Journal of Modern Physics
Vol. 2 No. 7 (2011) , Article ID: 5784 , 3 pages DOI:10.4236/jmp.2011.27076
Effect of Glass Composition on the Thermal Expansion of Relict Crystals of RuO2 in Doped Lead-Silicate Glasses (Thick Film Resistors)
Institute of Power Engineering, Uzbek Academy of Sciences, Tashkent, Uzbekistan
E-mail: gulmirzo@mail.ru
Received January 8, 2011; revised March 6, 2011; accepted March 17, 2011
Keywords: Ruthenium Dioxide, Anomalous Thermal Expansion, High Temperature X-Ray Diffraction, Anomalies of Resistivity and Thermopowe
ABSTRACT
The thermal expansion coefficients (TEC) of RuO2 crystallits in thick film resistor (TFR) composites, consisting of RuO2 dispersed in lead-silicate glass of various compositions, were evaluated from X-ray diffraction patterns at temperatures 298; 773; 973 and 1123 K corresponding to characteristic temperatures of resistivity and thermopower anomalies of the TFRs. It has been found that TEC of free RuO2 powder along a-axis has an anomaly at T > 973 K (expansion is replaced by constriction), whereas constriction along c-axes remains for all temperatures. This anomaly disappears in doped glass of simplest composition (2SiO2×PbO) but occurs in glasses of some complex compositions. Symmetry of unit cell of RuO2 is not changed in the temperature range investigated.
1. Introduction
The structure of TFRs consisting of glass frit mixed with conducting powders (CP) of ruthenium oxide or metal ruthenates (namely, RuO2, Pb2Ru2O6 and Bi2Ru2O7) was investigated to explain their conduction mechanism [1-4]. One of their peculiar behaviors is the fact that major portion of CP crystals remain in TFRs after firing. This is why many authors conclude that electrical conduction in TFR takes place due to infinite cluster(s) formed by linked CP particles.
This point of view and anomalies of the resistivity ρ(T) and thermopower S(T) of TFR at T > 700 K [5] as well as effect of CP content on sign and value of dρ/dT of the TFRs contradict one another.
Based on previous studies [6-8] and investigations of properties of RuO2 at T < 500 K, it is assumed that RuO2 does not exhibit the same anomalies.
The thermal expansion coefficient (TEC, α) of RuO2 is anisotropic [6,7]—the unit cell is expanded in the a direction (αa=100·10−7 K−1), while in the c direction it is constricted (αc= −23·10−7 K−1). Lead-silicate glasses used in experiments have α ≈ 70·10−7 K−1. So particles of RuO2 in TFR are deformed {anisotropically} because of temperature variations.
But, to our knowlwgde, this problem has not been investigated anywhere.
In addition, conduction of the RuO2 powders we used is semiconductor-like [9], due to nonstoichiometry of its composition, which may affect their thermal expansion as well. In this connection, we have investigated thermal expansion of RuO2 as free powder and in TFR, i.e. crystallites dispersed in glass, at 298; 773; 973 and 1123 K.
The samples we have investigated are free powders as well (i.e. without the substrate) and are prepared by standard technology without substrate (fired at 850˚C in 10 min—see, for example, [5]).
Temperature points mentioned above are characteristic for anomalies in the ρ(T) and S(T) of TFR [5]. The glasses examined have the following composition (weight %):
1) SiO2 33; PbO 67;
2) SiO2 33; PbO 63; Al2O3 4;
3) SiO2 27; PbO 67; BaO 4; MgO 2.
Content of CP was 16 mass% in all cases.
Using HTK-10 Anton PAAR high-temperature powder camera and CuKα radiation from Siemens D500 X-ray unit powder diffraction patterns were taken at temperatures 298; 773; 973 and 1123 K at the Institute of Catalysis of Siberian Branch of the Russian Academy of Sciences (Novosibirsk).
2. Experimental Results
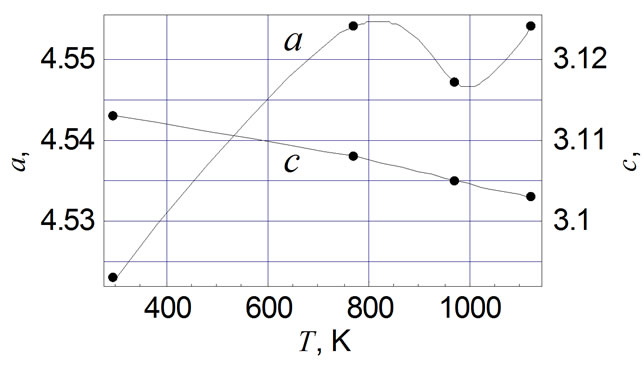
Table 1 shows the unit cell parameters at 298 K (accuracy of measurement is ±0.001 Å) of RuO2 free powder and crystallits in TFRs made with glasses of various compositions (see above). The sequence of main reflexes of RuO2 on X-ray patterns remains unchanged in all cases, indicating the symmetry of the unit cell is remained. Changes of unit cell parameters of free RuO2 powder are shown in Figure 1(a) in the temperature range 298 - 1123 K. Thermal expansion coefficients (TEC) αa and αc of free powder and crystallites in TFRs, evaluated in the temperature range 298 - 1123 K, are listed in Table 2, together with average values of TEC from [11,12]. It is characteristic that values of αa and αc we evaluated in temperature range 298 - 773 K are nearly 50 % higher than that of single crystal of RuO2 [11,12]. Partial substitution of PbO by Al2O3 in glass 1 does not change the variation of αa(T) characteristic for TFR based on glass 1 (Figure 1(b)) but changes the αc(T) (Figure 1(c)) —crystallites expand up to 973 K in the c direction instead of contraction, indicating that strengthening of the glass network by Al2O3 tends to force the RuO2 particles to extend in concordance with the glass. This conclusion is confirmed by Figure 1(d) showing a(T) and c(T) of TFR based on glass 3. Part of the SiO2 is replaced by BaO and MgO in this glass. BaO and MgO are not glass network formers in silicate glass and do not change usual properties of glass [10]. But partial depolymerisation of the glass network in this case allows RuO2 crystallites to extend more freely in comparison with TFR based on glass 2. This effect appears as nonmonotonic behavior of a(T) and c(T).
The values of a and c we obtained are significantly higher than those of [11-13], and indicate that our samples have the most defective structure and nonstoichiometric composition caused by oxygen enrichment. This might be a possible reason for the appearence of the semiconductor properties in these powders [9].
Changes of unit cell parameters of free powder of the RuO2 are shown in Figure 1(a) in the temperature range 298 - 1123 K. Temperature dependence of c(T) is monotonic and is in agreement with the results of other authors
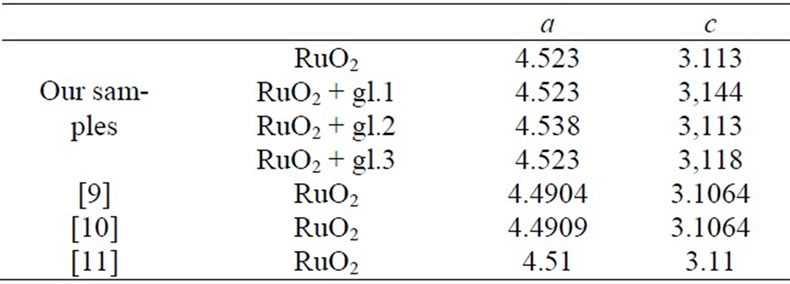
Table 1. Parameters of the unit cell of RuO2 (Å) at 298 K.
[11,12] while the minimum of a(T) occurs at 973 K in contrast to the results of [11,12].
The TEC αa and αc of the tree powder and relicts of RuO2 in TFR were evaluated in 298 - 1123 K temperature range and are listed in Table 2 where mean values of TEC from [11,12] in same temperature interval are listed for comparison as well.
It is characteristic that values of αa and αc we evaluated in temperature range 298 - 773 K are nearly 50 % larger than that for the single crystal of RuO2 [11,12].
The anomaly of αa disappears in the TFR made of the simplest lead-silicate glass 1 and its value decreases up to the half of the original value. Shape of the αc(T) is deformed slightly althought the module of αc is increased nearly fourfold.
Substitution of the part of PbO by Al2O3 in glass 1 does not change the characteristic variation of αa(T) of TFR based on the glass 1 (Figure 1(b)) but changes the αc(T) (Figure 1(c))—relicts of RuO2 in c direction expand up to 973 K instead of constriction. So strengthening of the glass frame due to substitute Al2O3 leads to the bond strengthening between RuO2 particles and glass so last forces their to extend in concord.
3. Conclusions
X-ray diffraction patterns of TFR at high temperature
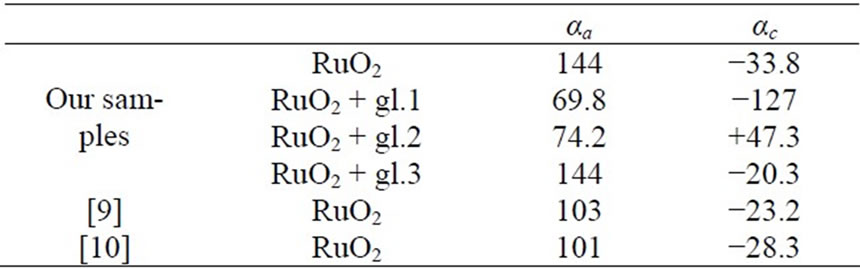
Table 2. TEC of RuO2 (10−7 K−1).
 (a)
(a)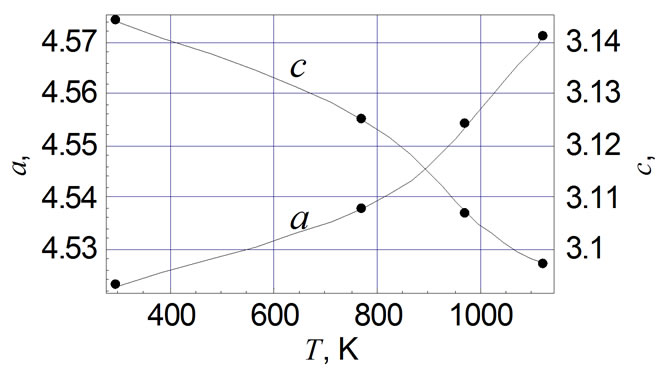 (b)
(b)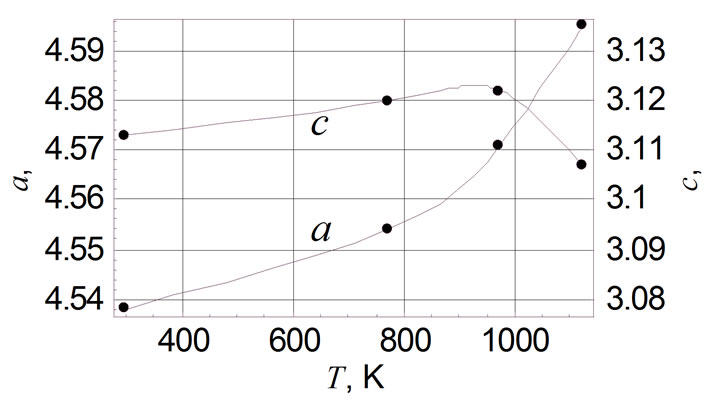 (c)
(c) (d)
(d)
Figure 1. Temperature dependence of unit cell parameters of RuO2: (a) free powder; (b) with glass 1; (c) with glass 2; (d) with glass 3.
show that embedded RuO2 crystallites are stressed due to mismatch of TEC of glass and RuO2 crystals. αa(T) of the latter is positive and almost twice as large as TEC of glass while αc(T) is negative with absolute value being half that of glass TEC. Composition of glasses used in TFR strongly affects TEC of RuO2 crystallites up to change of its sign. It is possible that enigmatic behavior of TFR, e.g. quadratic temperature dependence of resistance, is caused by thermal deformation of RuO2 crystallites.
4. Acknowledgements
The author acknowledges A. Ziborev from the Institute of Catalysis (Novosibirsk) for survey of X-ray patterns.
REFERENCES
- M. Prudenziati, Ed., “Thick Film Sensors,” Elsevier Science, Amsterdam, 1994.
- G. E. Pike and C. H. Seager, “Electrical Properties and Conduction Mechanisms of Ru-Based Thick-Film (Cermet) Resistors,” Journal of Applied Physics, Vol. 48, No. 12, 1977, pp. 5152-5169. doi:10.1063/1.323595
- K. Flachbart, V. Pavlík, N. Tomašovičová, C. J. Adkins, M. Somora, J. Leib and G. Eska, “Conduction Mechanism in RuO2-Based Thick Films,” Physica Status Solidi (b), Vol. 205, No. 1, 1998, pp. 399-404. doi:10.1002/(SICI)1521-3951(199801)205:1<399::AID-PSSB399>3.0.CO;2-X
- B. Morten, M. Prudenziati, M. Sacchi and F. Sirotti, “Phase Transitions in Ru Based Thick-Film (Cermet) Resistors,” Journal of Applied Physics, Vol. 83, No. 7, 1988, pp. 2267--2272. doi:10.1063/1.341119
- G. Abdurakhmanov and N. G. Abdurakhmanova, “High Temperature Anomalies in Resistivity and Thermoelectric Power of Thick Film Resistors,” Physica Status Solidi (b), Vol. 202, No. 9, 2005, pp. 1799-1803. doi:10.1002/pssa.200420036
- K. Adachi and H. Kuno, “Decomposition of Ruthenium Oxides in Lead Borosilicate Glass,” Journal of the American Ceramic Society, Vol. 80, No. 5, 1997, pp. 1055-1064. doi:10.1111/j.1151-2916.1997.tb02946.x
- K. Adachi, S. Iida and K. Hayashi, “Ruthenium Clusters in Lead-Borosilicate Glass in Thick Film Resistors,” Journal of Materials Research, Vol. 9, No. 7, pp. 1866-1878. doi:10.1557/JMR.1994.1866
- S. Gabani, K. Flachbart, V. Pavlik, A. Pietrikova and M. Gabaniova, “Microstructural Analysis and Transport Properties of RuO2-Based Thick Film Resistors,” Acta Physica Polonica A, Vol. 113, No. 1, 2008, p. 625.
- G. Abdurakhmanov and G. S. Vakhidova, “Conductivity Character of Ruthenium Dioxide Used in Thick Film Compositions,” Izvestiya Akademii Nauk UzSSR, Seriya fiz.-mat. Nauk, Reports of the Uzbek Academy of Sciences —Physics and Mathematics, in Russian, No. 2, 1989, p. 83.
- W. Eitel, “The Physical Chemistry of the Silicates,” The Russian Translation, Publishing House of Foreign Literature, Moscow, 1962, p. 1055.
- K. Rao and L. Iyengar, “X-Ray Studies on the Thermal Expansion of Ruthenium Dioxide,” Acta Crystallographica, Vol. 25, No. 2, 1969, pp. 302-303. doi:10.1107/S0567739469000465
- J. M. Fletcher, W. E. Gardner, B. F. Greenfield, M. J. Holdoway and M. H. Rand, “Magnetic and Other Studies of Ruthenium Dioxide and Its Hydrate,” Journal of the Chemical Society A, 1968, pp. 653-657. doi:10.1039/j19680000653
- Ruthenium Chemistry (Khimiya Ruteniya), Ed., “O. E. Zvyagintcev,” Nauka Publishing House, Moscow, 1965., p. 301.

