Advances in Bioscience and Biotechnology
Vol.4 No.3A(2013), Article ID:29277,6 pages DOI:10.4236/abb.2013.43A056
Use of Escherichia coli toxins in sepsis models
![]()
1Department of Pharmacology, Dalhousie University, Halifax, Canada
2Department of Anesthesia, Dalhousie University, Halifax, Canada
3Department of Microbiology & Immunology, Dalhousie University, Halifax, Canada
Email: *chlehmann@dal.ca
Received 6 January 2013; revised 5 February 2013; accepted 20 February 2013
Keywords: Sepsis; Endotoxemia; Lipopolysaccharide; LPS
ABSTRACT
The high prevalence of sepsis in intensive care units and emergency rooms, along with the high lethality of the sepsis cases makes the study of pathophysiology of sepsis critically important. As a preclinical model, endotoxemia is an important tool to study the pathophysiology of sepsis and septic shock. In this review, we discussed aspects of endotoxemia as an experimental model in sepsis research, including different techniques associated with the purification of the endotoxin of Escherichia coli, serotype dependency and dosage dependency of the experimental results.
1. INTRODUCTION
Despite advances in critical care medicine, development of new therapeutic approaches including new supportive care programs, establishment of new health care protocols and policies, severe sepsis and septic shock continue to be prevalent in intensive care units and emergency rooms. The lethality of patients severely affected by sepsis ranges from 20 to 50 percent [1], making sepsis the leading cause of death among patients in non-cardiac intensive care units [2]. In addition to being a critical health condition, severe sepsis also puts a heavy socioeconomic strain on the health care system [3]. It is reported in the literature that the care of patients with sepsis costs as much as $50,000 per patient [4], bringing an annual cost of the care to $17 billion, in the United States alone [5]. Furthermore, the survivors of severe sepsis have a substantial reduction in their overall quality of life [6,7].
One of the complicating factors in defining the epidemiology of severe sepsis is that the exact definition of sepsis is being debated and adjusted [5]. There are several studies conducted in United States, which evaluated the incidence of sepsis and attempted to project the mortality rates of sepsis. One of such studies [8] examined discharge data on approximately 750 million hospitalizations in the United States over a period between 1979 and 2000 and identified more than 10 million cases of sepsis, with the results of the study showing an annualized increase in the incidence of sepsis of 8.7 percent, from approximately 164,000 cases (82.7 per 100,000 population) to nearly 660,000 cases (240.4 per 100,000 population) [8]. Another study [5] projected that the number of sepsis cases will increase steadily at 1.5 percent per year and will yield 934,000 and 1,110,000 cases by the years 2010 and 2020 respectively. Letarte et al. [3] conducted a study investigated the costs of severe sepsis and septic shock in Quebec, Canada. The study estimated the burden of severe sepsis to be $36.4 to $72.9 million per year and concluded that the cost of severe sepsis is a significant burden to the Quebec health care system.
In this review, we discuss aspects of endotoxemia as an experimental model in sepsis research. We will discuss the different techniques associated with the purification of the endotoxin of E. coli. In addition, we will attempt to discuss the serotype dependency of the experimental results and present experimental data. Finally, we will discuss the dosage dependency of the experimental results with regard to clinical effects to be observed.
2. SEPSIS MODELS
The etiology of sepsis is related to the primary source of infection and the place of acquisition. Sepsis can potentially be caused by bacteria, fungi, parasites, or virus [9]. Literature reports that there is an increase of bloodstream infections over the last century, with interesting dynamics in the etiology—starting with gram-positive bacteria being the dominant cause in the beginning of the 20th century, then with a strong shift to a higher proportion of gram-negative bacteria induced sepsis to the current shift to fungi as an emerging pathogen [10]. A study conducted in Vincent et al. [11], surveyed the prevalence and outcomes of infection in intensive care units from 75 countries. The results of the study showed that the microbiological culture results were positive in 70% of the infected patients with 62% of the positive isolates being gram-negative organisms, 47% being gram-positive, and 19% being fungal in origin [11].
There is a clear need for preclinical models for the investigation of the pathogenesis of sepsis. It is important to note that in the preclinical models, sepsis is generally observed as an acute syndrome, which differs from the clinical situation, where a non-acute (subacute) or intermittent course may be possible [12]. Most of the preclinical models are systemic in their application, lacking an infectious focus and as a result do not have a localized infectious source, such as an infected organ or cavity from which the infection disseminates in clinical cases [12]. Some of the preclinical models are used because they replicate some of the symptoms observed during sepsis, whereas other preclinical models are used because they reproduce the laboratory findings that are found in septic patients [13-15]. However, most of these models can be essentially classified as bacterial infusion models, infection models and endotoxemia models, in which lipopolysaccharide (LPS) is infused [12].
A number of experiments use an approach where an animal receives an intravenous bolus or short-term continuous infusion of large doses of bacteria [13-15]. However, there are quite a few limitations with this particular model—large doses of bacteria are needed because most of the bacteria are rapidly killed after an intravenous challenge, in addition, this model does not correlate with clinical cases, in which an infectious focus from which bacteria continuously disseminate over time is present [13,14]. Moreover the survival is short, limiting the time for progression of the infection [13,15]. Finally, cytokine responses are transient and are greater in magnitude in mice, rats and baboons than in human patients, complicating the extrapolation of results from these studies to clinical cases [16].
There are also sepsis models that have an infectious focus from which a local infection develops and bacteria disseminate, these models are comparable to clinical cases. Some of the most commonly used bacterial sepsis models is murine pneumonia with Streptococcus pneumoniae, Klebsiella pneumoniae and Pseudomonas aeruginosa [12,17]. Another commonly used approach for a local sepsis infection model is cecal ligation and puncture model (CLP), in which fecal peritonitis is induced via a surgical procedure [13]. For experiments which use a peritonitis model and require a rapid dissemination—an intraperitoneal administration of virulent E. coli is utilized [18].
Another experimental approach that is frequently used as a sepsis model is an intravenous infusion of LPS. Continuous infusion of LPS results in a persistent physiologic response rather than an acute profound response
[13,15]. There are at least four distinct categories of LPSinfusion models that are more accurate at reproducing either compensated sepsis or septic shock in patients [15]. 1) Models that use small sublethal doses of LPS [16]; 2) models that utilize continuous infusion of LPS [19-23]; 3) models that provide aggressive resuscitation of intravascular volume [24,25]; and 4) models that utilize intraperitoneal administration of LPS [26]. Schultz and Van der Poll [12] report that these models are advantageous over the intravenous bacterial infusion models because LPS is specific and stable, and accurate doses of LPS can be administered as a bolus or continuous infusion, in a reproducible manner and the quantity of LPS-infusion can be easily varied. Similar to the bacterial infusion models, intravenous LPS models lack infectious focus [12]. It is important to briefly note a method that is related to the LPS-infusion model. It specifically utilizes the lipid component of the endotoxin molecule, called the lipid A, which is administered via an injection. This model is the furthest away from the clinical field and its application, however it is a very potent stimulant of the immune system [27].
3. E. COLI ENDOTOXINS
Lipopolysaccharide is usually referred to as endotoxin, a term introduced in the 19th century to specifically describe the toxic component of gram-negative bacteria, which was thought to be responsible for infections [28-30]. Although the terms endotoxin and LPS are used interchangeably, in the pharmaceutical industry LPS generally implies a purified form of the bacterial endotoxin. The general molecular structure of LPS typically consists of a hydrophobic domain, which is also known as Lipid A or endotoxin, a non-repeating oligosaccharide core component and a distal polysaccharide or O-antigen [27]. Lipid A, functionally acting as a hydrophobic anchor of LPS, is a highly-branched glucosamine-based phospholipid that is shown to make up the outer monolayer of the outer membranes of most Gramnegative bacteria [28,31-34]. Lipid A is required for growth of E. coli and most other Gram-negative bacteria [34,35], it is also crucial for the maintenance of an effective outer membrane barrier [36]. The core component consists of a hetero-oligosaccharide and has limited variability within different bacterial species [37] O-antigen structures are highly variable, compared to the structures of the core and lipid A and is thought to help bacteria evade the immune system [38,39] The O-antigen component can also differ in the monomer glycoses, the presence or absence of non-carbohydrate substituents and the position and stereochemistry of the O-glycosidic linkages [27]. The variability in O-antigen component of LPS is not limited to its chemical structure only, for example E. coli K-12 strain completely lacks the O-antigen component [38,39]. LPS and Lipid A are potent activators of macrophages, resulting in the rapid synthesis of inter-leukin 1 (IL 1) [40,41], tumor necrosis factor (TNF) [42-44] and other proteins [45].
4. ENDTOTOXIN PURIFICATION PROCESS
Before purified endotoxin or LPS may be used experimentally in research, it has to be extracted from strains of E. coli. Extraction techniques will produce LPS extracts that have a varying degree of contamination and result in different endotoxin activity of the end product. Thus, we may see different levels of LPS activity within the same serotype. Lipopolysaccharides are obtained from gramnegative bacteria via a variety of techniques [46]. These techniques include extraction with trichloroacetic acid at 4˚C, extraction with aqueous ether at 6˚C - 12˚C, extraction with water at 80˚C, and extraction with aqueous phenol [47]. Extraction with phenol-water is one of the most commonly used techniques not only because it can be utilized with many groups of bacteria, in addition to being a relatively simple technique, but also because it is one of the few techniques by which LPS may be extracted from R-mutant bacteria [47].
Once a conventional LPS preparation from bacteria is made, it is usually a multilayered monophasic suspension with multiple phases—a phenol phase containing mainly proteins, an interphase containing cell wall material and an aqueous phase containing LPS, polysaccharides, and nucleic acid [47]. The LPS preparation then needs to go through purification procedures to remove contaminants. Usually the purification procedures include digestion of crude LPS with nucleases [48], digestion with proteases [49], treatment with cationic detergents such as cetavlon and sequential ultracentrifugation [50]. Once an LPS preparation goes through a series of these procedures, the preparation may be counted as homogeneous, with the final LPS yields ranging from 0.05 to 2.0 percent of initial dry weight [47]. Purification process of bacterial LPS should be efficient, it is suggested that the bacterial cells are to be disrupted by mechanical or enzymic methods before extracting procedures [47]. While the lysozyme extraction method shows to be the most efficient, it has a major disadvantage in that the long treatment period results in degradative enzymes taking action and increasing the chances of non-specific absorption of cellular components into LPS [47].
Our own experimental findings indicate that when the serotype and the dosage (20 mg/kg) of the LPS are kept the same—the results are significantly affected by LPS being used from different batches from the supplier (Figure 1). We believe the difference to be due to the variance in endotoxin units of activity, which may be due
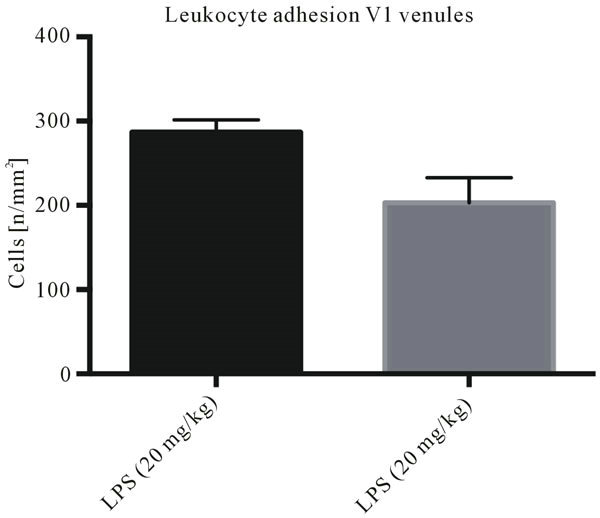
Figure 1. Endotoxemia as sepsis model—endotoxin activity dependency: Leukocyte adhesion in collecting (V1) venules of the intestinal submucosal layer assessed by intravital microscopy. Both groups of animals (n = 5 per group) received 20 mg/kg lipolpolysaccharide (LPS) with different endotoxin activity. Significant difference between both groups (p < 0.05).
to the variance in the utilized purification methods by the LPS supplier.
5. ENDTOTOXIN SEROTYPE DIFFERENCES
For experimental purposes, it is important to be consistent in the use of the endotoxin to obtain reproducible results. There are only a few reports on serotype-dependency of experimental results. Scientific literature reports that there is a close relationship between the serotype, outer membrane protein and lipopolysaccharide patterns [51]. In acute-phase reaction studies, where the body temperature is recorded, it was observed that two different serotypes of E. coli at the same dose produced variable thermoregulatory responses [52], providing support for the notion that it is important to account for serotype-dependency of the results when designing an experiment. Another study looked at the effects of various serotypes of E. coli LPSs on rectal temperature in a doseresponse study in rats, utilizing E. coli O55:B5, O127:B8, and O111:B4 serotypes [52] The same study then noted that the relative Lipid A content of each E. coli serotype LPS has not been defined yet and went on to hypothesize that Lipid A content of each serotype may be different. It then concluded that the serotype and the dose of LPS should be critical factors for the variability in results. Another study reports that different serotypes of LPS cause different increases in albumin extravasation in rats. In this study, serotypes O127:B8, known to lower interstitial fluid pressure and O111:B4, previously described not increasing the rate of albumin extravasation, were chosen [53]. The study concluded that after using two different serotypes of LPS, although the two induced the same amount of arterial hypotension, only one of the preparations increased transcapillary rate of albumin extravasation, following a lowering in interstitial fluid pressure.
6. ENDTOTOXIN DOSAGE DEPENDENCY
Another critical factor in experimental sepsis research is the choice of the LPS dosage. The main idea that is associated with this critical factor is having different clinical effects by varying the dosage of LPS administered, while keeping the LPS serotype (and type of purification) the same.
One study investigated the dose dependency and the individual variability of the LPS-induced immune response in cattle, which were challenged 3 times by intravenous injection of increasing doses of E. coli LPS [54]. The study found that the immune responses were dose dependent, with dose dependency changing over the course of the immune challenges.
Our experiments using intravital microscopy of leukocyte activation indicated that depending on the dosage, there is a difference in leukocyte adhesion to the endothelial wall in venous microvessels (Figure 2). If a lower dose of endotoxin (phenol extract from E. coli serotype O26:B6) is administered, we do not observe strong leukocyte activation. However, we did notice a difference in functional capillary density upon subjecting the tissue to
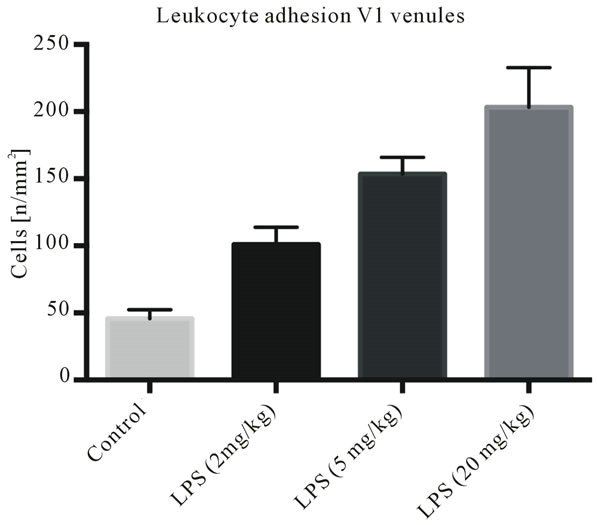
Figure 2. Endotoxemia as sepsis model—dose-response relationship: Leukocyte adhesion in collecting (V1) venules of the intestinal submucosal layer assessed by intravital microscopy. Using the same serotype and batch of LPS, while varying the dosage (0 mg/kg = control, 2 mg/kg, 5 mg/kg and 20 mg/kg). Significant differences between all groups (p < 0.05).
intravital microscopy, indicating that the immune response may be modulated by varying the dosage of endotoxin administered. Highest leukocyte activation was observed upon the administration of the largest LPS doses (20 mg/kg).
7. DISCUSSION
The high prevalence of sepsis in intensive care units and emergency rooms, along with the high lethality of the sepsis cases makes the study of pathophysiology of sepsis critically important. As a preclinical model, endotoxemia is an important tool to study the pathophysiology of sepsis and septic shock.
We examined aspects of endotoxemia as an experimental model in sepsis research. The different techniques associated with the purification of the endotoxin of E. coli may have a significant influence on the experimental results. The experimental data presented in our review provides evidence for the dependency of experimental results on endotoxin activity, with differences believed to be due to the variance in endotoxin units of activity, which may be due to the utilized purification methods used by the LPS supplier. In addition, the data indicated dosage-dependency of experimental results by showing that there is a difference in leukocyte adhesion to the endothelial wall in venous microvessels, based on the dosage of the administered endotoxin.
We believe that future studies which will be utlizing the endotoxemia model need to consider the 3 main factors related to the endotoxin molecule itself, which may influence the experimental results. First factor is the method of purification used to extract the endotoxin, with each method having a varying degree of contamination and resulting in different endotoxin activity. Second factor is the serotype of the endotoxin, with experimental results and clinical effects potentially being serotypedependent. Finally, the third critical factor is the dosage of endotoxin administered, with different clinical effects being associated with the varying dosages.
REFERENCES
- Wheeler, A.P. and Bernard, G.R. (1999) Treating patients with severe sepsis. The New England Journal of Medicine, 340, 207-214. doi:10.1056/NEJM199901213400307
- Parrillo, J.E., Parker, M.M., Natanson, C., Suffredini, A.F., Danner, R.L., Cunnion, R.E. and Ognibene, F.P. (1990) Septic shock in humans. Advances in the understanding of pathogenesis, cardiovascular dysfunction, and therapy. Annals of Internal Medicine, 113, 227-242.
- Letarte, J., Longo, C.J., Pelletier, J., Nabonne, B. and Fisher, H.N. (2002) Patient characteristics and costs of severe sepsis and septic shock in Quebec. Journal of Critical Care, 17, 9-49. doi:10.1053/jcrc.2002.33028
- Chalfin, D.B., Holbein, M.E., Fein, A.M. and Carlon, G.C. (1993) Cost-effectiveness of monoclonal antibodies to gram-negative endotoxin in the treatment of gram-negative sepsis in ICU patients. JAMA, 269, 249-254. doi:10.1001/jama.1993.03500020083037
- Angus, D.C., Linde-Zwirble, W.T., Lidicker, J., Clermont, G., Carcillo, J. and Pinsky, M.R. (2001) Epidemiology of severe sepsis in the United States: Analysis of incidence, outcome, and associated costs of care. Critical Care Medicine, 29, 1303-1310. doi:10.1097/00003246-200107000-00002
- Perl, T.M., Dvorak, L., Hwang, T. and Wenzel, R.P. (1995) Long-term survival and function after suspected gramnegative sepsis. JAMA, 274, 338-345. doi:10.1001/jama.1995.03530040066043
- Heyland, D.K., Hopman, W., Coo, H., Tranmer, J. and McColl, M.A. (2000) Long-term health-related quality of life in survivors of sepsis: Short Form-36: A valid and reliable measure of health-related quality of life. Critical Care Medicine, 28, 3599-3605. doi:10.1097/00003246-200011000-00006
- Martin, G.S., Mannino, D.M., Eaton, S. and Moss, M. (2003) The epidemiology of sepsis in the United States from 1979 through 2000. The New England Journal of Medicine, 348, 1546-1554. doi:10.1056/NEJMoa022139
- Bone, R.C., Balk, R.A., Cerra, F.B., Dellinger, R.P., Fein, A.M., Knaus, W.A., Schein, R.M. and Sibbald, W.J. (1992) Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM Consensus Conference Committee. American College of Chest Physicians/Society of Critical Care Medicine. Chest, 101, 1644-1655. doi:10.1378/chest.101.6.1644
- Salomao, R., Rigato, O., Pignatari, A.C., Freudenberg, M. A. and Galanos, C. (1999) Bloodstream infections: Epidemiology, pathophysiology and therapeutic perspectives. Infection, 27, 1-11. doi:10.1007/BF02565163
- Vincent, J.L., Rello, J., Marshall, J., Silva, E., Anzueto, A., Martin, C.D., Moreno, R., Lipman, J., Gomersall, C., Sakr, Y., et al. (2009) EPIC II group of investigators: International study of the prevalence and outcomes of infection in intensive care units. JAMA, 302, 2323-2329. doi:10.1001/jama.2009.1754
- Schultz, M.J., Knapp, S. and van der Poll, T. (2002) Regulatory role of alveolar macrophages and cytokines in pulmonary host defense. Yearbook of Intensive Care and Emergency Medicine, Springer Verlag, 65-76.
- Wichterman, K.A., Baue, A.E. and Chaudry, I.H. (1980) Sepsis and septic shock—A review of laboratory models and a proposal. Journal of Surgical Research, 29, 189- 201. doi:10.1016/0022-4804(80)90037-2
- Deitch, E.A. (1998) Animal models of sepsis and shock: A review and lessons learned. Shock, 9, 1-11. doi:10.1097/00024382-199801000-00001
- Fink, M.P. and Heard, S.O. (1990) Laboratory models of sepsis and septic shock. Journal of Surgical Research, 49, 186-196. doi:10.1016/0022-4804(90)90260-9
- Fink, M.P., Morrissey, P.E., Stein, K.L., Clement, R.E., Fiallo, V. and Gardiner, W.M. (1988) Systemic and regional hemodynamic effects of cyclo-oxygenase and thromboxane synthetase inhibition in normal and hyperdynamic endotoxemic rabbits. Circulatory Shock, 26, 41-57.
- Mehrad, B. and Standiford, T.J. (1999) Role of cytokines in pulmonary antimicrobial host defense. Immunologic Research, 20, 15-27. doi:10.1007/BF02786504
- Sewnath, M.E., Olszyna, D.P., Birjmohun, R., Ten Kate, F.J., Gouma, D.J. and van der Poll, T. (2001) IL-10-deficient mice demonstrate multiple organ failure and increased mortality during Escherichia coli peritonitis despite an accelerated bacterial clearance. The Journal of Immunology, 166, 6323-6331.
- Emerson Jr., T.E. and Gill, C.C. (1967) Effects of slow intravenous endotoxin infusion on hemodynamics and survival in dogs. Journal of Applied Physiology, 22, 874- 877.
- Fish, R.E. and Spitzer, J.A. (1984) Continuous infusion of endotoxin from an osmotic pump in the conscious, unrestrained rat: A unique model of chronic endotoxemia. Circulatory Shock, 12, 135-149.
- Fish, R.E., Burns, A.H., Lang, C.H. and Spitzer, J.A. (1985) Myocardial dysfunction in a non-lethal, non-shock model of chronic endotoxemia. Circulatory Shock, 16, 241- 252.
- Fish, R.E., Lang, C.H. and Spitzer, J.A. (1986) Regional blood flow during continuous low-dose endotoxin infusion. Circulatory Shock, 18, 267-275.
- Kurtz, H.J. and Quast, J. (1982) Effects of continuous intravenous infusion of Escherichia coli endotoxin into swine. American Journal of Veterinary Research, 43, 262- 268.
- Fink, M.P., Cohn, S.M., Lee, P.C., Rothschild, H.R., Deniz, Y.F., Wang, H., et al. (1989) Effect of lipopolysaccharide on intestinal intramucosal hydrogen ion concentration in pigs: Evidence of gut ischemia in a normodynamic model of septic shock. Critical Care Medicine, 17, 641-646. doi:10.1097/00003246-198907000-00009
- Breslow, M.J., Miller, C.F., Parker, S.D., Walman, A.T. and Traystman, R.J. (1987) Effect of vasopressors on organ blood flow during endotoxin shock in pigs. American Journal of Physiology, 252, 291-300.
- Fink, M.P., Fiallo, V., Stein, K.L. and Gardiner, W.M. (1987) Systemic and regional hemodynamic changes after intraperitoneal endotoxin in rabbits: Development of a new model of the clinical syndrome of hyperdynamic sepsis. Circulatory Shock, 22, 73-81.
- Raetz, C.R. and Whitfield, C. (2002) Lipopolysaccharide endotoxins. Annual Review of Biochemistry, 71, 635-700. doi:10.1146/annurev.biochem.71.110601.135414
- Rietschel, E.T., Kirikae, T., Schade, F.U., Mamat, U., Schmidt, G., Loppnow, H., Ulmer, A.J., Zähringer, U., Seydel, U. and Padova, D.F., et al. (1994) Bacterial endotoxin: Molecular relationships of structure to activity and function. FASEB Journal, 8, 217-225.
- Hinshaw, L.B. (1984) Handbook of endotoxin: Pathophysiology of endotoxin, Vol. II. Elsevier/North Holland Biomedical, Amsterdam.
- Suffredini, A.F., Fromm, R.E., Parker, M.M., Brenner, M. and Kovacs, J.A. (1989) The cardiovascular response of normal humans to the administration of endotoxin. The New England Journal of Medicine, 321, 280-287. doi:10.1056/NEJM198908033210503
- Raetz, C.R.H. (1990) Biochemistry of endotoxins. Annual Reviews in Biochemistry, 59, 129-170. doi:10.1146/annurev.bi.59.070190.001021
- Raetz, C.R.H. and Niedhardt, F.C. (1996) Escherichia coli and salmonella: Cellular and molecular biology. American Society for Microbiology, Washington DC, 1035- 1063.
- Zähringer, U., Lindner, B. and Rietschel, E.T. (1999) Endotoxin in health and disease. Marcel Dekker, Inc., New York, 93-114.
- Brade, H., Opal, S.M., Vogel, S.N. and Morrison, D.C. (1999) Endotoxin in health and disease. Marcel Dekker, Inc., New York, 950.
- Onishi, H.R., Pelak, B.A., Gerckens, L.S., Silver, L.L. and Kahan, F.M. (1996) Antibacterial agents that inhibit lipid a biosynthesis. Science, 274, 980-982. doi:10.1126/science.274.5289.980
- Vaara, M. (1993) Antimicrob. Agents Chemother, 37, 2255- 2260. doi:10.1128/AAC.37.11.2255
- Salomao, R., Brunialti, M.K., Rapozo, M.M., Baggio-Zappia, G.L., Galanos, C. and Freudenberg, M. (2012) Bacterial sensing, cell signaling, and modulation of the immune response during sepsis. Shock, 38, 227-242. doi:10.1097/SHK.0b013e318262c4b0
- Rietschel, E.Th. (1984) Handbook of endotoxin: Chemistry of endotoxin, Vol. I. Elsevier/North-Holland Biomedical, Amsterdam.
- Galanos, C., Rictschel, E.Th., Liideritz, O. and Westphal, O. (1977) International review of biochemistry: Biochemistry of lipids II. University Park Press, Baltimore, 239- 335.
- Dinarello, C.A. (1986) Interleukin-1. Reviews of Infectious Diseases, 6, 51-95. doi:10.1093/clinids/6.1.51
- Loppnow, H., Brade, H., Diirrbaum, I., Dinarello, C.A., Kusumoto, S., Rietschel, E.T. and Flad, H.D. (1989) IL-1 induction-capacity of defined lipopolysaccharide partial structures. The Journal of Immunology, 142, 3229-3238.
- Beutler, B. and Cerami, A. (1988) The history, properties, and biological effects of cachectin. Biochemistry, 27, 7575- 7582. doi:10.1021/bi00420a001
- Old, L.J. (1988) Tumor necrosis factor. Scientific American, 258, 59-75. doi:10.1038/scientificamerican0588-59
- Kiener, P.A., Marek, F., Rodgers, G., Lin, P.F., Warr, G. and Desiderio, J. (1988) Induction of tumor necrosis factor, IFN-gamma, and acute lethality in mice by toxic and non-toxic forms of lipid A. The Journal of Immunology, 141, 870-874.
- Wolpe, S.D., Davatelis, G., Sherry, B., Beutler, B. and Hesse, D.G. (1988) Macrophages secrete a novel heparin-binding protein with inflammatory and neutrophil chemokinetic properties. The Journal of Experimental Medicine, 167, 570-581. doi:10.1084/jem.167.2.570
- Luderitz, O.A., Staub, M. and Westphal. O. (1966) Immunochemistry of O and R antigens of salmonella and related enterobacteriace. Bacteriological Reviews, 30, 192- 255.
- Johnson, K.G. and Perry, M.B. (1975) Improved techniques for the preparation of bacterial lipopolysaccharides. Canadian Journal of Microbiology, 22, 29-34. doi:10.1139/m76-004
- Adams, G.A. (1972) Lipopolysaccharides. Preparation from gram-negative bacteria. In: Whistler, R.L. and BeMille, J.N., Eds., Methods in Carbohydrate Chemistry, Academic Press, Inc., New York, 157-161.
- Fensoma, H. and Gray, G.W. (1969) The chemical composition of lipopolysaccharide of Pseudonomas aeruginosa. Biochemical Journal, 114, 185-196.
- Leive, L. and Morris, D. (1972) Isolation of lipopolysaccharides from bacteria. In: Ginsburg, N., Ed., Methods in Enzymology, 254-262.
- Nishimura, L.S., Ferreira, L.C., Pacheco, A.B. and Guth, B.E. (1996) Relationship between outer membrane protein and lipopolysaccharide profiles and serotypes of enterotoxigenic Escherichia coli isolated in Brazil. FEMS Microbiology Letters, 143, 253-258. doi:10.1111/j.1574-6968.1996.tb08489.x
- Dogan, M.D., Ataoglu, H. and Akarsu, E.S. (2000) Effects of different serotypes of Escherichia coli lipopolysaccharides on body temperature in rats. Life Sciences, 67, 2319-2329. doi:10.1016/S0024-3205(00)00821-3
- Nedrebo, T. and Reed, R.K. (2001) Different serotypes of endotoxin (lipopolysaccharide) cause different increases in albumin extravasation in rats. Shock, 18, 138-141. doi:10.1097/00024382-200208000-00008
- Jacobsen, S., Andersen, P.H., Toelboell, T. and Heegaard, P.M. (2004) Dose dependency and individual variability of the lipopolysaccharide-induced bovine acute phase protein response. Journal of Dairy Science, 10, 3330-3339. doi:10.3168/jds.S0022-0302(04)73469-4
NOTES
*Corresponding author.

