Advances in Bioscience and Biotechnology
Vol.3 No.4(2012), Article ID:21851,6 pages DOI:10.4236/abb.2012.34044
Enhanced bio-catalytic and tolerance properties of an indigenous cellulase through xerogel immobilization
![]()
1Department of Chemistry and Biochemistry, University of Agriculture, Faisalabad, Pakistan
2Department of Chemistry, Government College University, Faisalabad, Pakistan
3Transgenic Lab Center of Agriculture Biochemistry and Biotechnology (CABB), University of Agriculture, Faisalabad, Pakistan
Email: *nasir_pk99@hotmail.com
Received 24 May 2012; revised 28 June 2012; accepted 4 July 2012
Keywords: Bio-catalysis; Cellulase; T. viride; immobilization; Characterization
ABSTRACT
Today, demand exists for cost-effective production of industrially important enzymes from entire scientific sectors. By keeping in mind the extensive industrial applications of cellulase, this study was performed to immobilize the indigenous enzyme produced from Trichoderma viride under pre-optimized SSF of an agricultural waste material, wheat straw. To enhance the bio-catalytic and tolerance properties of the present enzyme gel matrix immobilization engineering was applied. Previously, 2.33-fold purified novel cellulase was immobilized in to a xerogel matrix of TMOS and PTMS. FTIR spectroscopy confirmed the successful immobilization of cellulase. The free and immobilized cellulase was characterized and stability profile showed that after 24 h incubation, immobilization enhanced the thermo-stability up to 75% against 80˚C as compare to the free enzyme. Xerogel matrix immobilization enhanced the catalytic efficiency of entrapped enzyme than that of the free cellulase. Among activators/inhibitors SDS, EDTA, and Hg2+ showed inhibitory effect while, gel matrix immobilization enhanced 80% tolerance capacity of the cellulase against inactivating agents.
1. INTRODUCTION
The major bottle neck against comprehensive application of cellulases in industry is the high cost of the enzyme production. Currently, most commercial cellulases (including β-glucosidase) are produced by Trichoderma and Aspergillus species [1]. Cellulases are widely used in the textile industry for denim finishing; in the detergent market for color care and anti-deposition; in the food industry for mashing and in the pulp and paper industries for de-inking and fiber modification [1-4].
Cellulose degrading enzymes system is a complex of three enzymes that exhibit higher collective activity and degrade cellulose into glucose and other commodities by the phenomenon known as synergism [5]. The cellulase complex is comprised of three major components: cellualase (CMCases) or Endo-β-glucanase (EC 3.2.1.4), Exo-β-glucanase (EC 3.2.1.91) and β-glucosidase (EC 3.2.1.21) [1,6]. A variety of micro-organisms including Trichoderma, Aspergillus, Penicillium, and Fusarium have ability to produce enzymes like cellulases and hemicellulases under suitable growth conditions to hydrolyze insoluble polysaccharides to soluble oligomers, and subsequently to monomers [6,7].
From the last several years, enzyme immobilization has revolutionized the field of enzyme biotechnology. Several authors have been reported various immobilization methods including chemical engineering modification, mutation, gel entrapment and surface binding [8-11]. Among them, gel entrapment is preferred over others as this method is easier and cheaper and the structure of the enzyme remains secure. Moreover, xerogel polymers are nontoxic and do not swell in aqueous or organic solvents, preventing the leaching of the entrapped enzyme that allow the enzymes to maintain their native structure [8].
With respect to the factors affecting culture conditions, productivity and properties of cellulase, it was considered of significance to characterize this enzyme through kinetic studies including immobilization by studying the effect of varying pH, temperature and activators/inhibittors to explore that factors. In previous studies [1,6], we have successfully investigated the cellulase synthesis potential of indigenous fungal strain T. viride under SSF based on lignocellulosic substrate. In this paper we aimed to immobilize using xerogel matrix and further characterize the previously successfully purified cellulase from T. viride to investigate the factors affecting the activity and stability of the enzyme to present potential and possible application for industrial and biotechnological purposes.
2. MATERIALS AND METHODS
2.1. Cellulase Production and Activity Estimation
Physical and nutritional growth conditions have been successfully optimized previously to produce substantial amount of cellulase during SSF of wheat straw [1,6] and follow in the present study. Cellulase activity of the previously purified active fraction was determined using a UV/Visible spectrophotometer as described earlier [1,6].
2.2. Immobilization of Cellulase
TMOS and PTMS were used in molar ratios of 1:1 to prepare gel for cellulase entrapment into a gel matrix. Five different purified active fractions contained 2 - 10 mg/mL cellulase were suspended in de-ionized water and centrifuged at 3000 g for 10 min. The supernatant fluid from each fraction was added to a mixture contained aqueous sodium fluoride, polyvinyl alcohol and water followed by the addition of PTMS and TMOS respectively. The reaction mixture was shaken on a vortex mixer and placed in an ice bath until gelation occurred. At the end activity of gel entrapped cellulase was determined, as described in the previous section.
2.3. Characterization of Free and Immobilized Cellulase
2.3.1. Immobilization Confirmation
The FTIR spectra of the free and xerogel matrix immobilized cellulase were obtained by FTIR spectrometry. FTIR spectrum was recorded using a tablet form of thoroughly mixed mixture of 0.1 g KBr with 0.1 g xerogel matrix.
2.3.2. Effect of pH & Temperature on Cellulase Activity and Stability
The purified cellulase was subjected to characterization through kinetic studies by studying the effect of different pH (2 - 10) and temperature (20˚C - 80˚C) on free and immobilized enzyme. To investigate the effect of pH and temperature the free and immobilized enzyme was incubated for up to 24 h in the absence of substrate and residual activity of free enzyme was checked in comparison to the immobilized enzyme.
2.3.3. Determination of Km and Vmax
The Michalis-Menten kinetic constants Km and Vmax for cellulase were calculated from Lineweaver-Burk plot (created with Microsoft Excel Windows 7 via non linear curve fitting method) with varying concentrations (100 - 1000 µM) of carboxymethyl cellulose as substrate.
2.3.4. Effect of Activators/Inhibitors
For the first time effect of various compounds and metal ions (SDS, EDTA, Hg2+, Co2+ and Mn2+) as possible activators or inhibitors on the free and immobilized enzyme was studied. The enzyme activities for each case were determined under standard assay conditions as described earlier.
3. RESULTS AND DISCUSSION
3.1. Source of Cellulase
Cellulase produced by T. viride during SSF of wheat straw under pre-optimized growth conditions has been successfully purified and reported earlier [1,6]. Under pre-optimized conditions, T. viride produced 398 ± 2.43 U/mL of cellulase that was 2.33-fold purified by Sephadex G-100 gel filtration chromatographic technique with specific activity of 105 U/mg [1]. The previously purified novel cellulase was used in the present study based on the immobilization and characterization to enhance its bio-catalytic and tolerance properties.
3.2. Immobilization of Cellulase
The activity assay profile showed that the specific activity of gel immobilized cellulase was higher as compared to free enzyme and the results obtained are summarized in Table 1. The fraction contained 2 mg/mL enzyme concentration showed maximum immobilization efficiency (89.8%) and it was observed that the immobilization efficiency decreased up to the 49.2% with the increasing concentration of enzyme. In addition, the results obtained during the present immobilization study using xerogel matrix entrapment method are in the range of those attained by other immobilization methods such as the covalent bonding of the enzyme on to the siliceous cellular foams, sepabeads and Iron oxide MNPs [12-14].
3.3. Characterization of Free and Immobilized Cellulase
3.3.1. Immobilization Confirmation
The entrapment of cellulase into the gel matrix was confirmed by FTIR spectrometry. Figure 1 illustrated a typical FTIR spectra for free cellulase, and xerogel matrix immobilized cellulase. The characteristic bands of proteins at 1535 and 1562 cm−1 in the spectra of free and gel entrapped cellulase respectively indicates that cellu- lase was successfully present in the samples, confirming the immobilization of cellulase into the gel matrix of TMOS and PTMS. In an earlier study, Khoshnevisan et al. [10] also successfully used the FTIR spectrometry to confirm the binding of cellulase onto MNPs. In the same study Khoshnevisan et al. [10] reported characteristic bands of proteins at 1624, and 1408 cm−1, and 1624 and
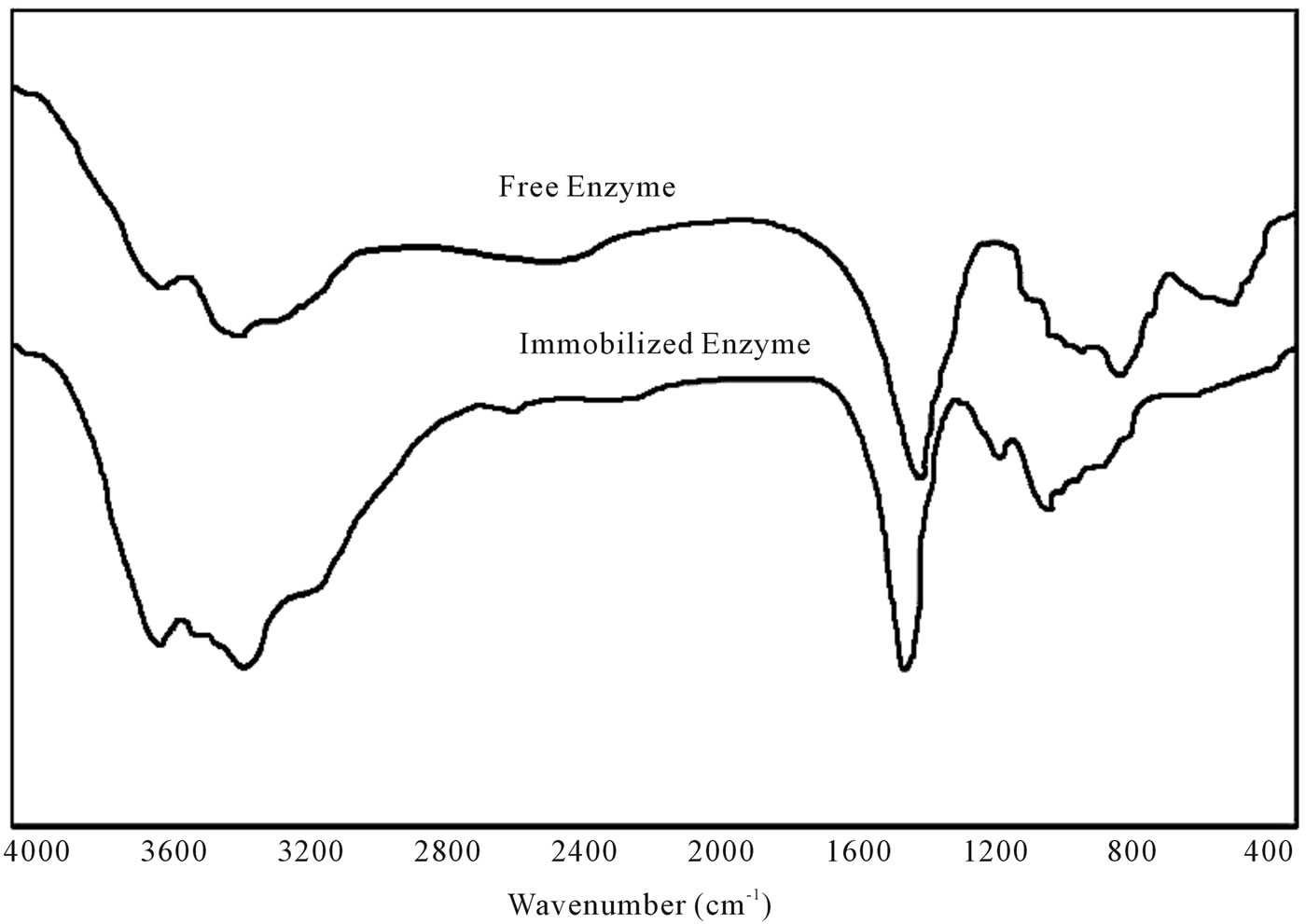
Figure 1. FTIR spectra of free and xerogel matrix immobilized cellulase.
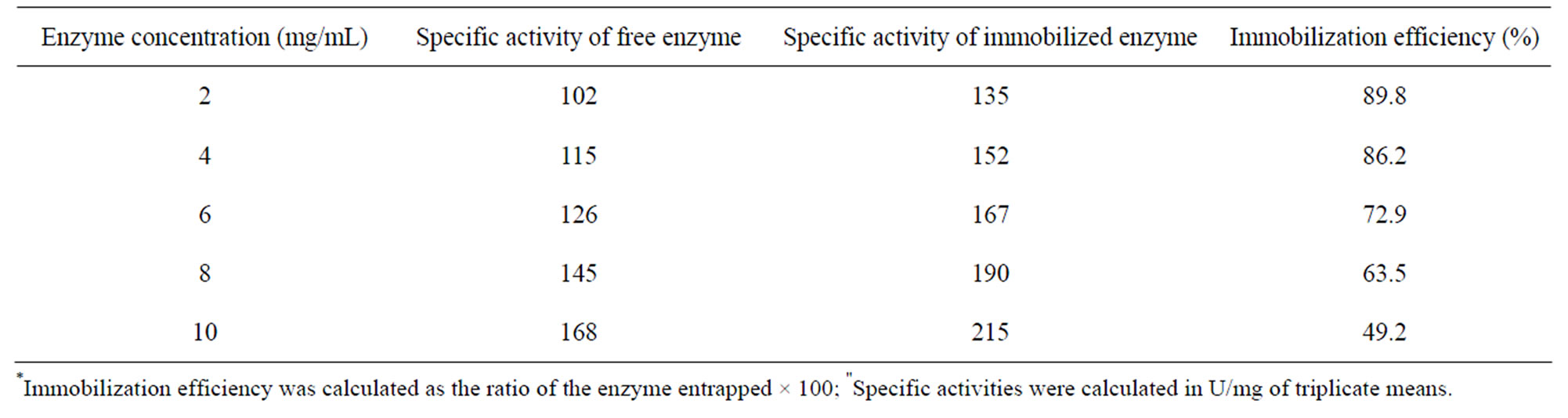
Table 1. Immobilization efficiency* and specific activities" of free and immobilized cellulase.
1552 cm−1 in the spectra of cellulase, and Fe3O4-cellulase respectively that well supported our study and prove the precise FTIR spectra of present investigation.
3.3.2. Effect of pH & Temperature on Cellulase Activity and Stability
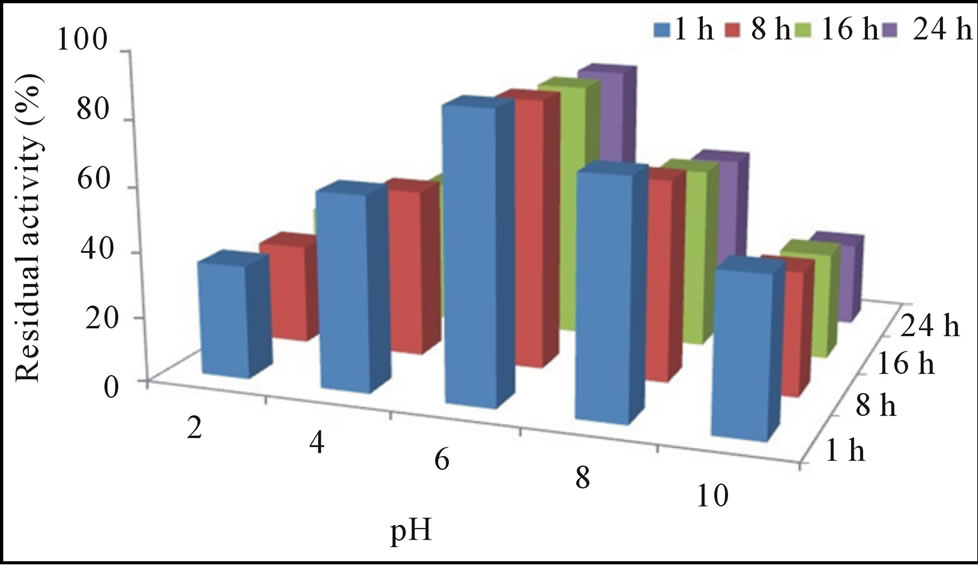
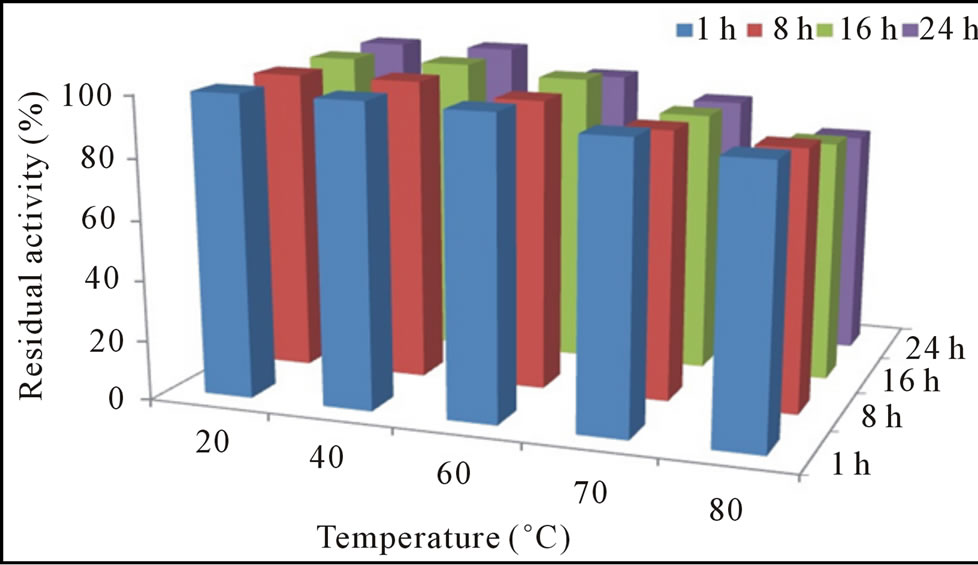
The pH-activity profile showed that free cellulase was optimally active at pH 8 whereas, immobilization slightly shift pH optima towards acidic i.e. 6. This phenomenon was also reported by Erdemir et al. [15], who observed a slight pH shift during lipase immobilization. Results of stability profile showed that free cellulase was only stable for 1 h in a pH range of 7.0 to 9.0 while, gel entrapment enhanced its stability up to 78% for 24 h that was much more than the free enzyme (Figures 2(a) and (b)). The purified cellulase was optimally active at 55˚C and further increase in temperature cause decrease in activity. Free cellulase was only 15% active at 80˚C while immobilize cellulase showed better thermo-stability and retained 75% original activity at 80˚C after 24 h incubation (Figures 3(a) and (b)). For a variety of industrial applications relatively high-thermo stability of an enzyme is an attractive and desirable characteristic [1,16]. Most of the earlier reported cellulases were found to lose their activities at temperatures around 60˚C or higher [10,12]. Cellulase produced from Cryptococcus sp. S-2 was optimally active in the range of 40˚C - 50˚C [17], whereas from Aspergillus niger and Mucor circinelloides was most active and stable at 55˚C [16,18].
3.3.3. Determination of Km and Vmax
Results obtained were plotted as activity against the concentration of substrate using Lineweaver-Burk double reciprocal plot. Immobilization enhanced the catalytic efficiency of the entrapped cellulase by lowering its Km value in comparison to the free enzyme (Figure 4). The lower value of Km (62 µM) for immobilized enzyme indicates that immobilization enhance the bio-catalytic
 (a)
(a)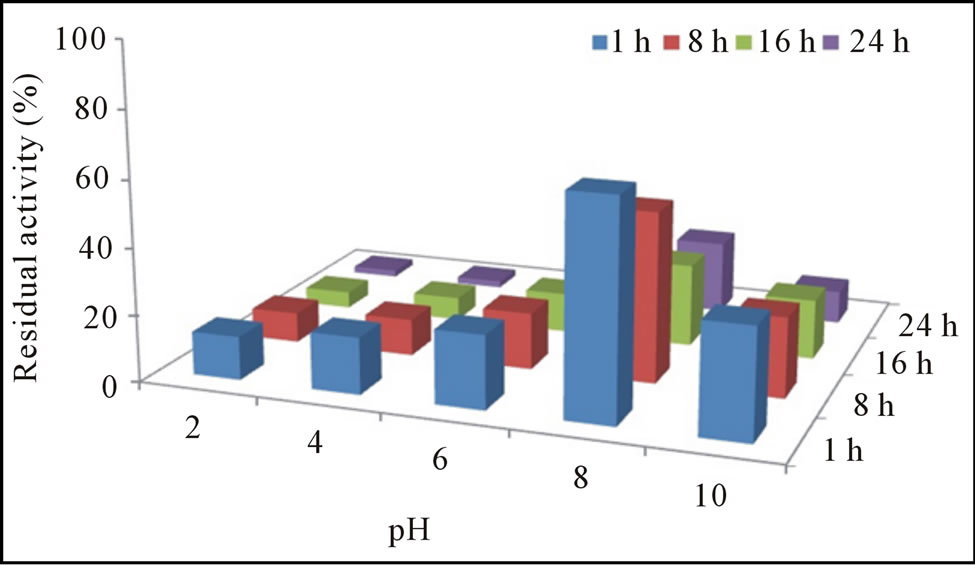 (b)
(b)
Figure 2. Residual activity and stability of free (a) and immobilized (b) cellulase at different pH.
 (a)
(a)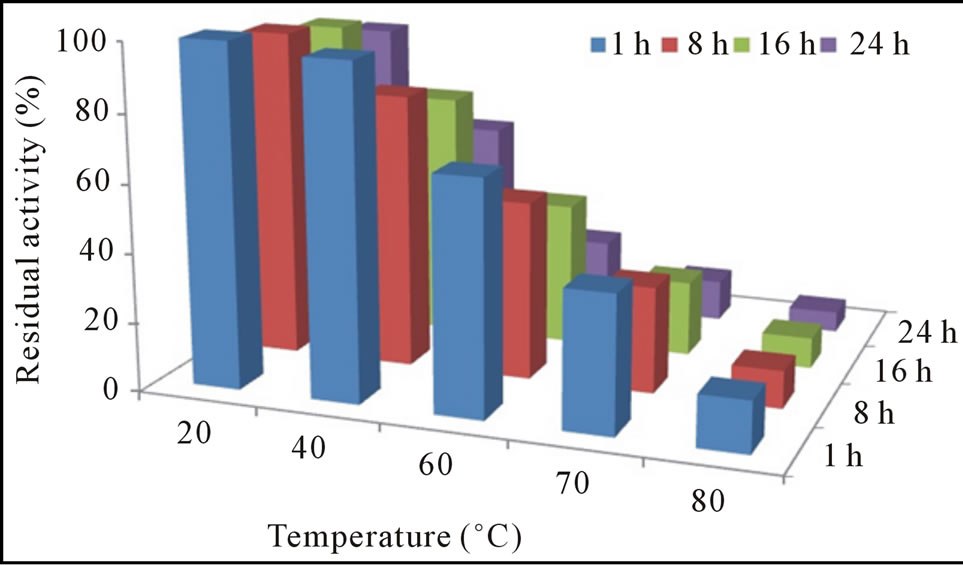 (b)
(b)
Figure 3. Residual activity and stability of free (a) and immobilized (b) cellulase at different temperatures.
substrate efficiency of immobilized cellulase as compare to free cellulase (68 µM). In earlier studies several authors reported different Km and Vmax for different fungal species such as A. anitratus and Branhamella sp. (4.97 and 7.90 mg/mL) [19] and Trichoderma reesei (1.1 mM) [20]. This slight difference in Km value of the presently investigated cellulase from Trichoderma viride and other reported fungal species may be due to the genetic variability among different species.
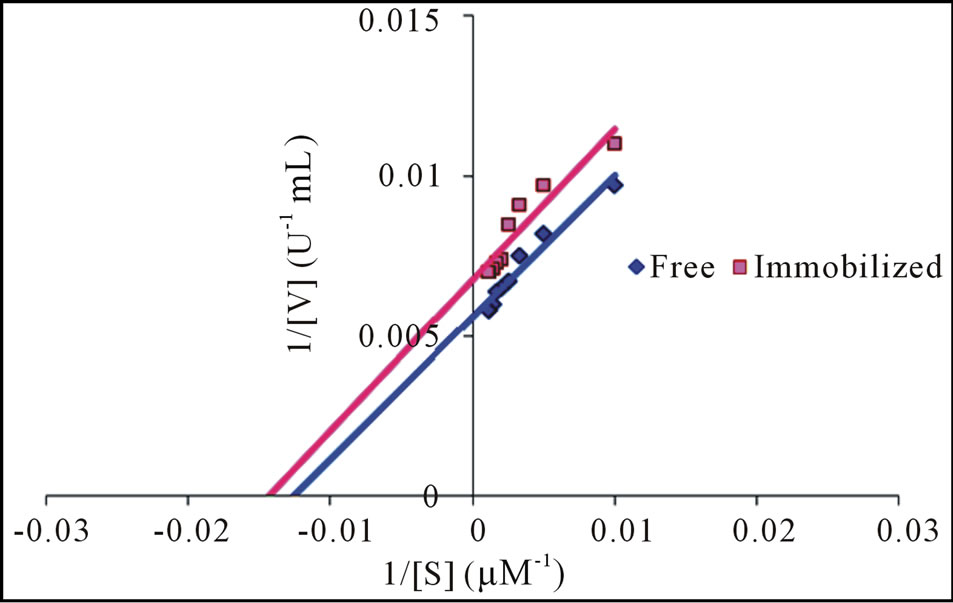
Figure 4. Lineweaver-Burk reciprocal plot: determination of Km and Vmax for free and immobilized cellulase.
3.3.4. Effect of Activators/Inhibitors
For the first time to investigate the effect of various compounds and metal ions as possible activators/inhibitors on the free and gel matrix immobilized cellulase was studied. Residual activity profile showed that free enzyme was completely inhibited by SDS and EDTA. Among the metal ions tested only Hg2+ caused complete enzyme inhibition whereas, cellulase retained 100% activity in the presence of Co2+ and Mn2+ (Figure 5). The gel entrapment was observed to present a noteworthy tolerance
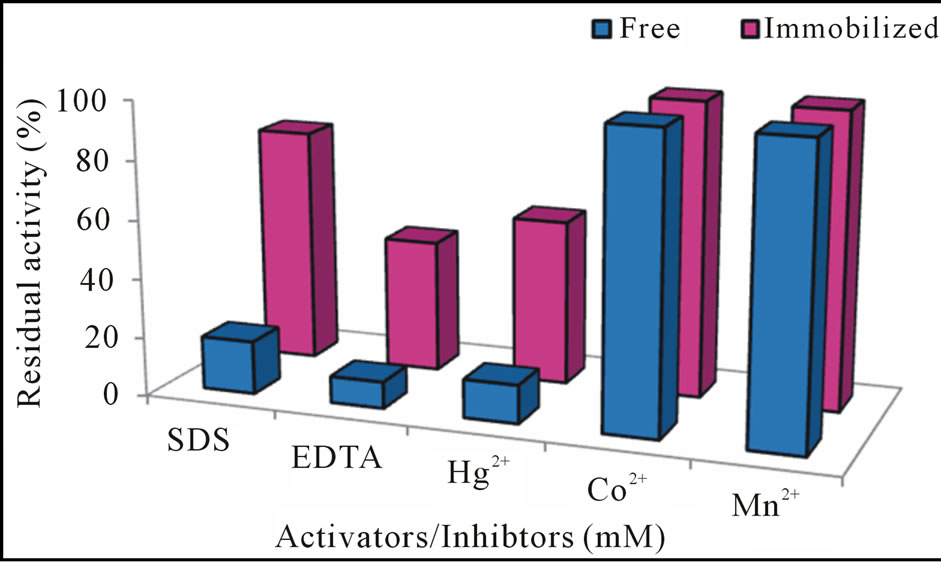
Figure 5. Residual activity of free and immobilized cellulase with different activators/inhibitors.
than the free enzyme against inactivation by SDS, EDTA, Hg2+ (residual activity of immobilized enzyme was increased from 18% to 80% for SDS, from 9% to 45% for EDTA and from 13% to 56% for Hg2+). EDTA is a metal chelating agent that has ability to form complex with inorganic groups of enzymes and therefore inhibitory effect was found [21]. Addition of Co2+, Mn2+ Fe3+, Ca2+ and Ni2+ into the enzyme production medium does not cause any alteration in the activity and under lower concentrations increased the enzymes activity [18,22].
4. CONCLUSION
In conclusion, the presently investigated indigenous strain T. viride showed incredible potential for cellulase synthesis in SSF of wheat straw in high titters (398 ± 2.43 U/mL) than other reported fungal species. Catalytic efficiency and extra thermo-stability of cellulase makes it a resourceful enzyme for various industrial and biotechnological applications. In this article, and for the first time, the effect of xerogel immobilization on the stability of the T. viride cellulase in the presence of different activators/inhibitors has been reported.
5. ACKNOWLEDGEMENTS
The reported work is an extract of the research training session supervised by H.M.N. Iqbal; on conducting voluntary trials Mr. I. Ahmed & M.T. Naveed are thankfully acknowledged. Authors are also grateful to the editor & reviewers, Advances in Bioscience and Biotechnology for their useful comments/recommendations over the work.
REFERENCES
- Iqbal, H.M.N., Ahmed, I., Zia, M.A. and Irfan, M. (2011) Purification and characterization of the kinetic parameters of cellulase produced from wheat straw by Trichoderma viride under SSF and its detergent compatibility. Advances in Bioscience and Biotechnology, 2, 149-156. doi:10.4236/abb.2011.23024
- Nagendran, S., Hallen-Adams, H.E., Paper, J.M., Aslam, N. and Walton, J.D. (2009) Reduced genomic potential for secreted plant cell wall degrading enzymes in the ectomycorrhizal fungus Amanita bisporigera, based on the secretome of T. reesei. Fungal Genetics and Biologya, 46, 427-435. doi:10.1016/j.fgb.2009.02.001
- Sun, H., Ge, X., Hao, Z. and Peng, M. (2010) Cellulase production by Trichoderma sp. on apple pomace under solid state fermentation. African Journal of Biotechnology, 9, 163-166.
- Yano, S., Ozaki, H, Matsuo, S., Ito, M., Wakayama, M. and Takagi, K. (2012) Production, purification and characterization of D-aspartate oxidase from the fungus Trichoderma harzianum SKW-36. Advances in Bioscience and Biotechnology, 3, 7-13.
- Gori, M.I. and Malana, M.A. (2010) Production of carboxymethyl cellulase from local isolate of Aspergillus species. Pakistan Journal of Life and Social Sciences, 8, 1-6.
- Ahmed, I., Zia, M.A. and Iqbal, H.M.N. (2010) Bio-processing of proximally analyzed wheat straw for enhanced production of cellulase through parameters optimization with T. viride under SSF. International Journal of Biological Sciences, 6, 164-170.
- Beukes, N. and Pletschke, B.I. (2006) Effect of sulfurcontaining compounds on Bacillus cellulosome associated “CMCase” and “avicelase” activities. FEMS Microbiology Letters, 264, 226-231. doi:10.1111/j.1574-6968.2006.00465.x
- Minovska, V., Winkelhausen, E. and Kuzmanova, S. (2005) Lipase immobilized by different techniques on various support materials applied in oil hydrolysis. Journal of the Serbian Chemical Society, 70, 609-624. doi:10.2298/JSC0504609M
- Torres, L.G., Velasquez, A. and Brito-Arias, M.A. (2011) Ca-alginate spheres behavior in presence of some solvents and water-solvent mixtures. Advances in Bioscience and Biotechnology, 2, 8-12. doi:10.4236/abb.2011.21002
- Khoshnevisan, K., Bordbar, A.-K., Zare, D., Davoodi, D., Noruzi, M., Barkhi, M. and Tabatabaei, M. (2011) Immobilization of cellulase enzyme on superparamagnetic nanoparticles and determination of its activity and stability. Chemical Engineering Journal, 171, 669-673. doi:10.1016/j.cej.2011.04.039
- Yoon, M., Hwang, H.J. and Kim, J.H. (2011) Immobilization of antibodies on the self-assembled monolayer by antigen-binding site protection and immobilization kinetic control. Journal of Biomedical Science and Engineering, 4, 242-247. doi:10.4236/jbise.2011.44033
- Ince, A., Bayramoglu, G., Karagoz, B., Altintas, B., Bicak, N. and Arica, M.Y. (2012) A method for fabrication of polyaniline coated polymer microspheres and its application for cellulase immobilization Chemical Engineering Journal, 189-190, 404-412. doi:10.1016/j.cej.2012.02.048
- Ren, Y., Rivera, J.G., He, L., Kulkarni, H., Lee, D.-K. and Messersmith, P.B. (2011) Facile, high efficiency immobilization of lipase enzyme on magnetic iron oxide nanoparticles via a biomimetic coating. BMC Biotechnology, 11, 63. doi:10.1186/1472-6750-11-63
- Boniello, C., Mayr, T., Bolivar, J.M. and Nidetzky, B. (2012) Dual-lifetime referencing (DLR): A powerful method for on-line measurement of internal pH in carrier-bound immobilized biocatalysts. BMC Biotechnology, 12, 11. doi:10.1186/1472-6750-12-11
- Erdemir, S., Sahin, O. and Uyanik, A. (2009) Effect of the glutaraldehyde derivatives of Calix[n]arene as cross linker reagents on lipase immobilization. Journal of Inclusion Phenomena and Macrocyclic Chemistry, 64, 273- 282. doi:10.1007/s10847-009-9562-5
- Fadel, M. (2000) Production physiology of cellulases and β-glucosidase enzymes of Aspergillus niger grown under solid state fermentation conditions. Online Journal of Biological Sciences, 1, 401-411.
- Thongekkaew, J., Ikeda, H., Masaki, K. and Iefuji, H. (2008) An acidic and thermo-stable carboxymethyl cellulase from the yeast Cryptococcus sp. S-2: Purification, characterization and improvement of its recombinant enzyme production by high cell-density fermentation of Pichia pastoris. Protein Expression and Purification, 60, 140-146. doi:10.1016/j.pep.2008.03.021
- Saha, B.C. (2004) Production purification and properties of endoglucanase from a newly isolated strain of Mucor circinelloides. Process Biochemistry, 39, 1871-1876. doi:10.1016/j.procbio.2003.09.013
- Ekperigin, M.M. (2007) Preliminary studies of cellulase production by Acinetobacter anitratus and Branhamella sp. African Journal of Biotechnology, 6, 028-033.
- Cascalheira, J.F. and Queiroz, J.A. (1999) Kinetic study of the cellobiase activity of Trichoderma reesei cellulose complex at high substrate concentrations. Biotechnology Letters, 21, 651-655. doi:10.1023/A:1005525015777
- Bakare, M.K., Adewale, I.O., Ajayi, A. and Shonukan, O.O. (2005) Purification and characterization of cellulase from the wild-type and two improved mutants of Pseudomonas fluorescens. African Journal of Biotechnology, 4, 898-904.
- Lucas, R., Robles, A., Garcia, M.T., Alvarez De Cienfuegos, G. and Galvez, A. (2001) Production, purification and properties of an endoglucanase produced by the hyphomycete Chalara (syn. Thielaviopsis) paradoxa CH32. Journal of Agricultural and Food Chemistry, 49, 79-85. doi:10.1021/jf000916p
NOTES
*Corresponding author.

