Materials Sciences and Applications
Vol.4 No.7(2013), Article ID:33805,7 pages DOI:10.4236/msa.2013.47052
Influence of Cr3+ Ion on the Dielectric Properties of Nano Crystalline Mg-Ferrites Synthesized by Citrate-Gel Method
![]()
1Department of Chemistry, Jayaprakash Narayan College of Engineering, Mahabubnagar, India; 2Department of Physics, Nizam College, Basheerbagh Osmania University, Hyderabad, India; 3Department of Chemistry, Osmania University, Hyderabad, India.
Email: *ravindergupta28@rediffmail.com
Copyright © 2013 M. Raghasudha et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received May 17th, 2013; revised June 18th, 2013; accepted June 27th, 2013
Keywords: Dielectric Constant; Dielectric Loss Tangent; Mg-Cr Nano Ferrites; LCR Meter
ABSTRACT
Mixed Mg-Cr Nano ferrites having the compositional formula MgCrxFe2−xO4 (where x = 0.0, 0.1, 0.3, 0.5, 0.7, 0.9 and 1.0) were synthesized using Citrate-Gel auto combustion method. Structural characterization was carried out by XRD Analysis which confirmed the formation of single phase cubic spinel structure without any impurity peak. The dielectric properties such as Dielectric constant (ε'), Dielectric Loss tangent (tan δ) and AC conductivity (σAC) of Mg-Cr nano ferrites were studied at room temperature in the frequency range of 2 Hz - 2 MHz using Agilent E4980A Precision LCR meter. The dielectric constant, loss tangent and AC conductivity shows a normal behavior with frequency. A qulitative explanation is given for composition and frequency dependance of the dielectric constant, dielectric loss tangent and AC conductivity of the nano ferrite. The loss tangent for the synthesized samples was found to be decreased from 0.09 to 0.054 in higher frequency region showing the potential applications of these materials in high frequency micro wave devices. On the basis of these results the explanation of dielectric mechanism in Mg-Cr ferrites is suggested.
1. Introduction
Nano ferrites have many applications in high frequency devices and they play a useful role in technological applications. One important characteristic of ferrites is their high values of resistivity, low magnetic and dielectric losses [1] which make them ideal for high frequency applications. Owing to the dielectric behavior, they are sometimes called multiferroics. They are commercially important because they can be used in, many devices such as Phase Shifter, high frequency transformer cores, switches, resonators, computers, TVs and mobile phones [2,3]. Dielectric properties of ferrites are dependent upon several factors, including method of preparation, chemical composition and grain size [4,5]. Since MgFe2O4 and related ferrites are widely used components in micro wave family due to their high electrical resistivity, low magnetic and dielectric losses [6,7] we investigated the dielectric behavior of these ferrites over wide range of frequencies at room temperature. In this study, we prepared nano sized MgCrxFe2−xO4 compounds containing different levels of Cr with the assumption that dielectric properties would be improved by substitution of Fe3+ ions with Cr3+ ions. Substitution of Magnesium Ferrites with Cr3+ ions at B site should be effective in enhancing the electrical resistivity. In the present work the aim of Cr3+ ion substitution for Fe3+ ions is to reduce dielectric loss. In this article we report the influence of Cr substitution on structural and dielectric properties of MgCrxFe2−xO4 ferrites synthesized by Citrate-gel auto combustion method as a function of frequency and composition at room temperature.
2. Experimental
2.1. Synthesis of Mg-Cr Ferrites
Ferrites with chemical formula MgCrxFe2−xO4 (x = 0.0, 0.1, 0.3, 0.5, 0.7, 0.9 and 1.0) have been prepared by the Citrate-gel auto combustion method using high purity AR grade of Magnesium Nitrate—(Mg(NO3)26H2O), Ferric Nitrate—(Fe(NO3)29H2O), Chromium Nitrate— (Cr(NO3)29H2O), Citric acid—(C6H8O7∙H2O), Ammonia —(NH3) as starting materials for the synthesis. Required quantities of metal nitrates were dissolved in a minimum quantity of distilled water and mixed together. Aqueous solution of Citric acid was then added to the mixed metal nitrate solution. Ammonia solution was then added with constant stirring to maintain PH of the solution at 7. The resulting solution was continuously heated on the hot plate at 100˚C up to dryness with continuous stirring. A viscous gel has resulted. Increasing the temperature up to 200˚C lead the ignition of gel. The dried gel burnt completely in a self propagating combustion manner to form a loose powder. The burnt powder was ground in Agate Mortor and Pistle to get a fine Ferrite powder. Finally the burnt powder was calcined in air at 500˚C temperature for four hours and cooled to room temperature.
2.2. Characterization
The structural characterization of powders was carried out by X-Ray Diffractometer to analyze the phase and crystallite size. For dielectric measurements the powders were added with a small amount 2% PVA as a binder to press the powders into circular pellets of diameter 13mm and thickness 1 mm applying a pressure of 5 tons. The prepared pellets were sintered at 500˚C for four hours in air in muffle furnace for the densification of the sample. For dielectric measurements silver paint was applied on both sides of the pellets and air dried to have good ohmic contact. The dielectric measurements were made using Agilent E4980A Precision LCR meter at room temperature in the frequency range 20 Hz to 2 MHz.
Using LCR meter the dielectric parameters such as Capacitance of the pellet, tan δ (loss tangent) and Capacitance of air with the same thickness as the pellet were measured.
The real part of the dielectric constant (ε') was determined from the following formula [8]
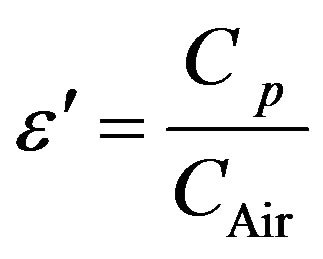
where ε' = Real part of dielectric constant; Cp = Capacitance of the Pellet in Faraday; CAir = Capacitance of Air in Faraday.
The imaginary part of the dielectric constant (ε") or dielectric loss was measured by using the following relation [9]
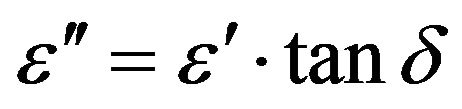
The Ac conductivity was calculated using the values of frequency (f) and loss tangent factor as [9]
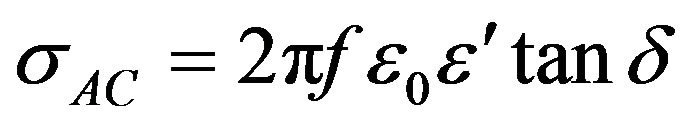
where ε0 = Constant permittivity of free space = 8.854 × 10−12 F/m; ε' = Real part of dielectric constant; tan δ = loss tangent.
3. Results and Discussion
3.1. Structural Characterization
The structural characterization of all the nano-ferrites was carried out by X-ray Diffraction. From the analysis the 2 theta and intensity data were used and graphs were plotted as shown in Figure 1. From the X-ray diffraction pattern, crystalline phases were identified by comparison with reference data from the ICSD card No. 71-1232 for Magnesium ferrites. All Bragg reflections have been indexed which confirms the formation of a well defined single phase cubic spinel structure without any impurity peak. The strongest reflection comes from (311) plane that indicates spinel phase. The XRD patterns of all the Chromium substituted Magnesium ferrites showed the homogeneous single phased cubic spinel belonging to the space group Fd3m (confirmed by ICSD Ref 71-1232).
The crystallite size (D) was calculated for all the compositions using high intensity peak (311) from Scherrer’s formula [10] and was in the range of 7 to 23 nm.
Lattice parameter (a) of the individual composition was calculated by using the following formula
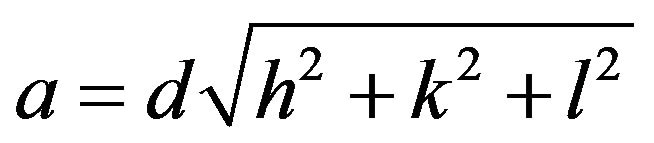
where a = lattice parameter; d = interplanar spacing; hkl = the miller indices.
The lattice parameter was found to decrease linearly with increase of Cr3+ ions in the Mg-Cr nano-ferrites system indicating that the system obeys Vegard’s law [11].
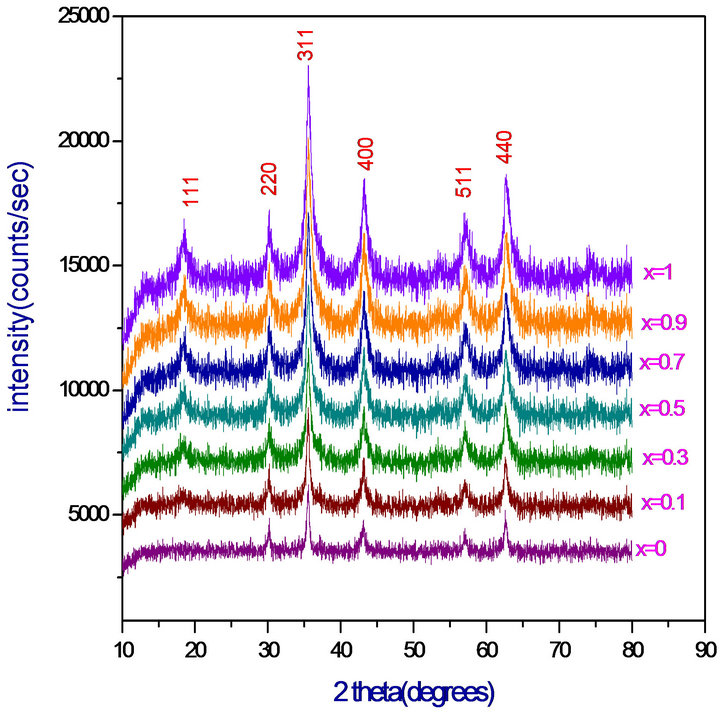
Figure 1. XRD patterns of MgCrxFe2−xO4 (x = 0.0, 0.1, 0.3, 0.5, 0.7, 0.9 and 1.0).
3.2. Dielectric Properties
3.2.1. Dielectric Constant (ε' and ε")
The frequency dependence of the real and imaginary part of dielectric constant (ε' and ε") for all the samples was studied at room temperature in the range of 20 Hz to 2 MHz. Figures 2 and 3 depict the variation of the real and imaginary part of the dielectric constant (ε' and ε") as a function of frequency for mixed ferrites MgCrxFe2−xO4 with different compositions (x = 0.0, 0.1, 0.3, 0.5, 0.7, 0.9 and 1.0). It is observed that all the samples have higher dielectric constant at lower frequency 20 Hz and there is a decreasing trend in value with increasing frequency from 1000 Hz to 2 MHz which is a normal behavior of ferromagnetic materials. The decrease in ε' is sharp initially from 20 Hz to 1000 Hz (lower frequency) and then ε' value decreases slowly with the increase in frequency and showed almost frequency independent behavior at high frequency regions [12]. Similar behavior was observed in our publications on Mg-Zn Ferrites (Ravinder and Latha, 1999), Li-Cd ferrites (Radha and Ravinder, 1995). This normal dielectric behavior of spinel ferrites was attributed to the lagging of existing charge carriers (hopping electrons) between Fe2+ and Fe3+ ions at localized sites, where the Fe2+ ions were formed in the samples during the sintering process at high temperature, responsible for polarization behind the applied field as its frequency increases [13,14]. The data revealed that none of the samples exhibit any anomalous behavior of peaking. The variation of dielectric constant with frequency may be explained on the basis of space-charge polarization phenomenon [15]. According to this, dielectric material has well conducting grains separated by highly resistive grain boundaries. On the application of electric field, space charge accumulates at the grain boundaries
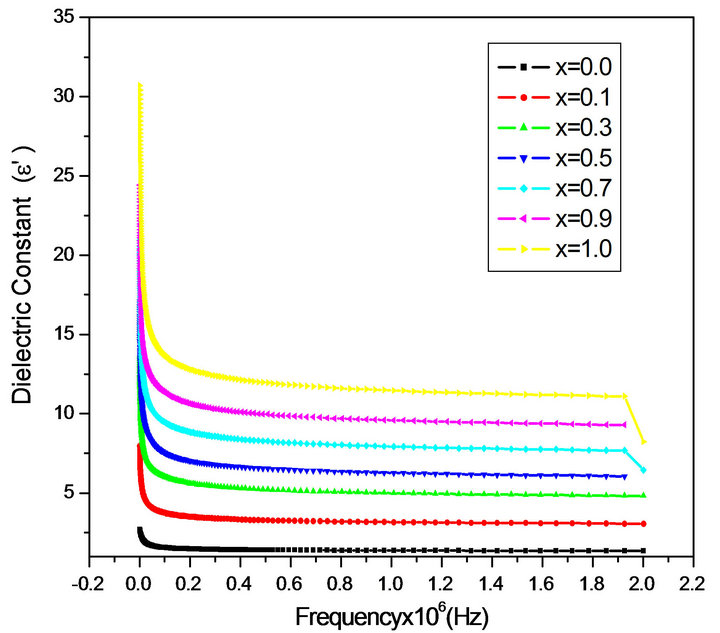
Figure 2. Variation of real part of dielectric constant ε' with frequency.
and voltage drops mainly at grain boundaries [16]. Koops proposed that grain boundary effect is more at low frequencies [16]. As the frequency increased beyond a certain limit the electron exchange between Fe2+ and Fe3+ ions does not follow the variations in applied field, so the value of dielectric constant becomes constant. According to Maxwell and Wagner [17,18] two layer model, spacecharge polarization is because of the inhomogeneous dielectric structure of the material. It is formed by large well conducting grains separated by thin poorly conducting intermediate grain boundaries. Rabinkin and Novikova [19] pointed out that polarization in ferrites is similar to that of conduction. The electron exchange between Fe2+ and Fe3+ ions results in local displacement of electrons in the direction of applied field that determines polarization. Polarization decreases with increasing value of frequency, and then reaches a constant value. It is due to the fact that beyond a certain frequency of external field, the electron exchange Fe2+↔ Fe3+ cannot follow the alternating field. The high value of dielectric constant at lower frequency is due to the predominance of the species like Fe2+ ions, oxygen vacancies, grain boundary defects, etc. [18] while the decrease in dielectric constant with frequency is natural that is any species contributing to the polarizability is found to show the applied field lagging behind at higher frequencies [20].
3.2.2. Loss Tangent (tan δ)
The value of tan δ measures the loss of electrical energy from the applied electric field into the samples at different frequencies. It is observed that the tan δ shows a decreasing trend with increasing in frequency. The loss tangent (tan δ) is defined as the ratio of the loss or resistive current to the charging current in sample. Also it is known that there is strong correlation, between the conduction
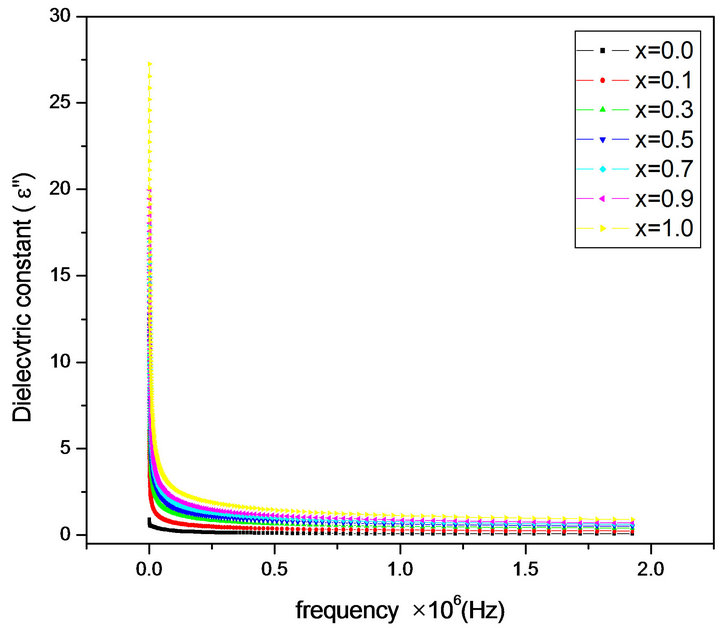
Figure 3. Variation of imaginary part of dielectric constant ε' with frequency.
mechanism and the dielectric constant behavior (Polarization mechanism) in ferrites. Variation of tan δ (the loss tangent) as a function of frequency (20 Hz to 2 MHz) at room temperature for all compositions is shown in Figure 4. The tan δ decreases with the increasing frequency which is normal behavior of any ferrite materials. The values of dielectric loss tangent decrease from 0.09 (x = 0.1) to 0.054 (x = 0.9) at 2 MHz. This shows that with increase in Cr concentration the energy losses decrease at high frequencies. The low loss values at higher frequencies show the potential applications of these materials in high frequency micro wave devices. From the figure it is clear that the loss decreases rapidly in the low frequency region while the rate of decrease is slow in high-frequency region and it shows an almost frequency independent behavior in high frequency region. The behavior can be explained on the basis that in the low frequency region, which corresponds to a high resistivity (due to the grain boundary), more energy is required for electron exchange between Fe2+ and Fe3+ ions, as a result the loss is high. In the high frequency region, which corresponds to a low resistivity (due to the grains), small energy is required for electron transfer between the two Fe ions at the octahedral site. Moreover, the dielectric loss factor also depends on a number of factors such as stoichiometry, Fe2+ content, and structural homogeneity which in turn depend upon the composition and sintering temperature of the samples [21].
3.2.3. AC Conductivity (σAC)
Conductivity is the physical property of a material which characterizes the conducting power inside the material. The electrical conductivity in ferrites is mainly due to the hopping of electrons between the ions of the same element presented in more than one valence state. Figure 5 shows the variation of the AC conductivity (σAC) of mixed MgCr ferrites of all compositions as a function of frequency
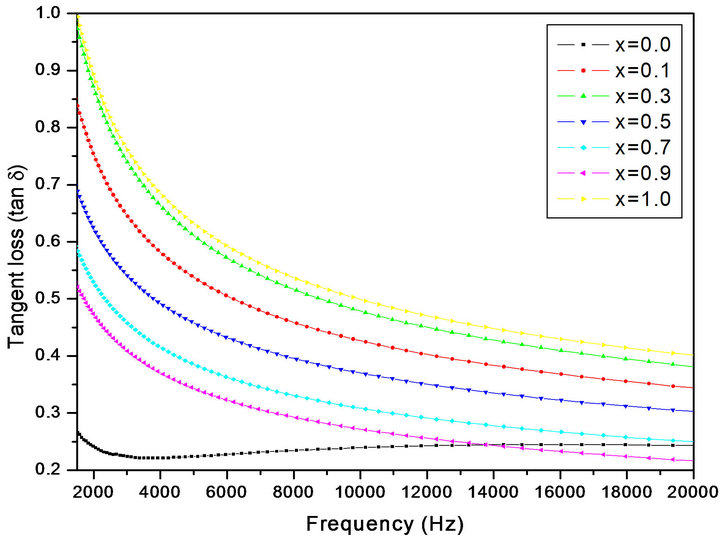
Figure 4. Variation of dielectric loss tangent (tan δ) with frequency.
in the range of 20 Hz to 2 MHz at room temperature. At low frequency range the AC conductivity was nearly independent of the frequency and showed an increasing trend with increase in frequency for all the samples. This behavior is akin to Maxwell-Wagner type. The dielectric structure of ferrites is given by Koops phenominologics theory and Maxwell-Wagner theory [18,22]. At lower frequencies the conductivity was found low due to the grain boundaries that are more active which acts as hindrance for mobility of charge carriers and hence the hopping of Fe2+ and Fe3+ ions is less at lower frequencies. As the frequency of applied field is increased, the conductive grains become more active thereby promoting the hopping between Fe2+ and Fe3+ ions and also responsible for creating charge carriers from different centers. These charge carriers take part in the conduction phenomenon thereby increasing the AC conductivity. The linear increase in conductivity was observed with frequency that confirms the polaron type of conduction. The frequency dependent conduction is attributed to small polarons [23]. At higher frequency where conductivity increases greatly with frequency, the transport is dominated by contributions from hopping infinite clusters.
3.2.4. Compositional Dependence of Dielectric Parameters (ε', ε", tan δ and σAC)
Figures 6-9 represent the variation of dielectric parameters (ε', ε", tan δ and σAC) as a function of Cr composition at selected frequencies (20 Hz, 1000 Hz, 2000 Hz, 3000 Hz, 1 MHz and 2 MHz) respectively. It can be seen that all the dielectric parameters ε', ε", tan δ and σAC increase upto 10% of Cr doping, thereafter, these parameters decrease with further doping of Cr which is clear from Table 1. It shows the values of dielectric parameters for different compositions of the Mg-Cr ferrite system at particular frequencies. The initial increase in dielectric constant up to x = 0.3 may be due to the formation of Fe3+
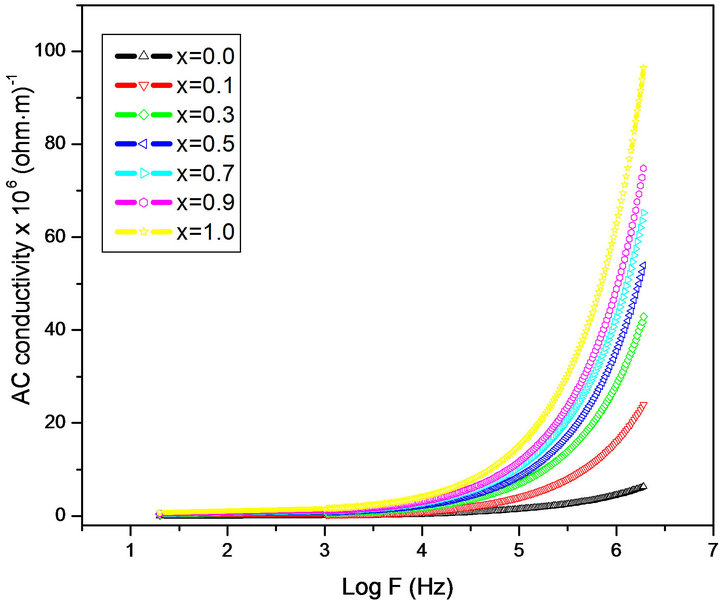
Figure 5. Variation of AC conductivity (σAC) with log F.
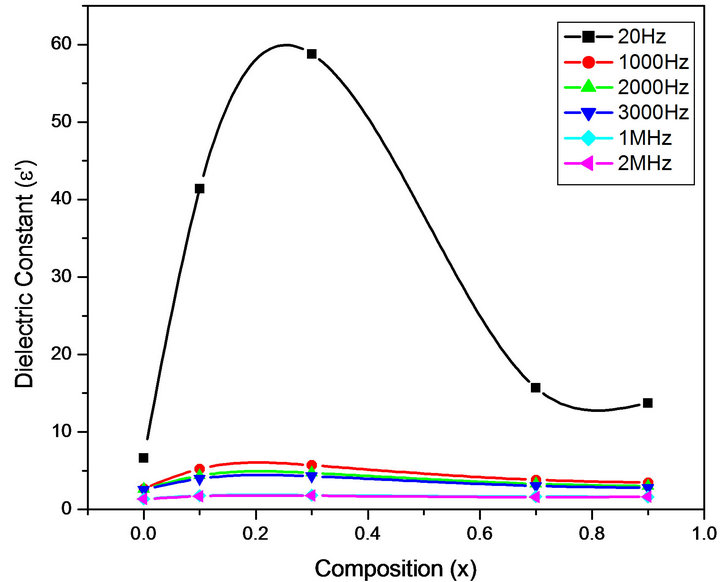
Figure 6. Variation of ε' with composition at selected frequencies.
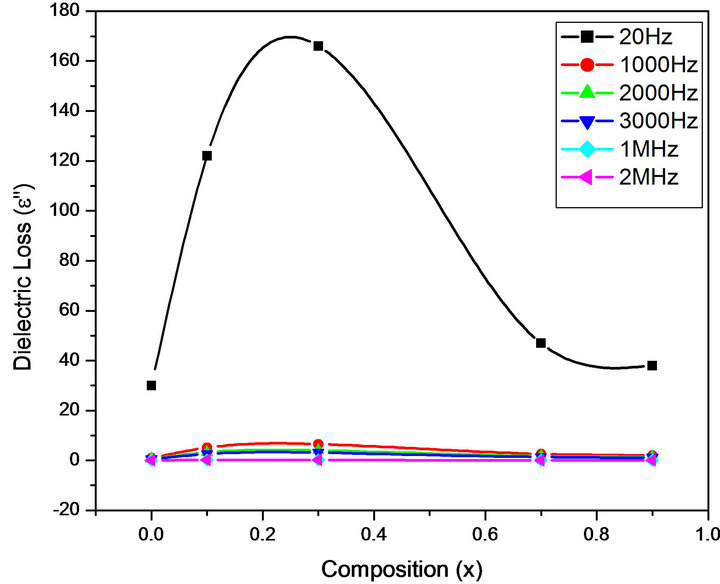
Figure 7. Variation of ε" with composition at selected frequencies.
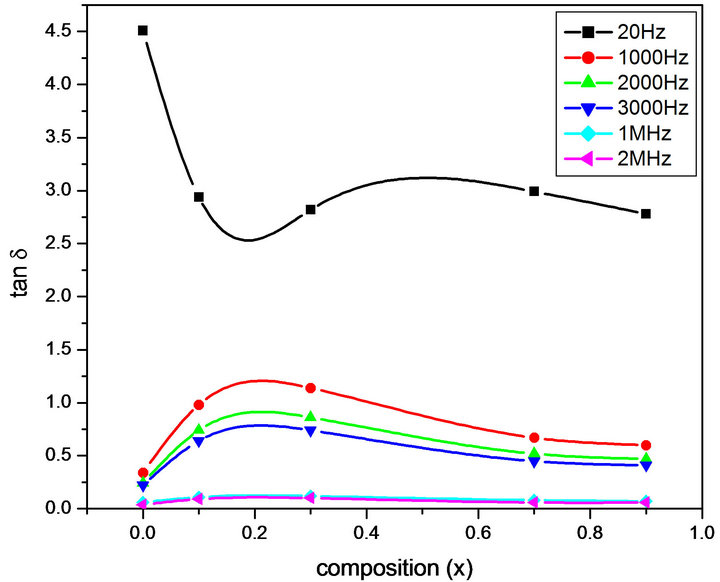
Figure 8. Variation of tan δ with composition at selected Frequencies.
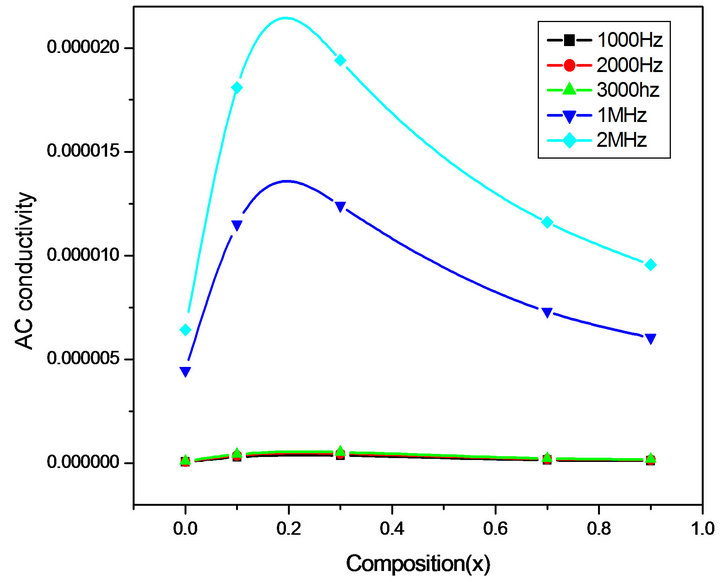
Figure 9. Variation of σAC with composition at selected frequencies.
ions on octahedral sites. This typical behavior can be explained on the basis that in Cr containing ferrites, Cr ions prefer to occupy the octahedral coordination until the ratio of Cr substitution becomes greater than 60%, whereafter, Cr ions may increase in tetrahedral sites causing migration of equal number ions to the octahedral sites [24]. The behavior can be explained by assuming that the mechanism of dielectric polarization is similar to that of the conduction in ferrites (Robinkin and Novikova, 1960). They observed that the electronic exchange interaction between Fe2+↔Fe3+ results in local displacement of the electrons in the direction of an electric field which determines the polarization of ferrites. The presence of Fe2+ ions in excess amount favors the polarization effect [25]. Thus more dispersion was observed in the sample with low Cr3+ ion substitution. This is because at low Cr3+ concentration the presence of Fe2+ ions is in excess amount. As the Cr3+ ion substitution increased it occupies the octahedral site in the ferrite system, thereby decreasing the number of Fe3+ ions and there is a least possibility of electronic exchange interaction between Fe2+↔Fe3+, which results in decrease in dielectric parameters with increasing Cr content in the present system. Table 1 shows the values of dielectric parameters for different compositions of the Mg-Cr ferrite system at selected frequencies.
4. Conclusions
We have successfully synthesized single phase MgCrxFe2−xO4 nano ferrites with cubic spinel structure through Citrate-gel auto combustion method with very fine crystallite size of the particles ranging from 7 - 23 nm.
• The dielectric constant of all the ferrites is very low at low frequency which may be due to low content of polarizable Fe2+ ions on the octahedral site.
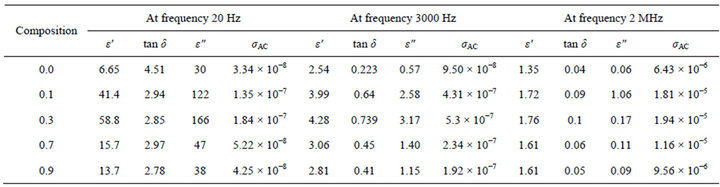
Table 1. Dielectric parameters of different compositions of MgCrxFe2−xO4 at selected frequencies (20 Hz, 3000 Hz and 2 MHz).
• A normal dispersion in dielectric parameters (ε', ε", tan δ) with frequency was observed for all samples and this has been explained on the basis of space charge polarization mechanism as discussed in MaxwellWagner model.
• AC conductivity measurement indicates that with increase in Cr3+ substitution Mg-Cr nano ferrites, AC conductivity increases linearly with frequency which suggest that the conduction in the present system may be due to the polaron hopping mechanism.
• The values of dielectric loss tangent decrease from 0.09 (x = 0.1) to 0.054 (x = 1.0) at 2 MHz. This shows that with increase in Cr concentration in Mg-Cr nano ferrites the energy losses decrease at high frequencies. The low loss values at higher frequencies show the potential applications of these materials in high frequency micro wave devices.
5. Acknowledgements
One of the authors (MRS) is grateful to K. S. Ravikumar, Chairman, Jayaprakash Narayan College of Engineering, Mahabunagar (Dist) for his support and continuous encouragement in carrying out research work. One of the authors (D.R) is grateful to Prof. T. L. N. Swamy, Principal Nizam College for his encouragement to carry out this research work. The authors are thankful to Prof. C. Gyana Kumari, Head, Department of Chemistry, Osmania University, Hyderbad for her encouragement in carrying out the research activities.
REFERENCES
- Y. Yamamoto and J. Makino, “Core Losses and Magnetic Properties of Mn-Zn Ferrites with Fine Grain Sizes,” Journal of Magnetism and Magnetic Materials, Vol. 133, No. 1-3, 1994, pp. 500-503. doi.10.1016/0304-8853(94)90607-6.
- J. Smit and H. P. G. Wijn, “Ferrites,” John and Wiley & Sons, Inc., Hoboken, 1959, p. 136.
- M. Sugimoto, “The Past, Present, and Future of Ferrites,” Journal of Ceramic American Society, Vol. 82, No. 2, 1999, p. 269.
- K. Kondo, T. Chiba and S. Yamada, “Effect of Microstructure on Magnetic Properties of Ni-Zn Ferrites,” Journal of Magnetism and Magnetic Materials, Vol. 541, 2003, p. 254.
- M. P. Reddy, G. Balakrishnaiah, W. Madhuri, M. Venkata Ramana, N. Rammanohar Reddy, K. V. Siva Kumar, V. R. K. Murthy and R. Ramakrishna Reddy, “Structural, Magnetic and Electrical Properties of NiCuZn Ferrites Prepared by Microwave Sintering Method Suitable for MLCI Applications,” Journal of Physis and Chemistry of Solids, Vol. 71, 2010, p. 1373.
- L. B. Kong, Z. W. Li, G. Q. Lin and Y. B. Gan, “Magneto-Dielectric Properties of Mg-Cu-Co Ferrite Ceramics: II. Electrical, Dielectric, and Magnetic Properties,” Journal of American Ceramic Society, Vol. 90, 2007, p. 2014. doi:10.1111/j.1551-2916.2007.01691.x
- Y. Konseoglu, H. Kavas and B. Aktas, “Surface Effects on Magnetic Properties of Superparamagnetic Magnetite Nanoparticles,” Physics Status Solidi A, Vol. 203, 2006, p. 1595. doi:10.1002/pssa.200563104
- S. L. Gupta and S. Gupta, “Electricity, Magnetism and Electronics,” Vol. 1, Jaiprakash Nath and Company, 1991, p. 60.
- E. Pervaiz and I. H. Gul, “Enhancement of Electrical Properties Due to Cr+3 Substitution in Co-Ferrite Nano Particles Synthesized by Two Chemical Techniques,” Journal of Magnetism and Magnetic Materials, Vol. 324, No. 22, 2012, pp. 3695-3703. doi.10.1016/j.jmmm.2012.05.050
- B. D. Cullity, “Elements of XRD,” Addison Weseley Publishing, Redading, 1959, p. 132.
- M. J. Iqbal, M. N. Ashiq, P. Hernandez-Gomezb and J. M. Munoz, “Synthesis, Physical, Magnetic and Electrical Properties of Al-Ga Substituted Co-Precipitated Nanocrystalline Strontium Hexaferrite,” Journal of Magnetism and Magnetic Materials, Vol. 320, 2007, No. 6, pp. 881-886. doi.10.1016/j.jmmm.2007.09.005
- B. Chandra Babu, V. Naresh, B. Jayaprakash and S. Buddhudu, “Structural, Thermal and Dielectric Properties of Lithium Zinc Silicate Ceramic Powders by Sol-Gel Method,” Ferro Electrics Letter, Vol. 38, 2011, p. 124.
- S. Sindhu, M. R. Antharaman, B. P. Thampi, K. A. Malini and Ph. Kurian, “Tailoring Magnetic and Dielectric Properties of Rubber Ferrite Composites Containing Mixed Ferrites,” Bulletin Materials Science, Vol. 24, 2001, pp. 623-629. doi.10.1007/BF02704011
- A. A. Zaky and R. Hawley, “Dielectric Solids,” Routledge and Kegan Paul Ltd., London, 1990, p. 21
- N. Rezlescu and E. Rezlescu, Physics Status Solidi A, Vol. 23, 1974, p. 575
- I. H. Gul, A. Maqsood, M. Naeem and M. Naeem Ashiq, “Optical, Magnetic and Electrical Investigation of Cobalt Ferrite Nanoparticles Synthesized by Co-Precipitation Route,” Journal of Alloys and Compounds, Vol. 507, No. 1, 2010, pp. 201-206. doi.10.1016/j.jallcom.2010.07.155
- J. C. Maxwell, “Electricity and Magnetism,” Vol. 1, Oxford University Press, Oxford, 1929.
- K. W. Wagner, “Zur Theorie der Unvolkommenen DiElektrika,” American Physics, American Physics, Vol. 40, 1913, p. 817.
- I. T. Rabinkin and Z. I. Novikova, “Ferrites,” IZV Acad. Nauk USSR Minsk, 1960.
- B. Baruwati, K. M. Reddy, V. Sunkara, R. K. Manorama, O. Singh and J. Prakash, “Tailored Conductivity Behavior in Nanocrystalline Nickel Ferrite,” Applied Physics Letters, Vol. 85, No. 14, 2004. doi.10.1063/1.1801685
- R. S. Devan and B. K. Chougule, “Effect of Composition on Coupled Electric Magnetic and Dielectric Properties of Two Phase Particulate Magnetoelectric Composite,” Journal of Applied Physics, Vol. 101, No. 1, 2007, Article ID: 014109
- C. G. Koops, Physics Revision, “On the Dispersion of Resistivity and Dielectric Constant of Some Semiconductors at Audiofrequencies,” Vol. 83, No. 1, 1951, pp. 121- 124. doi.10.1103/PhysRev.83.121
- D. Alder and J. Fienleib, “Electrical and Optical Properties of Narrow-Band Materials,” Physics Revision B, Vol. 2, 1970, p. 3112. doi.10.1103/PhysRevB.2.3112
- D. Elkony, “Egypt,” Journal of Solids, Vol. 27, No. 2, 2004, p. 285.
- S. E. Shirsath, B. G. Toksh, M. K. L. Mane, V. N. Dhage, D. R. Shengule and K. M. Jadhav, “Frequency, Temperature and In+3 Dependent Electrical Conduction in NiFe2O4 Powder,” Powder Technology, Vol. 212, No. 1, 2011, pp. 218-223. doi.10.1016/j.powtec.2011.05.019
NOTES
*Corresponding author.

