction: Part 1—Selection of a Synergistic SX System,” Hydrometallurgy, Vol. 104, No. 2, 2010, pp. 45-52. doi:10.1016/j.hydromet.2010.04.009
- R. K. Biswas, M. A. Habib and M. G. K. Mondal, “Kinetics and Mechanism of Stripping of Mn(II)-D2EHPA Complex by Sulfuric Acid Solution,” Hydrometallurgy, Vol. 80, No. 3, 2005, pp. 186-195. doi:10.1016/j.hydromet.2005.06.013

