Advances in Bioscience and Biotechnology
Vol.4 No.2A(2013), Article ID:28439,5 pages DOI:10.4236/abb.2013.42A037
Pectin shows antibacterial activity against Helicobacter pylori
![]()
1Faculty of Medicine, University of Balamand, El-Koura, Lebanon
2Tufts Medical Center, Boston, USA
3Faculty of Sciences, University of Balamand, El-Koura, Lebanon
Email: ziad.daoud@balamand.edu.lb
Received 15 October 2012; revised 17 November 2012; accepted 25 November 2012
Keywords: Pectin; Helicobacter pylori; Antibacterial Activity; Antibiotic; MIC; MBC
ABSTRACT
Pectins are the collective name for a group of heterogeneous, high molecular weight, branched polysaccharides that are found in the cell walls of higher plants. In this study, we intend to determine the antibacterial activity of pectin against reference strains and clinical isolates of Helicobacter pylori. The results show that pectin produced antibacterial effects on all the 16 clinical isolates and 2 reference strains of H. pylori with the greatest antibacterial effect at a low pH (5.0) versus higher pHs. The lowest Minimum Inhibitory Concentration recorded was of 0.016 µg/µl. Antibiotic resistance, therapy costs, and undesirable side effects stress the need for new antimicrobials or alternative therapies. The results from our study can further our goal of future eradication of H. pylori infection using new molecules.
1. INTRODUCTION
Pectins are a class of complex polysaccharides found in the cell walls of higher plants. They contribute to the firmness and structure of plant tissue both as part of the primary cell wall and as the main component in the middle lamella, which is responsible for cell-to-cell adhesion [1]. Pectin is a water-soluble fiber. Chemically, pectin is the collective name for a group of heterogeneous, high molecular weight, branched polysaccharides. They are composed of distinct structural domains linked together in characteristic patterns. They compromise acidic polysaccharides including homogalacturonan, rhamnogalacturonan I, rhamnogalacturonan II and neutral oligosaccharide chains such as galactans, arabinans, and arabinogalactans [2]. The most common pectin is isolated from citrus and is extensively branched.
An interest in this complex polysaccharide as a human dietary supplement has emerged due to its multiple health benefits including the lowering of blood cholesterol and serum glucose levels [3] and the potential inhibition of cancer growth and metastasis [4]. Due to its complex side-chains, pectin is a strong binding agent and this might explain its cholesterol lowering and detoxification properties. However due to pectins large size and molecular weight its actions are limited to its activity in the digestive tract.
Pectin is used in food as a gelling agent particularly in jams and jellies. It is also used in fillings, sweets, as a stabilizer in fruit juices and milk drinks and as a source of dietary fibers. The amount, structure and chemical composition of pectin differs between plants, within a plant over time and in different parts of a plant. During ripening, pectin is broken down by the enzymes pectinase and pectin-esterase; in this process the fruit becomes softer as the middle lamella breaks down and cells become separated from each other. Pectin is a natural part of human diet, and it is nowadays considered as food supplement. The daily intake of pectin from fruits and vegetables can be estimated to be around 5 g in a normal and balanced diet. In human digestion, pectin goes through the small intestine more or less intact; it is thus a soluble dietary fiber. Consumption of pectin has been shown to reduce blood cholesterol levels [5]. The mechanism appears to be an increase of viscosity in the intestinal tract, leading to a reduced absorption of cholesterol from bile or food [5].
Helicobacter pylori, a Gram-negative comma-shaped bacterium, is a common human pathogen and public health problem that causes gastritis and peptic ulcers. Infection with Helicobacter pylori has also been linked pathologically to the development of gastric cancer [6]. Worldwide, the infection with H. pylori is highly prevalent; approximately 50% of the world population is infected, with prevalence rates in countries ranging from 20% (Australia) to 80% (Mexico, central and south America, and Africa) [7]. Actual infection rates vary from nation to nation; the developing world has much higher infection rates than the West (Western Europe, North America, Australasia), where rates are estimated to be around 25% [7]. Infection with H. pylori may manifest as a number of clinical disorders, primarily of the gastro duodenal mucosa.
The antimicrobial activity of pectin was not extensively addressed in literature; however, some studies have shown that the consumption of plants and plant products, such as green tea, and high-fiber diets were associated with reduced risk for developing duodenal ulcer and gastritis [8-10]. On the other hand, increased consumption of allium vegetables has been suggested as a protective factor for stomach cancer [11-13]. Several studies have reported significantly reduced risk of H. pylori infection with increased consumption of fruits and/or vegetables, vitamin C, and beta-carotene [14-17].
In this study, we intend to determine the antibacterial activity of pectin against reference strains and clinical isolates of Helicobacter pylori. We believe this is important for a better understanding of the pathogenicity of this organism and of the environmental factors affecting its virulence. To our knowledge, this present study constitutes the first attempt to quantify the antibacterial activity of pectin against Helicobacter pylori, investigating therefore its potential role in the search for new molecules with antimicrobial properties.
2. MATERIAL AND METHODS
Bacterial strains: A total of 16 clinical isolates and 2 reference strains of H. pylori were used in this study. The reference strains were H. pylori ATCC 43,504 and H. pylori ATCC 26,695. The clinical strains were isolated from patients with documented duodenal or gastric ulcers at the Saint George Hospital-University Medical Center, Beirut between 2007 and 2010. After culturing the isolates were stored at −80˚C in Brucella broth (Becton Dickinson) containing 10% DMSO and 10% horse serum until used.
Antimicrobial activity of Pectin: Lyophilized Citrus Pectin was kindly provided by Megazyme (high-methoxyl pectin with Molecular weight of around 200,000 daltons). It was weighed and dissolved in sterile distilled water. The solutions were filtered through 0.22 µm sterile filter membranes and stored at 4˚C for further use. The concentration of the original solution of pectin was adjusted to 106 µg/ml. This was used as the stock solution from which the Minimum Inhibitory Concentration (MIC) series were prepared.
A micro broth dilution method was performed. Each well of a 96-well microplate was coated with twofold serial dilutions of the solution of pectin previously prepared and air-dried. A saline suspension of test strain equivalent to 2.0 McFarland standard (containing 1 × 107 to 1 × 108 c.f.u. ml−1), was prepared from a 72 h-old subculture of a blood agar plate. The suspension (0.5 ml) was put into 9.5 ml cation-adjusted Mueller-Hinton broth (Difco) supplemented with 5% horse serum to a density of 5 × 105 - 5 × 106 CFU/ml. A 100 μl volume of the suspension was added to each well, and the cultures were incubated at 35˚C for 3 days under a microaerophilic atmosphere (O2, 10%; CO2, 5%). The MIC was defined as the lowest concentration of a test antibiotic that completely inhibited visible bacterial growth. A routine bacterial count was performed in duplicates to verify the bacterial concentration. Positive and negative control wells were prepared in parallel. The negative control well consisted of 100 µl of cation-adjusted MuellerHinton broth supplemented with 5% horse serum; the positive well consisted of 100 µl of the same medium to which 100 µl of the above indicated bacterial suspension were added. All experiments were done in duplicates and when results were different within one dilution, the highest MIC was recorded. When results were different beyond one dilution, the experiment was repeated. With every set of MIC determination, an additional control for ampicillin and the tested strain of H. pylori was performed. The Minimum Bactericidal Concentration (MBC) was determined by sub-culturing samples from the tubes with concentrations above the MIC on new plates of Blood agar and incubated in microaerophillic conditions for 72 hours. The MBC corresponded to the lowest concentration of the extract associated with no bacterial culture. All experiments were performed three independent times in duplicate form. In cases where the MIC was recorded in two different tubes and the difference is only one dilution, the lowest concentration was recorded. When the difference was more than one dilution, the whole experiment was repeated. The MIC90 was defined as the Minimum Inhibitory Concentration below which 90% of the individual MICs were found, and the The MBC90 was defined as the Minimum Inhibitory Concentration below which 90% of the individual MBCs were found.
3. RESULTS
As shown in Table 1, pectin produced obvious antibacterial effects on all the strains of H. pylori including the ATCC strains and clinical isolates. The MICs were in general lower at pH 5.0 than at a higher pHs. The lowest MIC was recorded at a concentration of 0.016 µg/µl with 2 clinical isolates at pH 5.0. Values as low as 0.062 µg/µl were observed at pH 6.0 and 0.125 µg/µl at pH 7.0. The MIC90 was the lowest at pH 5.0 (0.162 µg/µl). However, the difference in MIC values at different pHs fell within one dilution, and subsequently a remarkable increase or decrease in antibacterial activity cannot be attributed to pH. An important point to highlight is that the MIC
Table 1. Minimum inhibitory concentrations of pectin against reference strains and clinical isolates of H. pylori.
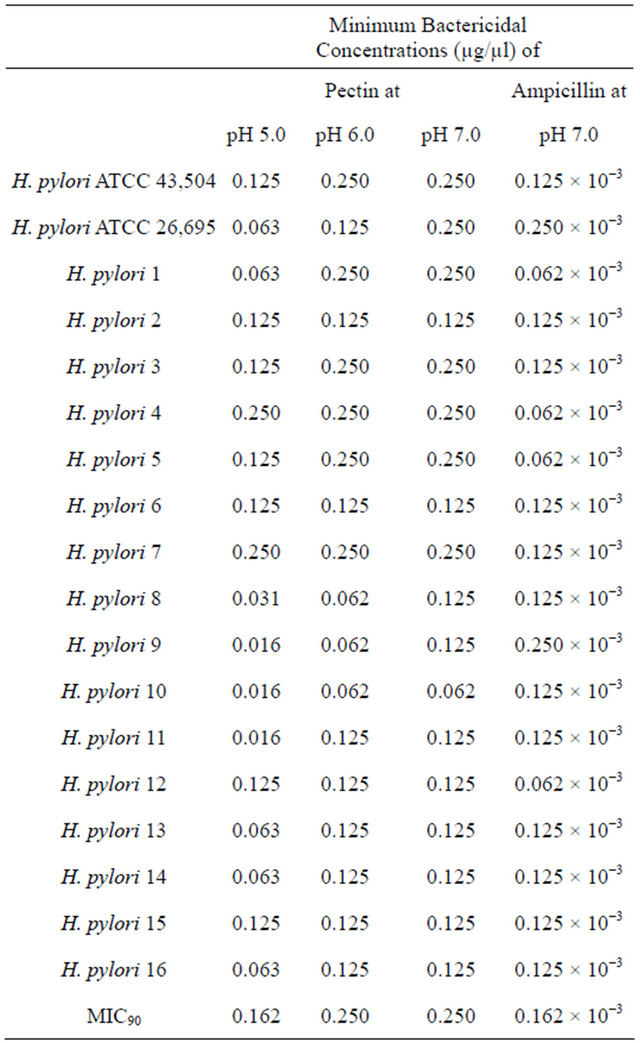
values were stable when the pHs varied between 5.0 and 7.0. These antibacterial activities could be roughly estimated to 1000 fold less than ampicillin with all strains. The MBC pattern was similar to that of MIC showing better activity at lower pH. As expected, the MBC fell within one or two dilutions higher than the MICs (Table 2).
4. DISCUSSION
There is a great need to increase our knowledge about the pathogenicity and virulence of H. pylori. The factors affecting the transmission of this organism and its occurrence, common in some people and not in others, are still being researched. Hereditary susceptibility to H. pylori infection has not been proven. However, studies do suggest that members of certain ethnic and racial groups including Hispanics and African-Americans have a higher rate of infection than Caucasians [18]; these differences however were not entirely explained by differ-
Table 2. Minimum bactericidal concentrations of pectin against reference strains and clinical isolates of H. pylori.
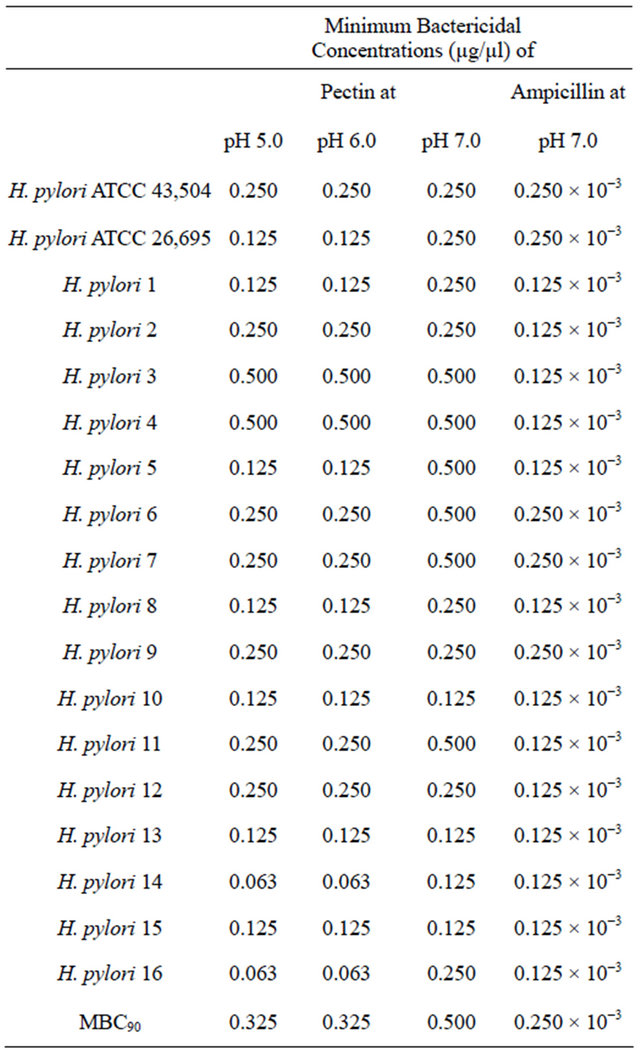
ences in socioeconomic status. Person-to-person transmission of H. pylori through either fecal/oral or oral/oral exposure seems most likely and humans appear to be the major reservoir of infection [19,20]. With the extent of the burden of H. pylori infection in the present world it is important to find a more natural and beneficial way to reduce the infection burden.
This study involves understanding at two levels, at one level it sheds light on the pathogenesis of Helicobacter infections by bringing new data that might help understanding how the diet can influence the ability of this organism to survive in a medium already hostile, like the stomach. In this context, our results suggest that pectin contributes to H. pylori killing mostly when the pH is more acidic (MIC90 of 0.162 µg/µl and MBC90 of 0.325 µg/µl). On the second level this study shows an obvious antibacterial activity of pectin, suggesting possibly a potential source of natural antimicrobial agents in an era of increasing bacterial resistance and limited new discovery of antibacterial molecules. Previous studies have shown a great deal of association between consumption of natural substances such as plants and vegetables and the prevention of H. pylori infection [8,12]. Pectin is one such natural substance available in the different types of food humans consume. One study has addressed the role of pectin as an anti-adhesive agent for H. pylori [21], adding therefore more evidence to the role of this molecule in decreasing the virulence of H. pylori by interfereence with its ability to colonize human tissues.
The aim of the present study was to investigate the antibacterial activity of pectin against H. pylori. The mechanism by which pectin exhibits its antibacterial activity needs more work and studies at a cellular, molecular, and chemical levels. In addition, our study offers relatively small data, however, enough to draw the attention to the potential of this molecule widely found in nature. Similar antimicrobial studies of pectin need to be conducted at a larger scale in larger populations and with different bacteria. On the other hand, the emergence of antibiotic resistant H. pylori strains has been increasing and the problems for the chemotherapy have also been growing. New and “safer” anti-H. pylori agents with high selective toxicity are urgently needed. Our preliminary results show that that pectin does not demonstrate significant antibacterial activity against E. coli and K. pneumoniae, organisms considered as representatives of the normal human intestinal flora. This makes pectin a compound with narrow antibacterial activity attacking specific organisms and avoiding therefore any secondary effect on the intestinal flora that could lead to diarrhea.
Although the anti-H. pylori activity should not be compared with the activity of ampicillin, this latter being included as a positive antibiotic control, the anti-Helicobacter activity is considerable in view of the low MICs it yielded. In one of the previous studies showing the use of T. Macroptera root extract against H. pylori infection and comparing its use with other antibiotics, the MIC90 for 92% of the H. pylori strains ranged from 200 to 25 µg/µl in which the MIC value of 200 µg/µl was considered the cut-off for H. pylori strain susceptibility [22]. Our data show a significant lower MIC90 of pectin (0.162 µg/µl) suggesting a potentially higher bactericidal effect. In our study, we did not investigate the mechanism by which pectin exhibits its antibacterial activity nor the cytotoxic activity of this molecule on the human cells. This is important to be considered for future research. It will help in understanding whether pectin could have a potential role as safe alternative for treatment of H. pylori becoming resistant to common antibiotic agents. In previous works, it has been shown that the bactericidal activity of pectin against Escherichia coli is related to its ionization state in that inhibition of growth was observed when the pH of the growth medium dropped below 5 [8]. Similarly, studies on Sphagnum (isolated from Sphanum moss, similar to pectin in its abundance of uronic acids) showed antibacterial activity at low pH which was contributed either to ionization or via cation-exchange capacity which may lead to its bactericidal effect [23,24].
Antibiotic resistance, therapy costs and undesirable side effects stress the need for new antimicrobials or alternative therapies. The significant results from our study can further our goal of future eradication of H. pylori infection. Pectin’s bactericidal activity against H. pylori, to our knowledge, is reported here for the first time and might be used further for standardization of pharmaceutical preparations.
REFERENCES
- Carpita, N.C. and Gibeaut, D.M. (1993) Structural models of primary cell walls in flowering plants: Consistency of molecular structure with the physical properties of walls during growth. The Plant Journal, 3, 1-30. doi:10.1111/j.1365-313X.1993.tb00007.x
- Knox, J.P., Linstead, P.J., King, J., Cooper, C. and Roberts, K. (1990) Pectin esterification is spatially regulated both within cell walls and between developing tissues of root apices. Planta, 181, 512-521. doi:10.1007/BF00193004
- Behall, K. and Reiser, S. (1986) Effects of pectin on human metabolism, chemistry and function of pectins. American Chemical Society, Washington, 248-265.
- Heitman, D.W., Hardman, W.E. and Cameron, I.L. (1992) Dietary supplementation with pectin and guar gum on 1,2-dimethylhydrazine-induced colon carcinogenesis in rats. Carcinogenesis, 13, 815-818. doi:10.1093/carcin/13.5.815
- Brouns, F., Theuwissen, E., Adam, A., Bell, M., Berger, A. and Mensink, R.P. (2012) Cholesterol-lowering properties of different pectin types in mildly hyper-cholesterolemic men and women. European Journal of Clinical Nutrition, 66, 591-599. doi:10.1038/ejcn.2011.208
- Kenneth, E.L. and McColl, M.D. (2010) Helicobacter pylori infection. New England Journal of Medicine, 362, 1597-1604.
- Pounder, R.E. (1995) The prevalence of helicobacter pylori infection in different countries, aliments pharmacology therapy. Department of Medicine, Division of Gastroenterology, Rhode Island Hospital and Brown University, Providence, 9, 33-39.
- El Nakeeb, M.A. and Youssef, R.T. (1970) Study of the antimicrobial action of pectin. II. Factors affecting the bactericidal activity of pectin. Planta Medica, 18, 295- 302. doi:10.1055/s-0028-1099782
- Rydning, A., Weberg, R., Lange, O. and Berstad, A. (1986) Healing of benign gastric ulcer with low-dose antacids and fiber diet. Gastroenterology, 91, 56-61.
- Setiawan, V.W., Zhang, Z.F., Yu, G.P., et al. (2001) Protective effect of green tea on the risks of chronic gastritis and stomach cancer. International Journal of Cancer, 92, 600-604. doi:10.1002/ijc.1231
- Lee, K.M., Yeo, M., Choue, J.S., et al. (2004) Protective mechanism of epigallocatechin-3-gallate against Helicobacter pylori-induced gastric epithelial cytotoxicity via the blockage of TLR-4 signaling. Helicobacter, 9, 632- 642. doi:10.1111/j.1083-4389.2004.00281.x
- Gao, C.M., Takezaki, T., Ding, J.H., Li, M.S. and Tajima, K. (1999) Protective effect of allium vegetables against both esophageal and stomach cancer: A simultaneous case-referent study of a high-epidemic area in Jiangsu Province, China. Japanese Journal of Cancer Research, 90, 614-621. doi:10.1111/j.1349-7006.1999.tb00791.x
- You, W.C., Blot, W.J., Chang, Y.S. et al. (1989) Allium vegetables and reduced risk of stomach cancer. Journal of the National Cancer Institute, 81, 162-164. doi:10.1093/jnci/81.2.162
- Brown, L.M., Thomas, T.L., Ma, J.L., Chang, Y.S., You, W.C., Liu, W.D., Zhang, L., Pee, D. and Gail, M.H. (2002) Helicobacter pylori infection in rural China: Demographic, lifestyle and environmental factor. International Journal of Epidemiology, 31, 638-645. doi:10.1093/ije/31.3.638
- Goodman, K.J., Correa, P., Tengana, A.H. et al. (1996) Helicobacter pylori infection in the Colombian Andes: A population-based study of transmission pathways. American Journal of Epidemiology, 144, 290-299. doi:10.1093/oxfordjournals.aje.a008924
- Goodman, K.J., Correa, P., Tengana, A.H., DeLany, J.P. and Collazos, T. (1997) Nutritional factors and Helicobacter pylori infection in Colombian children. Journal Pediatric Gastroenterolgy Nutrition, 25, 507-515. doi:10.1097/00005176-199711000-00004
- Jarosz, M., Dzieniszewski, J., Dabrowska-Ufniarz, E., Wartanowicz, M., Ziemlanski, S. and Reed, P.I. (1998) Effects of high dose vitamin C treatment on Helicobacter pylori infection and total vitamin C concentration in gastric juice. European Journal of Cancer Prevention, 7, 449-454. doi:10.1097/00008469-199812000-00004
- Graham, D.Y., Malaty, H.M., Evans, D.G., Evans Jr., D.J., Klein, P.D. and Adam, E. (1991) Epidemiology of Helicobacter pylori in an asymptomatic population in the United States. Effect of age, race, and socioeconomic status. Gastroenterology, 100, 1495-1501.
- Perry, S., de la Luz Sanchez, M., Yang, S., Haggerty, T.D., Hurst, P., Perez-Perez, G. and Parsonnet, J. (2006) Gastroenteritis and transmission of Helicobacter pylori infection in households. Emerging Infectious Diseases, 12, 1701-1708. doi:10.3201/eid1211.060086
- Mégraud, F. (1995) Transmission of Helicobacter pylori: Faecal-oral versus oral-oral route. Aliment Pharmacology Therapy, 9, 85-91.
- Lee, J.H., Shim, J.S., Lee, J.S., Kim, M.K., Chung, M.S. and Kim, K.H. (2006) Pectin-like acidic polysaccharide from Panax ginseng with selective antiadhesive activity against pathogenic bacteria. Carbohydrate Research, 341, 1154-1163.
- Silva, O., Viegas, S., de Mello-Sampayo, C., Costa, M.J., Serrano, R., Cabrita, J. and Gomes, E.T. (2012) Anti-Helicobacter pylori activity of Terminalia macroptera root. Fitoterapia, 83, 872-876. doi:10.1016/j.fitote.2012.03.019
- Stalheim, T., Balance, S., Christensen, B.E. and Granum, P.E. (2009) Sphagnan—A pectin-like polymer isolated from Sphagnum moss can inhibit the growth of some typical food spoilage and food poisoning bacteria by lowering the pH. Journal of Applied Microbiology, 106, 967-976. doi:10.1111/j.1365-2672.2008.04057.x
- Painter, T.J. (1991) Lindow man, Tollund man and other peat-bog bodies—The preservative and antimicrobial action of sphagnan, a reactive glycuronoglycan with tanning and sequestering properties. Carbohydrate Polymers, 15, 123-142. doi:10.1016/0144-8617(91)90028-B

