Advances in Bioscience and Biotechnology
Vol.4 No.5(2013), Article ID:31937,6 pages DOI:10.4236/abb.2013.45081
Expression of α6 integrin subunit in bovine oocyte and its potential role during fertilization
![]()
1Department of Animal Reproduction, College of Veterinary Medicine and Animal Science, São Paulo University, São Paulo, Brazil
2Department of Surgery, Division of Urology, Human Reproduction Section, São Paulo Federal University, São Paulo, Brazil
3Laboratory of Animal Endocrinology, College of Veterinary Medicine, São Paulo State University, Araçatuba, Brazil
Email: ona@usp.br
Copyright © 2013 Roseli Fernandes Gonçalves et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received 12 March 2013; revised 23 April 2013; accepted 10 May 2013
Keywords: Integrins; Bovine Oocytes; Fertilization; Sperm-Oocyte Binding
ABSTRACT
Fertilization in mammals requires the successful completion of a sequence of steps, starting with the transport of gametes in the reproductive tract and ending with sperm-egg membrane fusion to produce a zygote. Although some integrin subunits are known to be associated with the plasma membrane of some mammalian oocytes and spermatozoa, the presence of α6 integrin on bovine oocytes with intact zona pellucida has not been reported. The present study was undertaken to evaluate the expression of α6 integrin subunit in bovine oocyte and to determine if in vitro binding to the zona pellucida and fertilization were affected by treating oocytes with α6 integrin subunit antibody. The α6 integrin subunit was identified on the bovine oocyte by immunocytochemistry. In vitro fertilization was significantly decreased when in vitro matured bovine oocytes were pre-incubated with α6 integrin subunit antibody at concentration 5 and 20 µg/mL, and spermoocyte binding increased. These studies demonstrated the presence of α6 integrin subunit on bovine oocyte, and its importance in fertilization and polyspermy.
1. INTRODUCTION
Fertilization process is a highly complex and orchestrated event in the series of cellular mechanisms that pass the genome from one generation to the next and initiate development of a new organism. In mammals, fertilization is initiated by the direct interaction of sperm and egg membranes, a process mediated by gametes surface proteins [1].
Understanding the role in fertilization of gamete specific surface proteins and other surface proteins expressed, which both contribute to sperm-egg interactions is, at present, a pivotal area of research. Among the best characterized biomarkers for assessment of sperm-egg interactions are the integrins. Integrins are a family of heterodimeric cell adhesion molecules that mediate cell-cell and cell-extracellular matrix interactions. Up to now, 18 α subunits (120 - 180 kDa) and 8β subunits (90 - 110 kDa) were identified; these combine to make 24 different integrins [2,3]. Many integrins were able to recognize the tripeptide sequence arginine-glycine-aspartic acid (RGD). Integrins further attach to the cell, specifically to the extracellular matrix and participate in cell migration [1].
Integrins may be involved in the process of fertilization [1,4,5]. Coincubation of RGD-containing peptides with human sperm and zona-free hamster eggs or with hamster sperm and zona-free hamster eggs resulted in decreasing number of adherent sperm, egg penetration, and fertilization [4]. Also treatment of bovine sperm and oocytes with RGD peptide or with α5 or αV prior to fertilization significantly decreased in vitro sperm-egg binding and fertilization [1]. Oocytes and spermatozoa express a number of integrins and molecules that contain integrin recognition sites. Numerous integrin subunits, including α2, α3, α4, α5, α6, αV, αM, β1, β2, β3, and β5, have been detected in mammalian oocytes at the protein or RNA level [1-3,6-13]. Similarly, ejaculated spermatozoa and spermatogonial cells may express α4, α5, α6, αV, β1, β2, β3, and β5 [12-14]. Confocal microscopy and RT-PCR analysis indicated the presence of α6 and β3 integrin subunit at the plasma membrane of bovine oocytes [15].
Although some integrin subunits are known to be associated with the plasma membrane of some mammalian oocytes and spermatozoa, the presence of α6 integrin on bovine oocytes with intact zona pellucida has not been reported. The present study was undertaken to evaluate the expression of α6 integrin subunit in bovine oocyte and to determine if in vitro binding to the zona pellucida and fertilization were affected by treating oocytes with α6 integrin subunit antibody.
2. MATERIALS AND METHODS
2.1. Chemicals and Reagents
Unless otherwise stated, all chemicals were obtained from Sigma Chemical Co. (St. Louis, MO, USA). Tissue culture media 199 (TCM 199 Hepes and Bicarbonatebuffered), PBS, and fetal calf serum (FSC) were obtained from GibcoTM, Invitrogen Corporation (Grand Island, NY, USA).
2.2. Oocyte Collection and Maturation
Bovine ovaries were harvested at a local abattoir and transported to the laboratory in PBS at 25˚C - 30˚C. Once in the laboratory, ovaries were rinsed with warmed PBS. Follicles from 2 to 6 mm in diameter were aspirated using an 18-gauge needle attached to 5 mL syringe. The ovarian follicular fluid was pooled in 15 mL conical tubes and allowed to settle to the bottom of the tube for 10 - 15 min. Oocytes were selected based on the presence of at least one layer of intact cumulus cells for the sperm-oocyte binding experiments, and two or more intact cumulus cell layers for confocal microscopy and in vitro fertilization.
The cumulus-oocyte complexes (25/well) were matured in four-well culture plates (NunclonTM, Nunc, Roskilde, Denmark) in 500 µL TCM-199, 10% fetal calf serum, LH (6 µg/mL), FSH (8 µ/mL) (Sioux Biochemical®, Sioux Center, IA, USA), and penicillin (100 units/mL)— streptomycin (100 µg/mL) for 24 h at 39˚C, 5% CO2 in air (v/v). After maturation, oocytes were prepared for confocal microscopy, sperm binding and fertilization experiments as described below.
2.3. Localization of α6 Integrin Subunit by Confocal Microscopy
Cumulus cells were removed mechanically by vortexing, 10 min, in 1 mL of maturation medium. All oocytes were fixed with 3.5% (w/v) paraformaldehyde in PBS for 1 h at room temperature (RT). They were washed 3 times, 5 min each, with PBS/PVP, and permeabilized with 0.2% (v/v) Triton X-100 in PBS for 1 h at RT. Subsequently, they were incubated with 10% (v/v) goat serum (Invitrogen) in polyvinyl pyrrolidone (PVP, 0.1% (w/v) in PBS) solution for 30 min at 37˚C, with mouse MAB to α6 integrin subunit (Chemicon International®, Temecula, CA, USA) for 1 h. The oocytes were washed 4 times, 10 min each, in PBS with PVP. After that they were incubated with Alexa Fluor 488 goat-anti-mouse secondary antibody (Invitrogen®, Eugene, OR, USA) for 1 h at 37˚C. They were mounted in a droplet of ProLong® Gold Antifade Reagent with DAPI (Invitrogen®) and evaluated for the presence of α6 integrin subunit using an Olympus BX51 equipped with epifluorescence and a DP71 camera with a 12.5 MPixel CCD microscope.
2.4. Treatments
This study was conduct to determine whether in vitro sperm binding to the ZP of intact bovine oocytes and fertilization were affected by treating oocytes with α6 integrin subunit antibody. In vitro matured oocytes were incubated (39˚C, 5% CO2, in air) for 2 h in fertilization medium with: 1) no antibody; 2) 5 µg/mL α6 integrin subunit antibody; 3) 20 µg/mL α6 integrin subunit antibody; 4) a rabbit IgG against a non-bovine antigen, bacterial histidase (1:2000) [21]. Frozen-thawed bull semen from the same ejaculate was washed using Percoll gradient centrifugation. Briefly, 2 mL of a 90% Percoll solution in modified Tyrode’s medium (MTM; v/v) were placed in the bottom of a 15 mL polypropylene tube and 2 mL of a 45% Percoll solution in MTM (v/v) were gently overlaid on top of it. The straw content was layered onto the 45% solution and centrifuge for 30 min at 700 × g. The pelleted spermatozoa was recovered, assessed for motility and suspended in fertilization medium. Oocytes were inseminated with 10 × 106 washed spermatozoa in the fertilization medium supplemented with 2 µg/mL heparin and 20 µg/mL of PHE solution (20 µM penicillamine, 10 µM hypotaurine, 1 µM adrenaline). Oocytes and spermatozoa were co-incubated for 18 h at 39˚C in 5% CO2 in air (v/v).
2.5. Sperm-Oocyte Binding
After co-incubation oocytes were washed five times in TL-HEPES and placed, 10 per slide, under a coverslip mounted with paraffin wax and petroleum jelly at each corner. The coverslip was gently lowered over the oocytes until they burst, and the cytoplasm was rinsed away leaving the ZP behind. The ZP and spermatozoa attached to it were stained with Hoechst fluorescent dye 33,342 to determine the number of spermatozoa bound to each ZP using fluorescence microscope Olympus BX51 [1].
2.6. In Vitro Fertilization
In vitro matured oocytes were washed twice in HEPES medium, placed in fertilization medium with 2 µg/mL heparin and incubated as described above. After 18 h of co-incubation, oocytes were vortexed to remove cumulus cells and accessory spermatozoa, and washed twice in HEPES medium. The oocytes were placed 10 per slide under a coverslip mounted at the corners with paraffin wax and petroleum jelly. The coverslip was gently lowered over the oocytes and a adhered to the slide with rubber cement. Oocytes were fixed in acid alcohol for 24 h and stained with acetate-orcein [1] the presence of two pronuclei in the cytoplasm of the oocyte indicated normal fertilization.
2.7. Statistical Analysis
Each experiment was repeated four times and data from each experiment were polled. Approximately 40 - 50 oocytes per treatment for sperm binding, and 80 - 90 oocytes per treatment for fertilization were evaluated in each replicate. ANOVA using a general linear model was performed using mean number of spermatozoa bound per ZP for each treatment in the sperm-oocyte binding experiments, and a weighted mean based on the number of oocytes per treatment in the fertilization experiments. Least square means comparisons were used to assess sperm binding and weighted least square means were used to analyze fertilization data. The significance level for these tests was p < 0.05.
3. RESULTS
3.1. Localization of α6 Integrin Subunit by Confocal Microscopy
Expression of α6 integrin subunit could clearly be detected on the zona pellucida of in vitro matured bovine oocytes (Figure 1).
3.2. Binding of Sperm Incubated with α6 Integrin Subunit Antibody to Oocytes
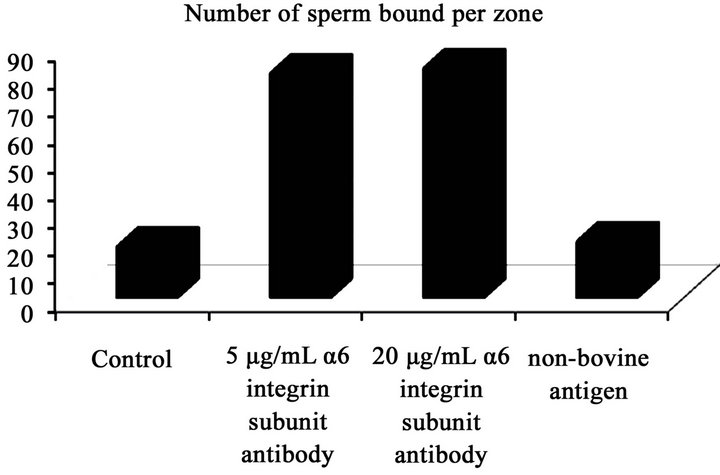
More sperm bound to oocytes treated with a 5 µg/mL and 20 µg/mL α6 integrin subunit antibody than oocytes incubated in control medium and a rabbit IgG against a non-bovine antigen, bacterial histidase (Figure 2(a)): control (18.3 ± 1.7) 5 µg/mL of α6 integrin subunit antibody (80.7 ± 1.9); 20 µg/mL of α6 integrin subunit antibody (82.5 ± 2.5); non-bovine antigen (20.2 ± 1.4).
3.3. In Vitro Fertilization
Treatment of oocytes with α6 integrin subunit antibody decreased fertilization compared to the untreated control (Figure 2(b)): 84% ± 1.5% (control); 40.2% ± 1.7% (5 µg/ mL of α6 integrin subunit antibody); 38.2% ± 1.5% (20 µg/ mL of α6 integrin subunit antibody); 82.8% ± 1.6% (non-bovine antigen).
4. DISCUSSION
The molecular events of sperm-oocyte binding and fusion have been studied extensively, but identification of the molecules involved in sperm-oocyte interaction and fertilization remains incomplete. Integrins are cell surface receptors that mediate cell-cell and cell-extracellular matrix interactions in many cell types. Many molecules have been characterized and suggested to participate in the fertilization process, having integrin as receptors such as CD46, members of the A Desintegrin and A Metalloprotease domains (ADAM) family, secreted prosphoprotein one (SSP1 or OPN) and molecules with RGD peptides sequence [1-6,9,11,18-22]. Inhibition of spermoocyte adhesion/fusion can be performed by monoclonal antibodies, RGD or desintegrin-like peptides and RNAimediated knockdown [16-19].
Integrins are expressed in an inactivated or activated state [23]. Monoclonal antibodies or ligands bind only the activated state. Integrin affinities advance is normally consistent with a conformational change in extracellular domain induced from inside the cell through cytoskeletal modifications or from outside the cell activation through cooperative ligand/receptor systems [24,25]. Several integrin subunits have been detected in mammalian oocytes at the mRNA or at the protein level, including sea urchin, mouse, hamster, human, pig, bovine [1-3,11,15, 26-33].
Bronson and Fusi (1990) were the first to show either with peptides containing the RGD sequence or with anti-integrin monoclonal antibodies that some integrin subunits were present on Syrian hamster unfertilized oocytes (α2, α5, α6β1, αVβ3) and on human oocytes (α2, α5, α6β1) [4,5,28]. Expression of α3, α5 and α6β1 were identified both at the protein and mRNA levels in unfertilized mouse oocytes [2,27]. De Nadia et al. (1996) have studied membrane receptor conservation among species, and through immunofluoresce labeling and Western blot analysis using a variety of mouse anti-integrin antibodies [26]. The same authors have demonstrated the presence of α2, α5, and β1 integrin subunits on sea urchin, hamster
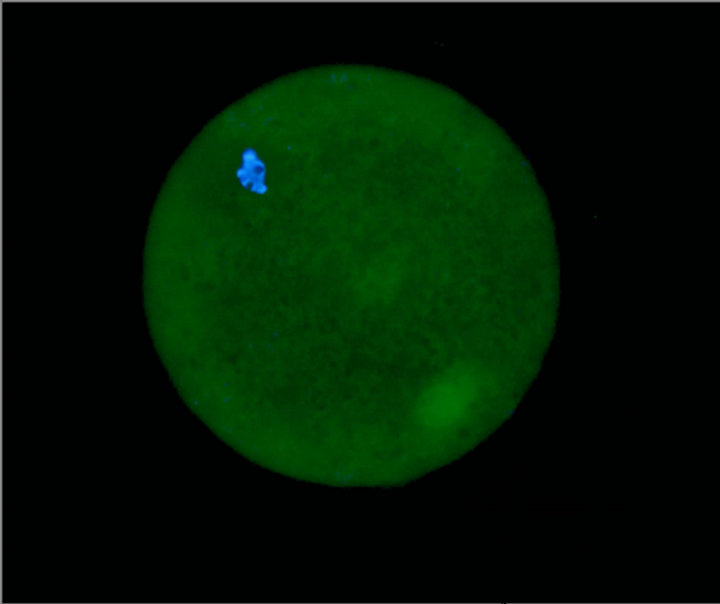
Figure 1. Cumulus denuded ZP intact bovine matured oocyte labeled with mouse MAB to α6 integrin subunit and Alexa Fluor 488 goat-anti-mouse combined with ProLong® Gold Antifade Reagent with DAPI. Bar = 25 µm.
 (a)
(a)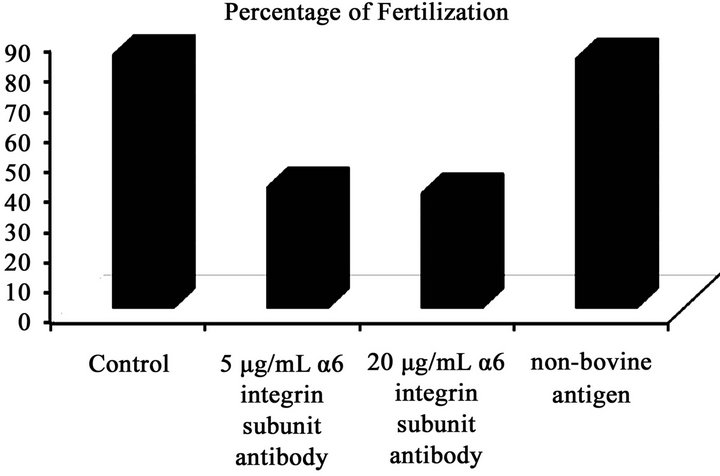 (b)
(b)
Figure 2. (a) Mean (±SEM) number of sperm bound zona pellucida (ZP); (b) mean percentage of fertilized eggs following exposure of oocytes to the treatments: control, 5 µg/mL α6 integrin subunit antibody, 20 µg/mL α6 integrin subunit antibody, a rabbit IgG against a non-bovine antigen, bacterial histidase (1:2000). The study was repeated four times using 40 - 50 (sperm-oocyte binding) and 80 - 90 (fertilization) oocytes per treatment.
and human unfertilized oocytes. They also have found by immunofluorescence studies that both hamster and human oocytes expressed αMβ2, a β2 class antigen, as already suggested by Anderson et al. (1993) [34]. Linfor and Berger (2000) have demonstrated the presence of β1, α1, α2, α3, α4, α5, α6, and αV integrin subunits on the plasma membrane of pig oocytes by immunocytochemistry and confocal microscopy [33].
Evidence for sperm-oocyte interaction through integrins have been established by the presence of α6, α4, α2, αV, β3, and β3 integrin subunits on the bovine oocyte plasma membrane and also supported by the ability of RGDcontaining peptides to induce intracellular calcium transients. Indeed, the parthenogenetic development and the decreased in vitro fertilization have both provided a pivotal role for integrins at oocyte surface, influencing spermoocyte interaction [1,29,31,32]. This is the first study showing the α6 integrin on the bovine oocytes with the zona pellucida intact by confocal microscopy. The binding of monoclonal antibody to α6 integrin subunit to the ZP of bovine maturated oocyte indicates that α6 integrin is part of the bovine oocyte.
The inhibition of sperm-oocyte plasma membrane binding and antibody recognizing of the α6 integrin subunit implies involvement of the α6 in sperm-oocyte interaction. This data also suggest that α6 integrin subunit may be involved in preventing polyspermy in bovine oocytes. Mammalian oocytes have capability to block polyspermy both at the zona pellucida and at the plasma membrane. While zona polyspermy blocking has been well characterized, little is known regarding molecular events surrounding the plasma membrane capabilities in avoiding polyspermy [19,35].
Bovine sperm bound in higher numbers to oocytes treated with anti-α6 integrin subunit than to oocytes incubated in control medium, nevertheless incidence of polyspermic fertilization also increased with antibody-treated oocyte. While it is possible that sperm bound in higher numbers to antibody-coated oocytes than control oocytes through IgG-ZP interactions, a decrease in fertilization rates coupled with an increase in polyspermic fertilizations suggests that α6 integrin subunit on bovine oocyte may participate in the induction of polyspermy blocks in bovine oocytes and in fertilization.
Bovine sperm bound in higher numbers to oocytes treated with anti-α6 integrin subunit than to oocytes incubated in control medium, nevertheless incidence of polyspermic fertilization also increased with antibodytreated oocyte. While it is possible that sperm bound in higher numbers to antibody-coated oocytes than control oocytes through IgG-ZP interactions, a decrease in fertilization rates coupled with an increase in polyspermic fertilizations suggests that α6 integrin subunit on bovine oocyte may participate in the induction of polyspermy blocks in bovine oocytes and in fertilization.
REFERENCES
- Gonçalves, R.F., Wolinetz, C.D. and Killian, G.J. (2007) Influence of arginine-glycine-aspartic acid (RGD), integrins (αV and α5) and osteopontin on bovine sperm-egg binding and fertilization in vitro. Theriogenology, 67, 468-474. doi:10.1016/j.theriogenology.2006.08.013
- Tarone, G., Russo, M.A., Hirsch, E., Odorisio, T., Altruda, F., Silengo, L. and Siracusa, G. (1993) Expression of beta 1 integrin complexes on the surface of unfertilized mouse oocyte. Development, 117, 1369-1375.
- Evans, J.P., Schultz, R.M. and Knopf, G.S. (1995) Identification and localization of integrin subunits in oocytes and eggs of the mouse. Molecular Reproduction and Development, 40, 220-221. doi:10.1002/mrd.1080400210
- Bronson, R.A. and Fusi, F. (1990) Evidence that an arggly-asp adhesion sequence plays a role in mammalian fertilization. Biology of Reproduction, 43, 1019-1025. doi:10.1095/biolreprod43.6.1019
- Bronson, R.A. and Fusi, F. (1990) Sperm-oolemmal interactions: Role of the Arg-Gly-Asp (RGD) adhesion peptide. Fertility and Sterility, 54, 527-529.
- Zuccotti, M., Giorgi Rossi, P., Fiorillo, E., Garagna, S., Farabosco, A. and Redi, C.A. (1998) Timing of gene expression and oolemma localization of mouse alpha6 and beta1 integrin subunits during oogenesis. Developmental Biology, 200, 27-34. doi:10.1006/dbio.1998.8923
- Burns, K.H., Owens, G.E., Fernandez, J.M., Nilson, J.H. and Matzuk, M.M. (2002) Characterization of integrin expression in the mouse ovary. Biology of Reproduction, 67, 743-751. doi:10.1095/biolreprod.101.000729
- Boissonnas, C.C., Montjean, D., Lesaffre, C., Auer, J., Vaiman, D., Wolf, J.P. and Ziyyat, A (2010) Role of sperm alphavbeta3 integrin in mouse fertilization. Developmental Dynamics, 239, 773-783. doi:10.1002/dvdy.22206
- White, K.L.; Pate, B.J. and Sessions, B.R. (2010) Oolema receptors and oocyte activation. Systems Biology in Reproductive Medicine, 56, 365-375.
- Campbell, S., Swann, H.R. Seif, M.W., Kimber, S.J. and Aplin, J.D. (1995) Cell adhesion molecules on the oocyte and preimplantation human embryo. Human Reproduction, 10, 1572-1578. doi:10.1093/HUMREP/10.6.1571
- Ji, Y.Z., Wolf, J.P., Jouannet, P. and Bomsel, M. (1998) Human gamete fusion can bypass beta 1 integrin requirement. Human Reproduction, 13, 682-689. doi:10.1093/humrep/13.3.682
- Sengoku, K., Takuma, N., Miyamoto, T., Horikama, M. and Ishikawa, M. (2004) Integrins are not involved in the process of human sperm-oolemmal fusion. Human Reproduction, 19, 639-644. doi:10.1093/humrep/deh095
- Ziyyat, A., Rubinstein, E., Monier-Gavelle, F., Barraud, V., Kulski, O., Prenant, M. Boucheix, C., Bomsel, M. and Wolf, J.P. (2006) CD9 controls the formation of clusters that contain tetraspaninas and the integrin alpha 6 beta 1, which are involved in human and mouse gamete fusion. Journal of Cell Science, 119, 416-424. doi:10.1242/jcs.02730
- Reddy, V.R.K., Rajeev, S.K. and Gupta, V. (2003) Alpha 6 beta 1 integrin is a potential clinical marker for evaluating sperm quality in men. Fertility Sterility, 79, 1590- 1600. doi:10.1016/S0015-0282(03)00368-6
- Glander, H.J. and Schaller, J. (1993) Beta 1 integrins of spermatozoa: A flow cytophometric analysis. International Journal of Andrology, 16, 105-111. doi:10.1111/j.1365-2605.1993.tb01162.x
- de Barros, F.R., Worst, R.A., Saurin, G.C., Mendes, C.M., Assumpção, M.E. and Visintin, J.A. (2012) α-6 integrin expression in bovine spermatogonial cells purified by discontinuous Percoll density gradient. Reproduction in Domestic Animals, 46, 887-890. doi:10.1111/j.1439-0531.2012.01985.x
- Pate, B.J., White, K.L., Winger, Q.A., Rickords, L.F., Aston, K.L., Sessons, B.R., Li, G.P., Campbell, K.D., Weimer, B. and Bunch, T.D. (2007) Specific integrin subunits in bovine oocytes, including novel sequences for alpha 6 and beta 3 subunits. Molecular Reproduction and Development, 74, 600-607. doi:10.1002/mrd.20649
- Primakoff, P. and Myles, D.G. (1983) A map of the guinea pig sperm surface constructed with monoclonal antibodies. Developmental Biology, 98, 417-428. doi:10.1016/0012-1606(83)90371-8
- Allen, C.A. and Green, D.P. (1995) Monoclonal antibodies which recognize equatorial segmente epitops de novo following the A23187-indiced acrosome reaction of guinea pig sperm. Journal of Cell Science, 108, 767-777.
- Toshimori, K., Saxena, D.K., Tanii, I. and Yoshinaga, K. (1998) An MN9 antigenic molecule, equatorial is required for successful sperm-oocyte fusion in mice. Biology of Reproduction, 59, 22-29. doi:10.1095/biolreprod59.1.22
- Evans, J.P. (2012) Sperm-egg interaction. Annual Review of Physiology, 74, 447-502. doi:10.1146/annurev-physiol-020911-153339
- Gonçalves, R.F., Barnabe, V.H. and Killian, G.J. (2008) Pre-treatment of cattle sperm and/or oocyte with antibody to lipocalin type prostaglandin D synthase inhibits in vitro fertilization and increases sperm-oocyte binding. Animal Reproduction Science, 106, 188-193. doi:10.1016/j.anireprosci.2007.12.019
- Gonçalves, R.F., Staros, A.L. and Killian, G.J. (2008) Oviductal fluid proteins associated with the bovine zona pellucida and the effect on in vitro sperm-egg binding, fertilization and embryo development. Reproduction in Domestic Animals, 43, 720-729. doi:10.1111/j.1439-0531.2007.00978.x
- Gonçalves, R.F., Wolinetz, C.G., Barnabe, V.H. and Killian, G.J. (2009) Influence of osteopontin in bovine uterine tube fluid on sperm binding and fertilization in RCA- 1 lectin-treated oocytes. Reproduction in Domestic Animals, 44, 152-155. doi:10.1111/j.1439-0531.2007.01011.x
- Hynes, R.O. (1992) Integrins: Versatility, modulation, and signaling in cell adhesion. Cell, 69, 11-25. doi:10.1016/0092-8674(92)90115-S
- Hazenbos, W.L., Van Den Berg, B.M. and Van Furth, R. (1993) Very late antigen-5 and complement receptor type 3 cooperatively mediate the interaction between Bordetella pertussis and human monocytes. Journal of Immunology, 151, 6274-6282.
- Fénichel, P. and Durand-Clément, M. (1998) Role of integrins during fertilization in mammals. Human Reproduction, 13, 31-46. doi:10.1093/humrep/13.suppl_4.31
- De Nadai, C., Fénichel, P., Donzeau, M., Epel, D. and Ciapa, B. (1996) Characterization and role of integrins during gametic interaction and egg activation. Zygote, 4, 31- 40. doi:10.1017/S0967199400002860
- Almeida, E.A., Huovila, A.P., Sutherland, A.E., Stephens, L.E., Calarco, P.G., Shaw, L.M., Mercurio, A.M., Sonnenberg, A., Primakoff, P., Myles, D.G. and White, J.M. (1995) Mouse egg. Cell, 81, 1095-1104. doi:10.1016/S0092-8674(05)80014-5
- Fusi, F.M., Vignale, M., Gailit, J. and Bronson, R.A. (1993) Mammalian oocytes exhibit specific recognition of the RGD (Arg-Gly-Asp) tripeptide and express oolemmal integrins. Molecular Reproduction and Development, 36, 212-219. doi:10.1002/mrd.1080360212
- Campbell, K.D., Reed, W.A. and White, K.L. (2000) Ability of integrins to mediate fertilization, intracellular calcium release and parthenogenic development in bovine oocytes. Biology of Reproduction, 62, 1702-1709. doi:10.1095/biolreprod62.6.1702
- Eto, K., Huet, C., Tarui, T., Kupriyanov, S., Liu, H.Z., Puzon-McLaughlin, W., Zhang, X.P., Sheppard, D., Engvall, E. and Takada, Y. (2002) Functional classification of ADAMs based on a conserved motif for binding to integrin alpha 9 beta 1: Implications for sperm-egg binding and other cell interactions. Journal of Biological Chemistry, 277, 17804-17810. doi:10.1074/jbc.M200086200
- Gonçalves, R.F., Bertolla, R.P., Mortara, R.A. and Barnabe, V.H. (2008) Identification of some integrin subunits on cattle (Bos indicus) oocytes. Reproduction in Domestic Animals, 43, 147.
- Pate, B.J., White, K.L., Winger, Q.A., Rickords, L.F., Aston, K.I. and Sessons, B.R. (2007) Specific integrin subunits in bovine oocytes, including novel sequences for alpha 6 and beta 3 subunits. Molecular Reproduction and Development, 74, 600-607. doi:10.1002/mrd.20649
- Linfor, J. and Berger, T. (2000) Potential role of alphav and beta1 integrin as oocyte adhesion molecules during fertilization in pigs. Journal of Reproduction and Fertility, 120, 65-72.
- Anderson, D.J., Abbot, A.F. and Jack, R.M. (1993) The role of complement C3b and its receptors in sperm-oocyte interaction. Proceedings of the National Academy of Science of the United States of America, 90, 10051- 10055. doi:10.1073/pnas.90.21.10051
- Gardner, A.J. and Evans, J.P. (2006) Mammalian membrane block to polyspermy: New insights into how mammalian eggs prevent fertilization by multiple sperm. Reproduction Fertility and Development, 18, 53-61.

