Advances in Bioscience and Biotechnology
Vol.3 No.2(2012), Article ID:18451,5 pages DOI:10.4236/abb.2012.32018
In vitro propagation of Aegle marmelos (L.) corr., a medicinal plant through axillary bud multiplication
![]()
1Department of Botany, BJB Junior College, Bhubaneswar, India
2Central University of Orissa, Koraput, India
Email: *puspapuhan@gmail.com
Received 11 January 2012; revised 18 February 2012; accepted 6 March 2012
Keywords: Aegle marmelos; Organogenesis; Rhizogenesis; Callus Culture; Plant Growth Regulators; Shoot Multiplication
ABSTRACT
A protocol for rapid in vitro propagation from nodal explants of the medicinal tree species Aegle marmelos (L.) corr. of family Rutaceae has been described. High frequency bud break were induced on Murashige and Skoog’s (1962) basal medium supplemented with 0.5 mg benzyladenine (BA)/l. After 10 days of culture, nodal explants with multiplied buds started callusing with restricted growth and defoliation. When the same nodal explants ware transferred into the same basal medium supplemented with 0.5 mg BA/l with different concentrations of either kinetin (KN) or gibberellic acid (GA3) or in combinations has shown healthy shoots with expanded shoot length. Excised shoots (2 cm - 3 cm long with 2 to 3 nodes) when grown on 1/2 MS basal medium with 2.5 mg/l indole-3-butyric acid (IBA) and 0.5% activated charcoal (A.C.)/l has shown rhizogenesis. After excision, in the second passage, the nodal explants also showed bud break when sub cultured on MS basal medium supplemented with 0.5 mg BA/l. These shoots also successfully rooted on the same above said medium.
1. INTRODUCTION
Aegle marmelos (L.) corr. (family-Rutaceae) is a forest cosmopolitan as well as an important medicinal plant. Different parts of A. marmelos have been investigated by several workers and found to contain coumarins, alkaloids, triterpenes, sterols and essential oils [1,2]. The essential oil has shown a broad spectrum of anti-bacterial and anti-fungal activities [3]. Bael has a wide therapeutic value in the treatment of diabetes, anaemia, fractures, healing of wound, swollen Joints, high blood pressure, Jundice, diarrhoea, troubles during pregnancy and typhoid. Pulp of the ripe fruit is taken during summer to keep the body and mind cool and to sharpen intellect and concentration of mind [4]. Roasted or dried pulp of ripe fruit is a purgative, astringent, digestive, tonic and stomachic. The leaves also help in controlling pollution by absorbing foul gases from the atmosphere and keep it clean and salubrious. Due to large-scale and unrestricted exploitation to meet increasing demands by the pharmaceutical industries coupled with limited cultivation and insufficient attempts for its reforestation, the stock of this important medicinal plant species has been markedly depleted. In recent years there has been an increased interest in in vitro culture techniques which offer a viable tool for mass multiplication and germplasm conservation of rare, endangered, aromatic and medicinal plants [5-8], as well as forest trees where the lifecycle takes a longer period [9,10].
In general, micropropagation through axillary shoot proliferation has proven to be a handy tool [11] An appreciable level of success has been achieved by many investigators in the case of many tropical medicinal plants through micropropagation as in case of Hemidesmus indicus ([12], Cleistanthus collinus [13], Gmelina arborea [14], Plumbago zeylanica [15] and Holarrhena antidysenterica [16]. Although there are techniques have been reported for in vitro propagation of Aegle marmelos from in vitro grown seedling parts, i.e. from roots [17], from hypocotyl [18], cotyledonary node [19] and excised leaf explants [20] but the works have more been concentrated on in vitro grown seedling explants. So in the present study trial has been taken to establish a protocol for regeneration and mass propagation of A. marmelos from mature tree explants i.e. from nodal explants through axillary bud multiplication.
2. MATERIALS AND METHODS
2.1. Explants Source and Surface Decontamination Methods
Young shoots from the crowns of mature tree (20 years old) growing in the B.J.B. College garden were collected.
Healthy nodal segments measuring 1.0 - 2.0 cm were cut, defoliated and with dormant axillary buds were used as explants. The nodal segments were initially rinsed thoroughly in running tap water for 14 minutes followed by immersing in a 5% (v/v) solution of detergent (Labolene, Qualigens, India) for 10 min. Then it was surface sterilized with 0.1% (w/v) mercuric chloride for (4 - 5 min) subjected to repeated washings in sterile distilled water. These surface-decontaminated explants were taken for inoculation.
2.2. Culture Medium and Shoot Multiplication
Murashige and Skoog’s (1962) basal medium [21] was used for culture. This medium was supplemented with various concentrations of only benzyladenine (BA) and Kinetin (KN) or in combinations with 2% (W/V) sucrose and gelled with 0.8% (W/V) agar (Bacteriological Grade, Himedia, Mumbai, India). Using 1N NaOH or 1N HCl, the pH of the medium adjusted to 5.8 before autoclaving. Routinely 15 ml each of molten medium was dispensed into sterile 25 × 150 mm culture tubes and tightly plugged with cotton. After that autoclaving was done at a temp of 121˚C and 15 Ib steam pressure for 15 min. The cultures were incubated at 25˚C ± 2˚C, 55% - 60% RH and 16-h photoperiod supplied by a bank of cool-white fluorescent tubes (PPF 45 mmol·m–2·s–1). After 2 weeks, the % of bud break, numbers of days required for bud break, number of shoots per explants and mean shoot length were measured. The culture tubes showing callusing and defoliation of leaves are also marked.
2.3. Elongation of Shoots
Nodal explants were transformed into the elongation medium (MS + different concentrations of BA, KN and GA3 either or in combinations) to restrict defoliation and callusing with healthy shoot growth. The cultures were sub cultured thrice at fortnightly intervals by transferring to fresh medium. The evaluations were made in terms of number of shoots per explants inducing healthy growth, shoot length after 4 and 6 weeks of culture and number of nodes per shoot.
2.4. Rooting
Shoots (2 cm or longer) excised and transferred into rooting medium. Rooting medium was MS (1962) basal medium supplemented with various concentrations of IBA, IAA with 0.5%, and 0.2% (W/V) activated charcoal. Rooting response also varied in terms of % of response, length and number of roots depending on the medium and source of material.
Autoclaving, incubation conditions were same for proliferation, differentiation and rooting medium as described above. For each treatment, 10 replicates were used and all the experiments were repeated three times.
All data were taken by Mean ± SE and significance of mean is tested by Duncan (1955) multiple range tests [22].
2.5. Hardening
After 4 weeks of culture the rooted plantlets were thoroughly washed with tap water and dipped in an antifungal solution 0.1% (w/v) Bavistin solution for 5 minutes, then washed and planted in plastic pots (5 cm diameter) containing autoclaved artificial soil (1 part vermiculite: 1 part perlite by v/v) and were supplied sterile distilled water for 4 weeks. Established plants were repotted in polythene bags (of the size 20 cm × 10 cm) filled with autoclaved sand, soil/red earth and farmyard manure (v:v:v) in the proportions of 1:1:1 and then to bigger earthen pots (25 cm diameter) containing a nonsterile sand: soil: compost mixture (1:3:1) and kept under shade (40% shade) in the garden for another 3weeks and watering was done as and when required. When the plants were established they were transferred to open light conditions in the botanical garden.
3. RESULTS AND DISCUSSION
The stem segments ware cultured on MS medium supplemented with different conc. of BA and KN. Multiple shoot buds appeared to arise directly from the node within 2 - 5 days of culture (Figure 1(a)). The frequency of bud producing explants increased with the increase of culture period and became 86.6 ± 3.33 at the end of 6th day of culture. The number of days taken for bud break is different with different growth regulators and it’s number increased with the increase in concentration of growth regulators used. The percentages of bud break, numbers of shoots and mean shoot length decreased with increase of concentrations of different growth regulators. Among different treatment combinations 0.5 mg BA/l was found to be the best for maximum bud break, number of shoots and mean shoot length (Table 1). Cultures of nodal explants with multiple shoots after 1 week and within the second week started callusing at the base of leaf and node and also started defoliation (Figure 1(b)). These primary cultures were then transferred into elongation/ induction medium for healthy growth of shoots by restricting callusing and defoliation. The induction medium was MS basal medium [21] supplemented with different concentrations of BA, KN and GA3 (Table 2).
The better response of explants in the medium containing benzyladenine with gibberellin and kinetin was shown because kinetin prevents senescence/defoliation by prevention of formation of hydrolases e.g. nucleases and proteases and causing immobilization of nutrients or their transport to cytokinin treated areas. Gibberellins

Table 1. Effect of plant growth regulators on axiliary bud multiplication of nodal explants of Aegle marmelos in terms of number of shoots, length of shoot and callusing.
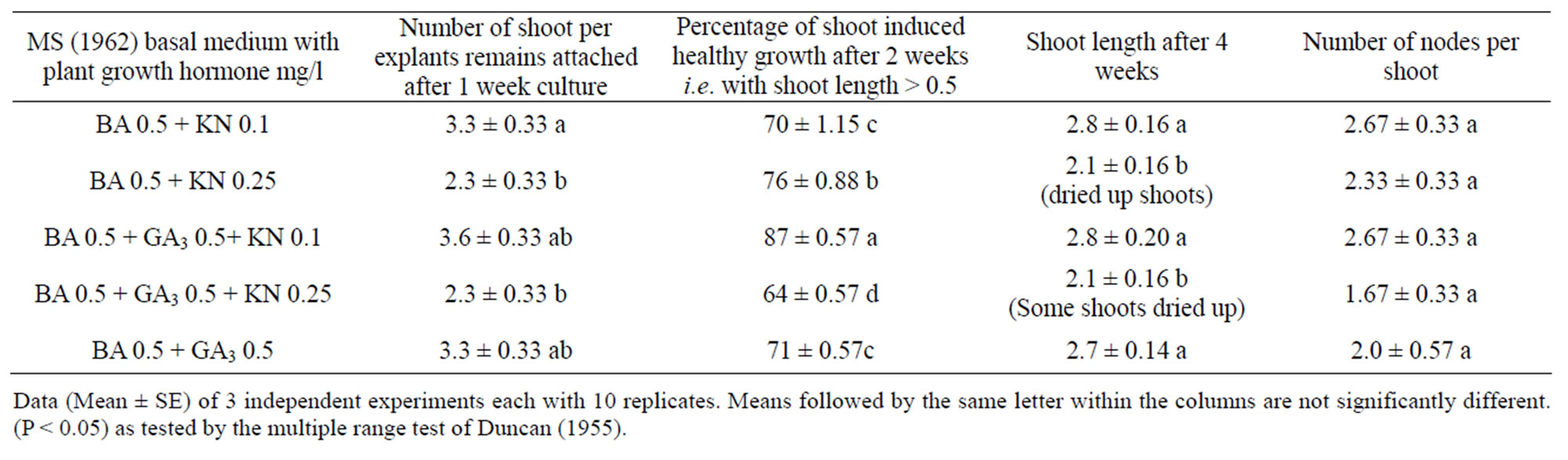
Table 2. Effect of different growth regulators for inducing healthy and elongated shoots growth by restricting callussing and defoliation of multiple axiliary bud of A. marmelos.
participate in cambium activity and differentiation of tissues by stimulating RNA, protein and DNA synthesis, which ultimately stimulates axillary bud elongation. But the application of higher concentration of GA3 and KN gave decreased response.
The stimulating effect of BA on bud break and multiple shoots formation has been reported earlier for several medicinal and aromatic plant species including Chlorophytum borivilianum [23], Withania somnifera [24], Litchi chinensis [25] and Anacardium occidentale [26]. Reduction in the number of shoots generated from each node at higher concentrations of BA than the optimum level was also reported for several medicinal plants [24].
It was found the induction medium with BA 0.5 mg/l + GA3 0.5 mg/l + KN 0.1 mg/l to be the most appropriate cocktail where maximum number of shoots per explants remains attached and out of this maximum percentage of shoots induced healthy growth with maximum shoot length, maximum number of number of nodes per shoot and expanded leaves (Figure 1(c)).
Excised shoots 2 cm or more were rooted on different strength of MS with different concentrations of IBA, IAA and activated charcoal. The percentage of rooting, root length is presented in Table 3.
Highest response was obtained from 1/2 MS + IBA 2.5 mg/l + 0.5% A.C. medium with maximum root length growth (Figure 1(d)). Different strength of MS basal medium with charcoal was used because explant on full strength MS medium without activated charcoal did not respond to the rhizogenesis. The shoots in the rooting medium devoid of charcoal dried up may be due to oxidation. Charcoal was used to avoid oxidation of polyphenols and reduce the light effect though it is a good absorbent of light (light enhances polyphenol oxidation which leads the blackening and death of explants). Previous investigators have also obtained comparable results on the effect of temp and light on rooting cv. Bridal pink [27,28] and lower concentration of MS salts in rooting [29].
Plantlet with six or seven fully expanded leaves and

Table 3. Rooting responses of Aegle marmelos in various concentration of IBA, IAA and activated charcoal.
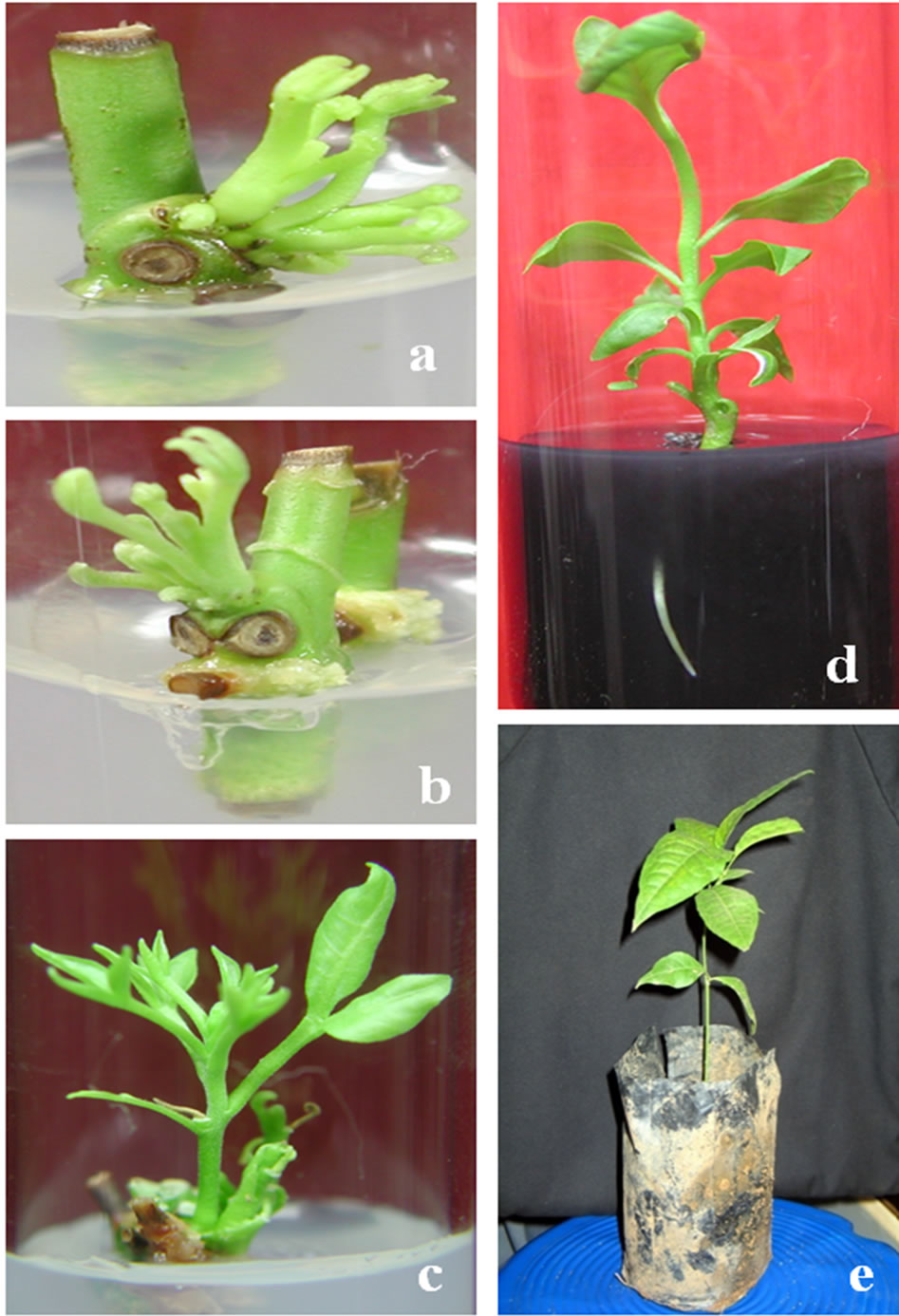
Figure 1. In vitro morphogenesis of nodal explants of Aegle marmelos and their derived plantlets. (a) Nodal explants showing bud break; (b) Nodal explants with multiple shoots starting callusing at the base of leaf and node within 1 week of culture; (c) Nodal explants with healthy shoot length growth in the induction medium; (d) Excised shoot showing well developed root and (e) Fully developed plantlets after 7weeks of hardening.
well developed roots were successfully hardened on vermiculite for 4 weeks and subsequently transferred to a mixture of sand:soil:farmyard manure (1:1:1) in poly bags (Figure 1(e)). Then these are transferred into bigger pots. The percentage of survival was 100%. All plants had normal leaf development and did not show any detectable differences in morphological or growth characteristics compared to the donor plants.
Trials for mass in vitro propagation of A. marmelos from a single node segments of a 20 years old tree which resulted 12.1 shoots of up to 5.2 cm length in 48% of the explants after 7 weeks of culture [30], 70% - 90% cuttings rooted and 85% of plants were established after hardening. In the present study 86.6% ± 3.33% response were obtained producing 10.0 ± 0.30 number of shoots out of which 3.6 ± 0.33 number of shoots gave 2.67 ± 0.33 cm shoot length after 2 week of primary culture. The rooted shoots gave 100% establishment after hardening and also in advantage, did not show any morphological differences to that of mother plant.
The nodal segments are the best explants as they could develop more multiple shoots [31]. The results obtained are highly reproducible, thus the method described is useful for clonal propagation of selected germplasm through axially bud multiplication.
4. ACKNOWLEDGEMENTS
The authors are grateful to the Director, Regional Research Laboratory, Bhubaneswar; Principal, B.J.B. College, Bhubaneswar and Head of the Department of Botany, Utkal University, Bhubaneswar for the Laboratory and Library facilities provided by them.
REFERENCES
- Karawya, M.S., Mirchom, Y.W. and Shehuata, I.A. (1980) Sterols, triterpenes, Coumarins and alkaloids of Aegle marmelos correa, cultivated in Egypt. Egyptian Journal of Pharmaceutical Sciences, 21, 239-248.
- Tokitomo, Y., Shimono, Y., Kobayashi, A. and Yamanishi, T. (1982) Aroma components of bael fruit (Aegle marmelos correa). Agricultural Biology and Chemistry, 46, 1873-1877. doi:10.1271/bbb1961.46.1873
- Pattnaik, S., Subramanyam, V.R. and Kale, C. (1996) Antibacterial and anti-fungal activity of ten essential oils in vitro. Microbios, 86, 237-246.
- Parichha, S. (2004) Bael (Aegle marmelos) nature’s most natural medicinal fruit. Orissa Review, 9, 16-17.
- Bera, T.K. and Roy, S.C. (1993) Micropropagation of Tylophora Indica (Burmn f.) Merr. by multiple bud formation from mature leaf explants without callus intervention. Botanical Bulletin of Academia Sinica, 34, 83-87.
- Sudha, G.C. and Seeni, S. (1994) In vitro multiplication and field establishment of Adhatoda beddomei CB clarke, a rare medicinal plants. Plant Cell Reports, 13, 203-207. doi:10.1007/BF00239893
- Sahoo, Y. and Chand, P.K. (1998) Micropropagation of Vitex negundo L., a woody aromatic medicinal shrub, through high frequency axillary shoot proliferation. Plant Cell Reports, 18, 301-307. doi:10.1007/s002990050576
- Wawrosch, C., Maskay, N. and Kopp, B. (1998) Micropropagation of the threatened nepalese medicinal plant Swertia chirwta Buch.-Ham-ex. wall. Plant Cell Reports, 18, 997-1001. doi:10.1007/s002990050697
- Ahuja, M.R. (1991) Biotechnology of forest trees. Plant Research and Development, 33, 106-120.
- Shankar, S. and Mohan Ram, H.Y. (1990) In vitro plantlet regeneration in the leguminous tree Sesbania sesban. Phytomorphology, 40, 43-52.
- Malathy, S. and Pai, J.S. (1998) Micropropagation of Ixora Singaporensiss (Linn.)—An ornamental shrub. Current Science, 75, 545-547.
- Patnaik, K. and Debata, B.K. (1996) Micropropagation of Hemidesmus Indicus (L.) R. BR: Through axillary bud culture. Plant Cell Reports, 15, 427-430. doi:10.1007/BF00232069
- Quraishi, A., Koche, V. and Mishra, S.K. (1996) In vitro micropropagation from nodal segments of Cleistanthus collinus. Plant Cell, Tissue and Organ Culture, 45, 87-91. doi:10.1007/BF00043433
- Thirunavoukkarasu, M. and Debata, B.K. (1998) Micro propagation of Glemelina arborea Roxb through axillary bud culture. Indian Journal of Plant Physiology, 3, 82-85.
- Selvakumar, V., Anbudurai, R.P. and Balkumar, T. (2001) In vitro propagation of the medicinal plant Plumbago zeylanica L. through nodal explants. In Vitro Cellular & Developmental Biology—Plant, 37, 280-284. doi:10.1007/s11627-001-0050-x
- Raha, S. and Roy, S.C. (2001) In vitro plant regeneration in Holarrhena antidysenterica wall, through high frequency axillary shoot proliferation. In Vitro Cellular & Developmental Biology—Plant, 37, 232-236. doi:10.1007/s11627-001-0041-y
- Bhati, R., Sekhwatt, N.S. and Arya, H.C. (1992) In vitro regeneration of Plant lets from root segments of Aegle marmelos. Indian Journal of Experimental Biology, 30, 844-845.
- Hossain, M., Islam, R., Islam, A. and Joarder, O.I. (1995) Direct organogenesis in cultured hypocotyl explants of Aegle marmelos corr. Plant Tissue Culture, 5, 21-25.
- Arumugam, A. and Rao, M.V. (1996) In vitro production of plantlets from cotyledonary node cultures of Aegle marmelos L. Corr. Advances in Plant Sciences, 9, 181- 186.
- Islam, R., Hossain, M., Joarder, O.I. and Karim, M.R. (1993) Adventitious shoot formation on excised leaf explants of in vitro grown seedlings of Aegle marmelos. Corr. Journal of Horticultural Science, 68, 495-498.
- Murashige, T. and Skoog, F. (1962). A revised medium for rapid growth and bioassays with tobacco tissue culture. Plant Physiology, 15, 473-497. doi:10.1111/j.1399-3054.1962.tb08052.x
- Duncan, D.B. (1955) Multiple range and multiple F test. Biometrics, 11, 1-42. doi:10.2307/3001478
- Purohit, S.D., Dave, A. and Kukda, G. (1995) Micropropagation of safed musli (Chlorophytum borivilianum), a rare medicinal herb. Plant Cell, Tissue and Organ Culture, 39, 93-96. doi:10.1007/BF00037596
- Sen, J. and Sharma, A.K. (1991) Micropropagation of Withania somnifera from germinating seeds and shoot tips. Plant Cell, Tissue and Organ Culture, 26, 71-73. doi:10.1007/BF00036108
- Das, D.K., Shiva Prakash, N. and Bhalla-Sarin, N. (1999) Multiple shoot induction and plant regeneration in litchi (Litchi chinensis Sonn.). Plant Cell Reports, 18, 691-695. doi:10.1007/s002990050644
- Bogetti, B., Jasik, J. and Mantell, S. (1999) In vitro multiplication of cashew (Anacardium occidentale L.) using shoot node explants of glasshouse-raised plants. Plant Cell Reports, 18, 456-461. doi:10.1007/s002990050603
- Khosh-Khui, M. and Sink, K.C. (1982) Callus induction and culture of Rosa. Scientia Horticulturae, 17, 361-370. doi:10.1016/0304-4238(82)90117-0
- Khosh-Khui, M. and Sink, K.C. (1982) Rooting enhancement of Rosa hybrida for tissue culture propagation. Scientia Horticulturae, 17, 371-376. doi:10.1016/0304-4238(82)90118-2
- Rout, G.R., Debata, B.K. and Das, P. (1990) In vitro Clonal multiplication of roses. Proceedings of the National Academy of Sciences (India), 60, 311-318.
- Ajithkumar, D. and Seeni, S. (1998) Rapid clonal multiplication through in vitro axillary shoot proliferation of Aegle marmelos (L.) Corr., a medicinal tree. Plant Cell Reports, 17, 422-426.
- Len, L.C. and Kong, L.S. (1985) Micropropagation of Lagestroemia speciosa (L.) Pers (lythraccae). Gardens’ Bull, 38, 175-184. doi:10.1007/s002990050418
NOTES
*Corresponding author.

