Advances in Bioscience and Biotechnology
Vol.3 No.3(2012), Article ID:19883,5 pages DOI:10.4236/abb.2012.33027
Sulfotransferase 1A1 G638A polymorphism, cigarette smoking and bladder cancer risk in Taiwan
![]()
1Department of Urology, Chi-Mei Medical Center, Tainan, Chinese Taipei
2Department of Urology, Shuang Ho Hospital, Taipei Medical University, Taipei, Chinese Taipei
3Division of Urology, Department of Surgery, Shuang Ho Hospital, Taipei Medical University, Taipei, Chinese Taipei
4Department of Urology, Shin-Kong Wu Ho-Su Memorial Hospital, Taipei, Chinese Taipei
Email: #m000733@ms.skh.org.tw
Received 6 February 2012; revised 25 March 2012; accepted 17 April 2012
Keywords: Bladder Cancer; Cigarette Smoking; Polymorphism; SULT1A1
ABSTRACT
Cigarette smoking is a major risk factor for bladder cancer (BC). Sulfotransferase 1A1 (SULT1A1), a phase II enzyme, plays an important role in the metabolism of several carcinogens contained in cigarettes. The aim of this study was to investigate the relationship between SULT1A1 G638A polymorphism, cigarette smoking and bladder cancer risk in Taiwan. A total of 150 BC patients and 150 cancer-free controls were recruited from February 2002 to February 2009. Genotyping of the SULT1A1 G638A polymerphism was determined using the polymerase chain reaction-restricted fragment length polymorphism (PCRRFLP) method. The odds ratio (OR) and 95% confidence interval (CI) were calculated as a measure of the combined effect of cigarette smoking and the SULT1A1 G638A polymorphism on BC risk. In the present study, we found that study subjects with the G/G genotype of the SULT1A1 gene had a significantly higher BC risk of 1.7 (95% CI = 1.3 - 3.2) compared with those carrying the combination of G/A and A/A genotypes. Moreover, ever smokers who carried the G/G genotype of the SULT1A1 gene had a significantly increased UC risk of 3.5 (95% CI = 2.5 - 10.2) compared with never smokers who carried the G/A and A/A genotypes as the reference group. In conclusion, our findings suggest that SULT1A1 G638A polymorphism is associated with the development of BC, especially among cigarette smokers.
1. INTRODUCTION
Bladder cancer (BC) is the eighth most common diagnosed malignancy among men in Taiwan [1]. Cigarette smoking is considered to be the key risk factor for bladder cancer [2,3]. Cigarettes contain about fifty-five carcinogens that have been evaluated by the International Agency for Research on Cancer [4]. Among these carcinogens, polycyclic aromatic hydrocarbons (PAHs), aromatic amines and N-nitroso compounds are considered to be major risk factors for the development of urothelial cancer. Additional risk factors include occupational exposure to carcinogenic chemicals, inflammatory reactions to parasites (such as schistosomiasis) or other chronic infections, and exposure to arsenic in drinking water are known risk factors for bladder cancer [5,6].
Genetic polymorphisms of the enzymes that catalyze xenobiotically-produced carcinogens may determine individual susceptibility to cancer. Most chemical carcinogens in cigarettes require metabolic activation by phase I enzymes and detoxification by phase II enzymes. Metabolic activation of PAHs by phase I enzymes leads to oxidized products, including quinones, resulting in reactive oxygen species (ROS) [7]. In contrast, detoxification of certain carcinogens leads to less toxic and more hydrophilic derivatives, which are more readily excreted.
Sulfotransferases (SULTs), a family of multifunctional enzymes, catalyze sulfonate conjugation. This is an important pathway in the metabolism of several chemicals that are exogenous (e.g. mutagens from diet and environment) or endogenous (e.g. hormones and neurotransmitters). In addition to its important role in metabolic detoxification, SULT1A1 may act to bioactivate dietary and environmental procarcinogens and promutagens [8,9]. In particular, SULT1A1 appears to be a key phenol SULT because of its abundance and distribution in a wide range of tissues [10]. The common polymorphism of SULT1A1 involves a single nucleotide G to A transition at nucleotide 638 (codon 213) in exon 7, which results in an arginine (Arg) to histidine (His) amino acid substitution and this polymorphism may lead to a lower enzyme activity and thermostability [11]. Epidemiological studies have shown inconsistent results for the association between the SULT1A1 G638A polymorphism and several malignancies including bladder cancer, lung cancer and breast cancer [12-15]. Moreover, due to its abundance in human tissues and significantly different expression among various genetic polymorphisms, SULT1A1 is considered to be a potentially cancer-predisposing gene.
Therefore, we conducted a hospital-based case-control study to explore the role of SULT1A1 G638A polymorphism in the development of bladder cancer in Taiwan. We also sought to investigate the combined effect of the SULT1A1 G638A polymorphism and cigarette smoking.
2. MATERIALS AND METHODS
2.1. Study Population
We studied a total of 150 histologically confirmed patients with bladder cancer (BC), diagnosed at the ChiMei Medical Center and the Department of Urology of the Shin Kong Wu Ho-Su Memorial Hospital between February 2002 and February 2009. A total of 150 cancer-free controls, frequency-matched with BC patients for age (±3 years) and gender, were selected from individuals who admitted to the same hospitals for a health examination and had no history of urological malignancies. All subjects were given an explanation of the present study, and then informed consents were obtained. All participants were interviewed by a well-trained interviewer using a structured questionnaire to collect information including a history of cigarette smoking. The study protocol was approved by the institutional review board at both collaborated hospitals.
2.2. Genotyping of the SULT1A1 G638A Polymorphism
A venous blood sample (6 - 8 ml) was drawn into an EDTA vial for each participant. Genomic DNA was extracted from peripheral lymphocytes by proteinase K digestion and phenol/chloroform method, which was stored at a –80˚C for further genotyping. We used a previously described polymerase chain reaction-restriction fragment length polymorphism (PCR-RFLP) method to determine the genotypes of SULT1A1 G638A polymerphism at codon 213 of exon 7 was also performed according to a previously published PCR-RFLP method [12]. The frequent homozygous genotype (G/G) yielded two band (168 and 165 bp), the heterozygous genotype (G/A) yielded three bands (333, 168 and 165 bp), and the variant homozygous genotype (A/A) yielded one band (333 bp). To ensure the quality, positive and negative tests were used in each experiment.
2.3. Statistical Analysis
The Hardy-Weinberg equilibrium (HWE) test was evaluated by comparing the observed with the expected genotype frequencies among controls using a Chi-square test. Study subjects who consumed more than 100 cigarettes during their lifetime were defined as ever smokers, while those who consumed less than 100 cigarettes were defined as never smokers. The number of pack-years was calculated using the formula: pack-years = (cigarettes per day/20) ´ (smoked years). The odds ratio (OR) and 95% confidence interval (CI) were calculated as a measure of the effect of the SULT1A1 G638A polymorphism on BC risk. Furthermore, the combined effect of the SULT1A1 G638A polymorphism and cigarette smoking on BC risk was estimated by a multivariate-adjusted logistic regression. Statistical Analysis Software (SAS, Version 9.1; SAS Institute, Cary, NC) was used for all analyses. The differences between BC cases and controls were considered significant if the p-values were less than 0.05.
3. RESULTS
3.1. The Distribution of Basic Characteristics
The distribution of basic characteristics for BC cases and controls is shown in Table 1. Among BC cases, 94 were male (mean age ± SD, 63.5 ± 12.3 years) and 56 were female (64.5 ± 12.3 years). Among controls, 97 were male (mean age ± SD, 63.6 ± 11.6 years) and 53 were female (61.9 ± 11.3 years). There were no significant differences in the distribution of age and gender between BC cases and controls. We found a significantly increased BC risk in ever smokers (OR = 1.8; 95% C = 1.4 - 3.2). The median value of pack-years among controls who had smoked was 30 pack-years. A significantly increased BC risk was also found in study subjects who smoked more than 30 pack-years (OR = 1.8; 95% CI = 1.3 - 3.7).
3.2. Comparison of SULT1A1 G638A Polymorphism
The distribution of the observed genotype frequencies was in Hardy-Weinberg equilibrium for SULT1A1 (P = 0.728). Study subjects who carried the G/G genotype of SULT1A1 gene have a non-significant higher BC risk (OR = 1.7; 95% CI = 0.8 - 16.4), compared with those with the A/A genotype. However, compared with individuals who carried the combination of SULT1A1 A/A and G/A genotypes, those with the G/G genotype had a significantly higher BC risk of 1.7 (95% CI = 1.3 - 3.2) (Table 2).
3.3. Combination Analysis of SULT1A1 G638A Polymorphism and Smoking
Because we hypothesized that SULT1A1 G638A polymorphism would modulate the effect of chemical carcinogens in cigarettes on BC, we examined the combined effect of SULT1A1 G638A polymorphism and cigarette smoking. Table 3 outlines the relationship between the SULT1A1 G638A polymorphism and BC risk by stratification of cigarette smoking. Comparing with never smokers who carried the G/A and A/A genotypes of the SULT1A1 gene as the reference group, significantly increased BC risks of 2.1 (95% CI = 1.2 - 5.0), 2.4 (95% CI = 1.3 - 8.5) and 3.5 (95% CI = 2.5 - 10.2) were found for never smokers with the G/G genotype, ever smokers
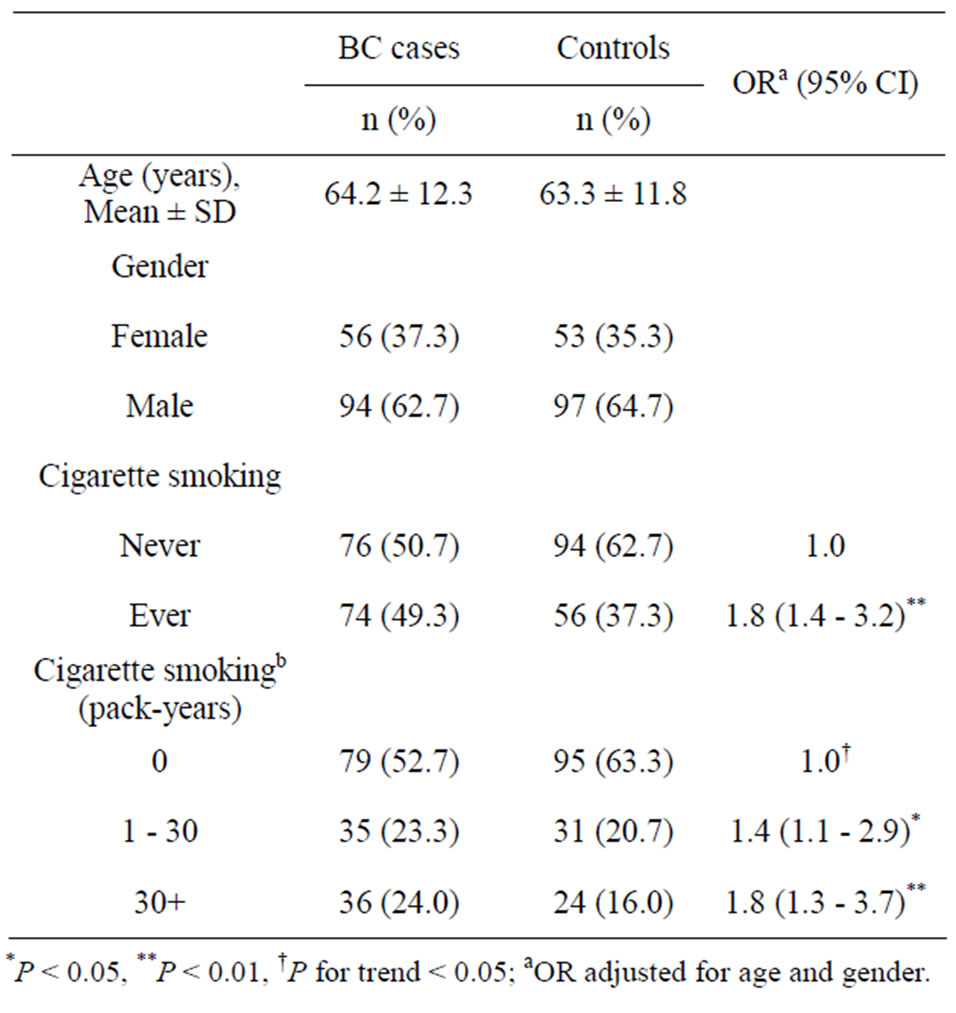
Table 1. Distribution of basic characteristics for BC cases and controls.
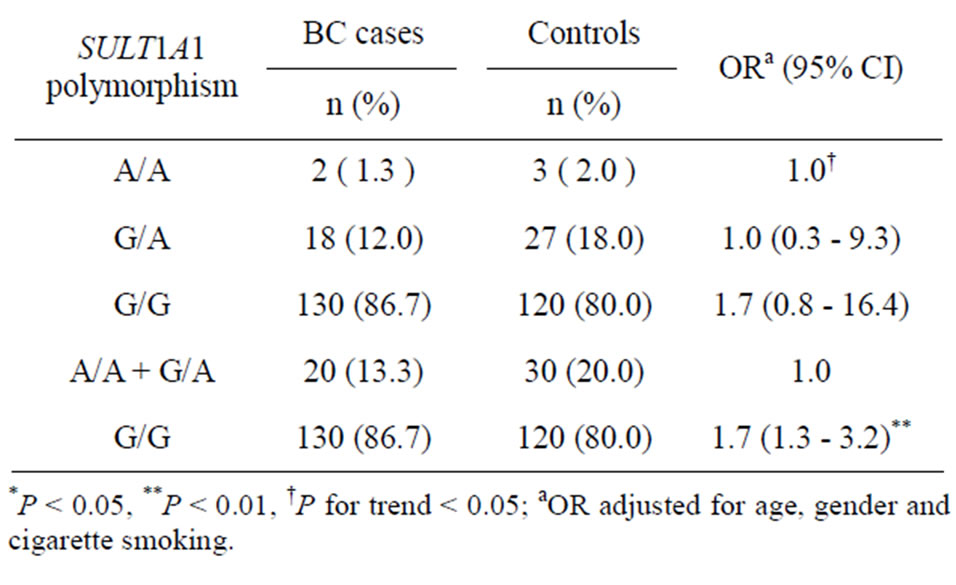
Table 2. Distribution of SULT1A1 G638A polymorphism in BC cases and controls.
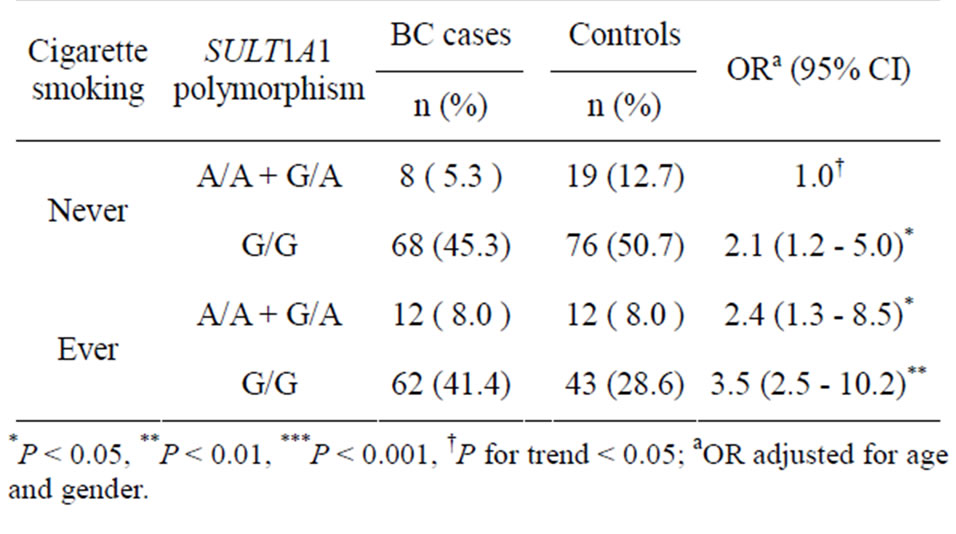
Table 3. The combined effect of SULT1A1 G638A polymorphism and cigarette smoking on BC risk.
with the G/A and A/A genotypes and ever smokers with the G/G genotype, respectively.
4. DISCUSSION
Our study evaluated whether the SULT1A1 G638A polymorphism is associated with bladder cancer risk. We also investigated a potential interaction of the SULT1A1 G638A polymorphism with cigarette smoking. Several studies have elucidated the association between cigarette smoking and bladder cancer [2,3,16]. We found that ever smokers have a significantly increased risk of BC, especially among those who had smoked over 30 pack-years.
Sulfonation is thought to be a detoxification pathway for various xenobiotics, and it is also involved in the bioactivation of several carcinogens by O-esterification to form DNA-damaging toxic metabolites [8,11]. The G to A polymorphism at position 638 of SULT1A1 may result in reduced enzyme activity and reduced thermostability. Few prior studies had investigated the effect of SULT1A1 on risk of cigarette-related cancer and no consistent results were observed [12,14,15]. We found that individuals carrying the G/G genotype of the SULT1A1 gene have a significantly increased risk (OR = 1.7) for bladder cancer. Ever smokers with the G/G genotype of the SULT1A1 gene have a significantly higher BC risk of 3.5. Our findings, however, differ from previous reports, which have observed an increased cancer risk or null results associated with the A allele of SULT1A1 [13,15, 17]. Positive association was also reported for the A allele of SULT1A1 in esophageal cancer (OR = 3.5; 95% CI = 2.12 - 5.87) [18]. However, a Japanese study found a marginally protective effect of the A allele of the SULT1A1 gene on urothelial carcinoma [14]. Furthermore, a four-fold increased risk of colorectal adenomas was observed in cigarette smokers carrying the SULT1A1 G/G genotype compared to never smokers with the G/A and A/A genotypes of SULT1A1 [19]. The increased BC risk we observed may be due to the dual role of SULTs, such as SULT1A1, which are involved in both the detoxification and bioactivation of several carcinogens. SULT1A1 activity is also known to depend upon tissue or organ specificity. SULTs have substrate-dependent effects and the SULT1A1 can activate both heterocyclic amines and aromatic amines to become DNA-binding species. This activity may be greatly reduced in those with the A/A phenotype [12]. Therefore, the association between SULT1A1 and the risk for cancer development may be explained by wide substrate specificity and different distribution of the enzyme within the tissue.
We noted that the divergent genetic background and differing carcinogen exposure in various populations may explain the differing risk assessments related to the genetic polymorphisms [20-22]. Our study is weakened somewhat by recall bias, in that it can be difficult to recall carcinogen exposure at time points in the past, and cases are more motivated than controls to recollect past exposures.
In conclusion, the present study provides epidemiologic evidence that the SULT1A1 G638A polymorphism can modulate individual susceptibility to BC, in combination with cigarette smoking. Further large studies are needed to estimate the effect of differing candidate alleles on carcinogens-metabolized enzymes and to provide more rigorous data on potential risk factors for BC.
5. ACKNOWLEDGEMENTS
This work was supported by the grant from the Shin Kong Wu Ho-Su Memorial Hospital (SKH-8302-99-DR-20).
REFERENCES
- Republic of China, Taipei Department of Health the Executive Yuan (2010) Department of health, the executive Yuan: Cancer registry annual report.
- Samanic, C., et al. (2006) Smoking and bladder cancer in Spain: Effects of tobacco type, timing, environmental tobacco smoke, and gender. Cancer Epidemiology, Biomarkers & Prevention, 15, 1348-1354. doi:10.1158/1055-9965.EPI-06-0021
- Zeegers, M.P., Goldbohm, R.A. and van den Brandt, P.A. (2002) A prospective study on active and environmental tobacco smoking and bladder cancer risk (The Netherlands). Cancer Causes Control, 13, 83-90. doi:10.1023/A:1013954932343
- IARC (1986) IARC monographs on the evaluation of the carcinogenic risk of chemicals to humans: Tobacco smoking. WHO, Lyon.
- Pelucchi, C., Bosetti, C., Negri, E., Malvezzi, M. and La Vecchia, C. (2006) Mechanisms of disease: The epidemiology of bladder cancer. Nature Clinical Practice Urology, 3, 327-340. doi:10.1038/ncpuro0510
- Kalble. T. (2001) Etiopathology, risk factors, environmental influences and epidemiology of bladder cancer. Urologe A, 40, 447-450.
- Kellen, E., et al. (2007) Does occupational exposure to PAHs, diesel and aromatic amines interact with smoking and metabolic genetic polymorphisms to increase the risk on bladder cancer? The Belgian case control study on bladder cancer risk. Cancer Letters, 245, 51-60. doi:10.1016/j.canlet.2005.12.025
- Glatt, H. (2000) Sulfotransferases in the bioactivation of xenobiotics. Chemico-Biological Interactions, 129, 141- 170. doi:10.1016/S0009-2797(00)00202-7
- Ginsberg, G., et al. (2010) Genetic polymorphism in metabolism and host defense enzymes: Implications for human health risk assessment. Critical Reviews in Toxicology, 40, 575-619. doi:10.3109/10408441003742895
- Glatt, H., et al. (2001) Human cytosolic sulphotransferases: Genetics, characteristics, toxicological aspects. Mutation Research, 482, 27-40. doi:10.1016/S0027-5107(01)00207-X
- Pereira, W.O., et al. (2005) Genetic polymorphism in the sulfotransferase SULT1A1 gene in cancer. Cancer Genetics and Cytogenetics, 160, 55-60. doi:10.1016/j.cancergencyto.2004.12.005
- Zheng, L., Wang, Y., Schabath, M.B., Grossman, H.B. and Wu, X. (2003) Sulfotransferase 1A1 (SULT1A1) polymorphism and bladder cancer risk: A case-control study. Cancer Letters, 202, 61-69. doi:10.1016/j.canlet.2003.08.007
- Tang, D., et al. (2003) Sulfotransferase 1A1 (SULT1A1) polymorphism, PAH-DNA adduct levels in breast tissue and breast cancer risk in a case-control study. Breast Cancer Research and Treatment, 78, 217-222. doi:10.1023/A:1022968303118
- Tsukino, H., et al. (2004) Cytochrome P450 (CYP) 1A2, sulfotransferase (SULT) 1A1, and N-acetyltransferase (NAT) 2 polymorphisms and susceptibility to urothelial cancer. Journal of Cancer Research and Clinical Oncology, 130, 99-106. doi:10.1007/s00432-003-0512-0
- Liang, G., Miao, X., Zhou, Y., Tan, W. and Lin, D. (2004) A functional polymorphism in the SULT1A1 gene (G638A) is associated with risk of lung cancer in relation to tobacco smoking. Carcinogenesis, 25, 773-778. doi:10.1093/carcin/bgh053
- Bjerregaard, B.K., et al. (2006) Tobacco smoke and bladder cancer-in the European prospective investigation into cancer and nutrition. International Journal of Cancer, 119, 2412-2416. doi:10.1002/ijc.22169
- Zhang, C., et al. (2011) Lack of association of SULT1A1 R213H polymorphism with colorectal cancer: A metaanalysis. PLoS One, 6, e19127. doi:10.1371/journal.pone.0019127
- Wu, M.T., et al. (2003) SULT1A1 polymorphism and esophageal cancer in males. International Journal of Cancer, 103, 101-104. doi:10.1002/ijc.10805
- Tiemersma, E.W., et al. (2004) Effect of SULT1A1 and NAT2 genetic polymorphism on the association between cigarette smoking and colorectal adenomas. International Journal of Cancer, 108, 97-103. doi:10.1002/ijc.11533
- Kotnis, A., Kannan, S., Sarin, R. and Mulherkar, R. (2008) Case-control study and meta-analysis of SULT1A1 Arg213His polymorphism for gene, ethnicity and environment interaction for cancer risk. British Journal of Cancer, 99, 1340-1347. doi:10.1038/sj.bjc.6604683
- Yu, X., et al. (2010) Functional genetic variants in the 3’-untranslated region of sulfotransferase isoform 1A1 (SULT1A1) and their effect on enzymatic activity. Toxicological Science, 118, 391-403. doi:10.1093/toxsci/kfq296
- Li, K., et al. (2012) SULT1A1 Arg213His polymorphism and susceptibility of environment-related cancers: A metaanalysis of 5915 cases and 7900 controls. Molecular Biology Report, 39, 2597-2605. doi:10.1007/s11033-011-1012-y
NOTES
*These authors (K.-H. Shen, C.-C. Wu and Y.-H. Wang) contributed equally to this work.
#Corresponding author.

