Journal of Environmental Protection
Vol.08 No.10(2017), Article ID:79124,12 pages
10.4236/jep.2017.810068
Residual Effects from Occupational Mercury Exposure Include a Proposed Mercury Tremor Biomarker or “Fingerprint”
Linda Jones
School of Psychology, Massey University, Wellington, New Zealand

Copyright © 2017 by author and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY 4.0).
http://creativecommons.org/licenses/by/4.0/



Received: August 16, 2017; Accepted: September 15, 2017; Published: September 18, 2017
ABSTRACT
The study investigated residual effects of high levels of occupational mercury exposure, 30 years after a cohort of women worked in public service dentistry. They had all used copper amalgam in a pellet form that required heating and handling, and silver amalgam before the encapsulated form was available. Mercury handling practices changed in the mid-1970 when the workforce was urine tested and mercury poisoning became apparent. The aim was to compare control group and exposed group scores on tasks from a neurobehavioural test battery; plus survey results from a composite health, work history and environmental influences survey. The findings showed that the exposed and control groups were equivalent not only on those variables that one would want to be matched (age, alcohol consumption), but also on many of the cognitive and psychomotor test scores. The present paper focuses on psychomotor skill and tremor patterns. Tremor patterns were seen as generating new evidence of long term effects of the historic mercury insult. Data also suggest that there may be a distinctive mercury “fingerprint”, in samples of sinusoidal waveforms that may have potential as a non-invasive sub-clinical biomarker for adverse effects of mercury exposure, in screening or workplace monitoring.
Keywords:
Mercury, Tremor, Dentistry, Women, Psychomotor Tests

1. Introduction
In New Zealand in the 1960s and 1970s every primary school had a state funded dental clinic, staffed by women who were specifically trained in a limited range of conservative dentistry techniques for treating children. The filling materials were copper amalgam for deciduous teeth, and silver amalgam for permanent teeth. By the mid-1970s it was found that 10% of the staff had urine-mercury levels over 50 ug/l [1] [2] . Many had been medically diagnosed with mood disorders that were incorrectly attributed the notion that women were emotionally labile, rather than suffering from mercury poisoning1. Copper amalgam was withdrawn from use in 1975 and although the use of silver amalgam has persisted, an encapsulated form was introduced with mechanical mixing. This has created a natural experimental design for investigating three little-reported aspects of occupational mercury safety: possible long term effects, effects on woman, and studies of metals including the heavy metal, mercury, in dental materials generally.
One historical difficulty for a retrospective study is that although a total working population sample of 1300 women were urine tested in one year, few were given their results. There was no treatment for women with elevated results, only enforced time off work. The government agency responsible for urine-testing was not required to produce a report, and only minimal information on the episode can be found in Department of Health archived records. Hence it is not possible to produce a model that quantifies individual mercury exposure and residual effects. However a model of exposure can be constructed from four other variables: a description of mercury use in the New Zealand School Dental Service at the time; the volume of amalgam use from filling placement data in Department of Health’s annual reports; a description of the clinic environment and gazetted instructions to the women about mercury hygiene; and finally, survey responses on the women’s recall of working with mercury.
Description of Mercury use. Copper amalgam, 70% mercury to 30% copper, was supplied in pellet form. Pellets required heating over a flame to soften the copper and release the mercury so the amalgam could be mixed (see Figure 1).
Figure 1. Heated copper amalgam pellets with mercury released, ready for trituration using a glass mortar and pestle.
The mercury vapor levels in the ambient air peaked at these times. Frykholm [3] reported on experimental testing of air levels in such a situation, and suggested it was possible to reach 3000 µg Hg/m3 in air around the burner. He suggested that copper amalgam could only safely be used with a ventilation and exhaust system. In the New Zealand scenario, the burner was close to both the operator and patient. There was no assisted ventilation. After mixing to reincorporate the copper and mercury, the amalgam was scooped up by hand using a cotton “squeeze cloth” and excess mercury was wrung out over the mortar. Droplet spills were common.
Silver amalgam was supplied in the separate ingredients of silver alloy powder and liquid mercury. These were weighed on a small balance scale in the ratio of five parts silver six parts mercury, with the mercury poured from a wooden “dropper” bottle. Frykholm’s experiments described this situation also, reporting 400 µg Hg/m3 in the air above the silver amalgam mix [3] . As with the copper mix, the powdered silver and room temperature mercury were mixed to a paste in the mortar and pestle, and again when the desired consistency was reached, excess mercury was wrung out by hand. Waste amalgam and mercury from the mortar were placed in water filled and lidded glass jars for future collection and recycling.
Peak mercury in ambient air level as measured by Frykholm [3] shows there was a constant and continual risk of mercury poisoning for the women, when compared with the generally accepted international value is 25 µg/m3 [4] or the German MAK2 value of 10 µg/m3 [5] .
Volume of amalgam use. There were just over 1300 women, mostly in full time positions, staffing school dental clinics in the years 1974 to 1976. That each prepared on average 10 amalgam fillings per day can be extrapolated from data in the official Department of Health annual report to government (Table 1). This showed that collectively the women performed more than 2 million fillings during the 44 weeks per year when clinics were open. This would require hand contact with mercury on at least 10 occasions per day, and a constant high level of ambient air mercury.
Dental clinic environments. School dental clinics were built to a standard design with one large window to let in light, but it did not open. Smaller side-wall windows could be. Variation and fluctuations in New Zealand’s seasonal
Table 1. Filling Output, by Staff and Year.
Source: AJHR, 1975, 1976.
temperatures meant that the departmental instruction for routine mercury risk prevention―to keep windows open―was impossible to follow [6] . Women reported that in cold weather the clinic windows were kept closed and heaters turned on. There was no air conditioning or exhaust ventilation system. Women were not trained to wear, nor supplied with, protective clothing such as gloves or masks; and were sufficiently uninformed about mercury toxicity that they gave spilled droplets of mercury to their child patients in empty burr boxes as a reward for cooperative behavior [7] .
The high levels and frequent peaks of mercury in air, the manual measurement of liquid mercury for silver fillings, the naked-hand expression of excess mercury from amalgam mixes and clean-up of accidental spills, the absence of any protective clothing, and the sheer volume of work, demonstrates that even the most conscientious woman would be at risk of mercury poisoning in this occupational environment.
The neurobehavioural effects of mercury exposure are well documented for people who have chronic or continued exposure. These include psychosomatic illness experience; and deficits or changes in affect, cognitive skills, psychomotor skills and sensory function. Meta-analysis has established exposure and test performance relationships for different domains with common neurobehavioural tests [5] . Studies specifically in dentistry have found adverse neurobehavioral effects from chronic low-level mercury exposure [8] [9] [10] [11] [12] . From these there is no clear evidence from which to predict the long-term health outcomes for dentists who may at one time have experienced neurobehavioural changes from occupational mercury exposure.
There are few longitudinal studies of mercury exposed workers, and in those studies findings are not consistent. [13] [14] [15] [16] . Ratcliffe et al. critically assessed the methodology and analysis of 164 mercury exposure studies and concluded that “the evidence for association between neurologic effects and inorganic mercury is irrefutable” p. 238, [17] , yet some uncertainty remains about long term effects once exposure ceases. Enduring health and psychomotor function changes are seen in some chloralkali factory workers, but the possibility remains that other deficits are reversible once exposure ceases [18] .
In addition, women have been neglected in neurobehavioural studies of occupational mercury exposure. There are three reports specifically of women and occupational mercury exposure and a limited number of studies that include gender more broadly. In total, at the time data were collected for the present study, there were neurobehavioural data on fewer than 30 women [11] [19] [20] .
The aim of the present paper is to report on psychomotor assessment, including tremor analysis, from a neurobehavioural test battery, with women who were occupationally exposed to mercury as young women, at least 30 years prior to their assessment, and matched controls with no history of occupational exposure to heavy metals (Findings from other tests are reported elsewhere [21] ). Further, tremor analysis is explored as a potential non-invasive, early warning technique for people currently occupationally exposed to mercury in any form.
2. Materials and Method
Participants were approached by mail with a request to volunteer with a matched control. The mercury exposed group was 43 women ( 2.2 years, SD 1.2). Inclusion criteria included graduation between 1968 and 1971 with a minimum of three year’s work experience with the New Zealand school dental service. The response rate was 40.2%. The control group were 13 sisters and 19 women friends (total 32) of the mercury-exposed group ( 51.4 years, SD 4.5). The power analysis formula was that recommended in the Adult Environmental Neurobehavioral Test Battery [22] to detect a 20% between groups difference across the psychomotor tests.
Alcohol intake and tobacco smoking were measured on a 5-point self-report scale, from 0 = none or not at all to 4 = heavily or very frequently. Alcohol intake was generally low, and few participants from either group smoked: alcohol-exposed group ( 1.76, SD 0.9); control group ( 1.78, SD 0.75); tobacco smoking-exposed group ( 0.32, SD 0.82); control group ( 0.83, SD 0.87). Most women reported they were in very good or excellent health in a 7-point scale, see Idler & Kasl [23] : exposed group ( 6.05, SD 0.83); control group ( 6.02, SD 0.81.7).
The screening survey included questions on injury that might confound the psychomotor test results, other environmental influences on health, and work history. For the exposed group there were additional questions on mercury hygiene practices and recall of mercury spill accidents.
The 2-hour neurobehavioural test battery was designed to be culturally appropriate for a cohort of New Zealand mid-life women. It contained a dual purpose memory and malingering test. Psychomotor tests included a computerised simple reaction time test, the O’Connor Tweezer Dexterity Test, the Grooved Pegboard, the Jamar dynamometer for grip strength, and the Tremorometer® for finger tremor.
3. Procedure
The assessments were conducted in English, in most case, in participants’ homes. All the standardised tests had developers’ instructions and these were followed.
For finger tremor the accelerator sensor was taped to the first finger for trials in three positions for each hand. Each trial lasted 21 seconds with 100 recordings per second. The Tremorometer measured frequency, amplitude and intensity of tremor on three-axes, and was supported by a computer software package to provide summary statistical data and Fast Fourier Transformed (FFT) power spectra. The hand positions tested related to tremors defined by Findley and Koller, p. 123 [24] . The first trial was for “postural tremor”, a tremor that occurs when maintaining an action against gravity. The participant extended an arm in front, at shoulder height. The second trial was for “rest tremor”, a tremor that occurs with the hand inactive, and in a hanging-relaxed position. The third trial was for “action tremor”, and was measured as the voluntary action of the hand against gravity and holding a light load. In this case the participant repeated the posture position with an added 135 g weight.
Psychomotor data were analysed for between group differences using Statistical Package for Social Sciences (SPSS) v10, with Bonferroni adjustment to significance levels for multiple testing of the same participants. Tremor data were analysed with the TremorLab® analytical software package.
The project was assessed and approved by the Massey University Human Ethics Committee (MUHEC), protocol number PN 01/125.
4. Results
Participants were assigned to four levels of mercury exposure based on reported work history, with all controls in the no exposure level, and exposed women bands of up to five years exposure, five to10 years of exposure and more than 10 years. One participant reported traumatic brain injury and was excluded from analysis. Occupational overuse syndrome (OOS) was reported by 32.5% of exposed group and 06.7% of the control group. All participants from the exposed group reporting OOS had worked in dentistry more than 5 years. This was a significant between groups difference (c2 = 6.80, (1, N = 70), p < .01). There was no evidence of malingering.
Simple reaction time For each participant, from 60 trials, reaction times in milliseconds were rank ordered and the slowest 10 and fastest 10 trials were excluded to minimise the effects of inattention and anticipation respectively. Comparing the central 40 trials by mean scores there was no significant difference between the exposed group ( = 292.78, SD = 32.43) and the control group ( = 292.14, SD = 29.05), t = 0.8, p = 0.93 (two tailed), df = 67.
O’Connor Tweezer Dexterity Test. Time in minutes and seconds for placement of 100 pins was converted to minutes and decimal fractions of a minute (min), and contributed to the group data. Levene’s test for equal variances showed that data for exposed and control groups did have significantly different variance (F = 8.15, p = .006) with the control group scores negatively skewed. With the assumption of unequal variances there was no significant difference between the exposed group ( = 6.24 min, SD = 0.86 min) and the control group ( = 6.66 min, SD = 1.36 min), t = 1.46, p = .15 (two tailed), df = 46.55.
Grip strength For each participant, raw scores from three trials with each hand were averaged and scores recorded for dominant and non-dominant hands in kilograms force. Comparing the mean scores for the dominant hand, there was no significant difference between the exposed group ( = 28.96, SD = 6.79) and the control group ( = 28.88, SD = 4.24), t = 0.06, p = 0.95, (two tailed), df = 71. Comparing the mean scores for the non-dominant hand, there was no significant difference between the exposed group ( = 27.89, SD = 5.25) and the control group ( = 27.77, SD = 4.85), t = 0.10, p = .92, (two tailed), df = 71.
Grooved Pegboard Scores for the Grooved Pegboard were recorded in seconds and centi-seconds for time taken to place correctly all 25 pegs in the board. Comparing the mean scores, there was no significant difference between the exposed group ( = 69.01, SD = 9.26) and the control group ( = 68.28, SD = 10.75), t = 0.31, p = 0.76, (two tailed), df = 70.
Pearson’s r test was used to correlate the mean scores on the Grooved Pegboard with the O’Connor Tweezer Dexterity Test. This showed that for the exposed group, the scores had a moderate positive correlation, r = +0.50, while the control group had a weaker positive correlation, r = +0.32. The difference was not significant.
Tremor Finger tremor patterns showed a significant, unilateral, negative correlation between duration of exposure and tremor mean frequency. All these data were collected with the one meter. Correlations between duration of exposure and three hand positions were: “right rest” r = −0.68, p = 0.03; “right posture” r = −0.78, p = 0.003; “right load” r = −0.74, p = 0.002. Duration correlations used exposed group data only as the control group had no exposure. There were no significant correlations between tremor frequency and duration of exposure in the left (non-dominant) hand. A second instrument was used during subsequent data collection but there was no ability to calibrate the two meters so this is not reported or discussed beyond Table 2.
With the final data set, a GLM ANOVA was conducted to examine the effect on tremor frequency, measured in Hz, from several independent variables: meter (two levels), position (three levels), hand (2 levels), and mercury exposure (four levels). None of the IVs are repeated measures as there was too much missing data from meter 2 to impute the values necessary for a mixed measure ANOVA. There was a significant main effect found for meter and for position but no significant interactions (see Table 2). All participants were right-hand dominant.
Table 2. Between-Groups ANOVA: mean tremor frequencies (Hz).
A separate analysis with repeated measures for hand and for position was conducted on data from meter 1. There was a main effect for position and hand, with the right hand showing a significant decrease in frequency between the mean posture and rest positions, 8.8 Hz and 8.7 Hz respectively, and the load position mean of 7.4 Hz.
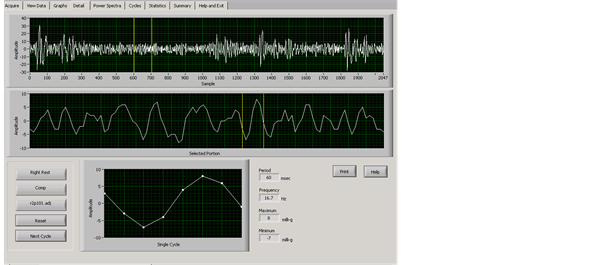
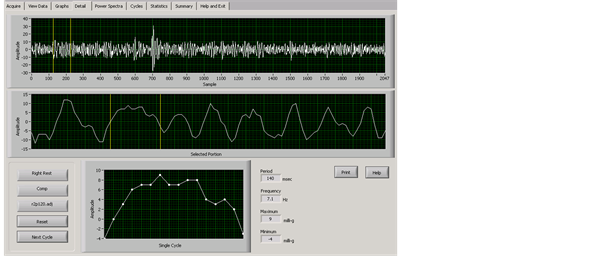
An in-depth analysis of the sinusoidal waveforms was conducted. The TremorLab provided graphs of the 20 second tremor samples by three axes, as a composite recording, and these could be broken into one second blocks and single cycles, as well as frequency and power spectra displays. From the visual data display, with exposed group data only, there was an apparent break or stall in the waveforms. Figure 2 and Figure 3 show tremor samples from the dominant
Figure 2. Control participant’s 20 second composite tremor sample, right hand, rest position; with a one-second area selected for magnification (yellow lines), and a single “normal” cycle.
Figure 3. Exposed participant’s 20 second composite tremor sample, right hand, rest position; with a one-second area selected for magnification (yellow lines), and a single cycle illustrating a “stalled” waveform.
hand in a rest position. Figure 2 is a control participant, and Figure 3 the mercury exposed participant. In both figures the top line shows the 20 second sample with yellow lines highlighting a one-second section selected to illustrate the differences; a middle line showing the highlighted one-second section displayed in detail, and beneath that a single cycle. The one-second display and one single cycle show a normal waveform in the control participant, and a stalled cycle in the mercury exposed participant
5. Discussion
Throughout the mercury poisoning literature, reports of damage to the peripheral nervous system are common, and the specific psychomotor tasks of grip strength, the Grooved Pegboard and simple reaction time were included for this reason. These assessment tools are well established for mercury exposure use [24] [25] . However no residual psychomotor skill differences were shown between the exposed group and controls, although a within group test of the exposed group reported significantly greater experience of OOS the longer the exposure period. While mercury may be implicated, other factors may be responsible such as the effects of years of holding small vibrating devices [26] .
The inclusion of the O’Connor Tweezer Dexterity test was related to the exposed group’s practice of dentistry and added an additional challenge to the skill required for the Grooved Pegboard. The exposed group had used tweezers for much of their detailed daily work, and some residual transfer of training may have occurred. Finger dexterity, assessed with the “pins-mins” test, differentiated mercury exposed and controls with practicing dentists [27] [28] [29] , but even with the finer hand-eye coordination required with tweezers, no differences were found in the present study. This supports the assertion that there were no residual hand-eye coordination difficulties for the exposed group. Data were collected in 2003 and initially the no-difference finding meant the psychomotor results were not reported in other publications of the study; but the tremor analysis continued.
Tremor is the single most cited symptom of mercury poisoning yet there has been very little standardisation of visual analytical techniques or equipment. A Danish device that incorporated a two-axes accelerometer in the tip of a pen-like instrument had gained acceptance at the time of data collection, [30] but the Tremorometer [31] , while largely untested, offered a three axis accelerometer, so was preferred . However differences in the two meters used, accounted for 16% of variance in tremor. Not having a single meter or being able to use them both with the same participant meant reliability was not assured, and this is a limitation of the study.
What the Tremorometer could do was show a frequency window for mercury-tremor, and tremor characteristics; and through the analytic software, had the potential for differential diagnosis of mercury tremor from essential tremor and Parkinsonism tremor, as described by others [32] [33] [34] when using an axis by axis, cycle by cycle view of a sample of tremor (see Figure 2 and Figure 3). The three axis recordings could also be separated and compared with the power spectra images but at this point in the analysis there was nothing unexpected. However throughout the exposed group there was an apparent sinusoidal wave stall that, if investigated further, may be a mercury biomarker or “fingerprint”. It is in this that the main contribution from the present study may be made. Since tremor testing is non-invasive and brief, the identification of such a fingerprint could be useful in monitoring people currently occupationally exposed to mercury for changes in individual tremor response at a sub-clinical level, as well as in group studies. The author invites comment on this point, particularly from researchers investigating other heavy metals.
6. Conclusion
In a 30-year follow-up study of mid-life women occupationally exposed to mercury, there was little evidence to show they had been compromised in psychomotor skill by their earlier exposure. The exception was in the self-reporting of OOS, which was strongly and positively correlated with length of time working with mercury. Sub-clinical dominant hand tremor was found in the exposed group women, but although mercury and tremor are known to be associated, in this sample, there were other factors that could not be ruled out. However from the tremor data the possibility of a unique mercury “fingerprint” was noted, and if this could be corroborated by other researchers, may prove highly valuable as a screening or monitoring tool in occupational health and safety where mercury is concerned.
Cite this paper
Jones, L. (2017) Residual Effects from Occupational Mercury Exposure Include a Proposed Mercury Tremor Biomarker or “Fingerprint”. Journal of Environmental Protection, 8, 1075-1086. https://doi.org/10.4236/jep.2017.810068
References
- 1. Appendices to the Journal of the House of Representatives (New Zealand) (1975) 33-34, 88-89.
- 2. Appendices to the Journal of the House of Representatives (New Zealand) (1976) 31, 40, 90.
- 3. Frykholm, K. (1957) Mercury from Dental Amalgam: Its Toxic and Allergic Effects. Acta Odontologia Scandinavica, 15, 1-108.
- 4. World Health Organisation, International Program on Chemical Safety. http://www.who.int/ipcs/en/ Accessed 08/14/2017
- 5. Meyer-Baron, M., Schaeper, M. and Seeber, A. (2002) A Meta-Analysis for Neurobehavioural Results due to Occupational Mercury Exposure. Archives of Toxicology, 76, 127-136. https://doi.org/10.1007/s00204-002-0327-9
- 6. Short, A. (1974) Use of Mercury in Restorative Dentistry (Editorial Notes). New Zealand School Dental Service Gazette, 6, 63-64.
- 7. Jones, L. (2009) We Gave the Good Patients Mercury in Burr Boxes. A View of Mercury from Its Earlier Use in the School Dental Service to Contemporary Dental Practice. New Zealand Dental Journal, 105, 47-50.
- 8. Echeverria, D., Heyer, N., Martin, M., Naleway, C., Woods, J. and Bittner, A. (1995) Behavioural Effects of Low-Level Exposure to Hgo among Dentists. Neurotoxicology and Teratology, 17, 161-168. https://doi.org/10.1016/0892-0362(94)00049-J
- 9. Ngim, C., Foo, S., Boey, K. and Jeyaratnam, J. (1992) Chronic Neurobehavioural Effects of Elemental Mercury in Dentists. British Journal of Industrial Medicine, 49, 782-790.
- 10. Shapiro, I., Cornblath, D., Sumner, A., Uzzell, B., Spitz, L., Ship, I. and Bloch, P. (1982) Neurophysiological and Neuropsychological Function in Mercury-Exposed Dentists. The Lancet, 319, 1147-1150. https://doi.org/10.1016/S0140-6736(82)92226-7
- 11. Uzzell, B. and Oler, J. (1986) Chronic Low-Level Mercury Exposure and Neuropsychological Functioning. Journal of Clinical and Experimental Neuropsychology, 8, 581-593.
- 12. Iyer, K., Goodgold, J., Eberstein, A. and Berg, P. (1976) Mercury Poisoning in a Dentist. Archives of Neurology, 33, 788-790. https://doi.org/10.1001/archneur.1976.00500110056011
- 13. Albers, J., Kallenbach, L., Fine, L., Langolf, G., Wolfe, R., Donofrio, P., et al. (1988) Neurological Abnormalities Associated with Remote Occupational Elemental Mercury Exposure. Annals of Neurology, 24, 651-659. https://doi.org/10.1002/ana.410240510
- 14. Letz, R., Gerr, F., Cragle, D., Green, R., Watkins, J. and Fidler, A. (2000) Residual Neurologic Deficits 30 Years after Occupational Exposure to Elemental Mercury. Neurotoxicology. 21, 459-474.
- 15. Frumkin, H., Letz, R., Williams, P., Gerr, F., Pierce, M., Sanders, A., et al. (2001) Health Effects of Long-Term Mercury Exposure among Chloralkali Plant Workers. American Journal of Industrial Medicine, 39, 1-18. https://doi.org/10.1002/1097-0274(200101)39:1<1::AID-AJIM1>3.0.CO;2-N
- 16. Powell, T. (2000) Chronic Neurobehavioural Effects of Mercury Poisoning on a Group of Zulu Chemical Workers. Brain Injury, 14, 797-814. https://doi.org/10.1080/026990500421912
- 17. Ratcliffe, H., Swanson, G. and Fischer, L. (1996) Human Exposure to Mercury: A Critical Assessment of the Evidence of Adverse Health Effects. Journal of Toxicology & Environmental Health, 49, 221-270. https://doi.org/10.1080/00984108.1996.11667600
- 18. Cavalleri, A. and Gobba, F. (1998) Reversible Colour Vision Loss in Occupational Exposure to Metallic Mercury. Environmental Research, 77, 173-177. https://doi.org/10.1006/enrs.1997.3814
- 19. Triebig, G. and Schaller, K. (1982) Neurotoxic Effects in Mercury-Exposed Workers. Neurobehavioral Toxicology & Teratology, 4, 717-720.
- 20. Wood, R., Weiss, A. and Weiss, B. (1973) Hand Tremor Induced by Industrial Exposure to Inorganic Mercury. Archives of Environmental Health, 26, 249-252. https://doi.org/10.1080/00039896.1973.10666268
- 21. Jones, L., Bunnell, J. and Stillman, J. (2007) A 30-Year Follow-Up Study of Women’s Occupational Mercury Exposure: An Analysis of Residual Effects. Human and Experimental Toxicology, 26, 367-374. https://doi.org/10.1177/0960327107076824
- 22. Ambler, R., Anger, K. and Sizemore, O. (1995) Adult Environmental Neurobehavioural Test Battery. Department of Health and Human Services, Agency for Toxic Substances and Disease Registry, Atlanta.
- 23. Idler, E. and Kasl, S. (1991) Health Perceptions and Survival: Do Global Predictions of Health Status Really Predict Mortality? Journal of Gerontology, 46, 55-65. https://doi.org/10.1093/geronj/46.2.S55
- 24. Findley, L. (1996) Classification of Tremors. Journal of Clinical Neurophysiology, 13, 122-132. https://doi.org/10.1097/00004691-199603000-00003
- 25. Hartman, D. (1995) Neuropsychological Toxicology: Identification and Assessment of Human Neurotoxic Syndromes. 2nd Edition, Plenum Press, New York. https://doi.org/10.1007/978-1-4615-1849-5
- 26. Heaver, C., Goonetilleke, K., Ferguson, H. and Shiralkar, S. (2011) Hand-Arm Vibration Syndrome: A Common Occupational Hazard in Industrialized Countries. Journal of Hand Surgery (Eur), 36, 354-363. https://doi.org/10.1177/1753193410396636
- 27. Bittner, A., Echeverria, D., Woods, J., Aposhian, H., Naleway, C., Martin, M., et al. (1998) Behavioral Effects of Low-Level Exposure to Hg0 among Dental Professionals: A Cross-Study Evaluation of Psychomotor Effects. Neurotoxicology & Teratology, 20, 429-439. https://doi.org/10.1016/S0892-0362(98)00006-3
- 28. Echeverria, D., Aposhian, H., Woods, J., Heyer, N., Aposhian, M., Bittner, A., et al. (1998) Neurobehavioural Effects from Exposure to Dental Amalgam Hgo: New Distinctions between Recent Exposure and Hg Body Burden. FASEB Journal, 12, 971-980.
- 29. Gonzalez-Ramirez, D., Maiorino, R., Zuniga-Charles, M., Xu, Z., Hurlbut, K., Junco-Munoz, P., et al. (1995) Sodium 2,3-Dimercaptopropane-1-sulfonate Challenge Test for Mercury in Humans: Urinary Mercury, Porphyrins and Neurobehavioral Changes of Dental Workers in Monterrey, Mexico. Journal of Pharmacology & Experimental Therapeutics, 272, 264-274.
- 30. Galinski, T., Rosa, R. and Wheeler, D. (1990) Assessing Muscular Fatigue with a Portable Tremor Measurement System Suitable for Field Use. Behavioral Research Methods, Instruments, and Computers, 22, 507-516. https://doi.org/10.3758/BF03204434
- 31. Tripp, R. (2002) Tremerometer User Manual. FlexAble Systems Inc., Fountain Hills, AZ.
- 32. Beuter, A and de Geoffrey, A. (1996) Can Tremor Be Used to Measure the Effects of Chronic Mercury Exposure in Human Subjects? Neurotoxicology, 17, 213-228.
- 33. McCullough, J., Dick, R. and Rutchik, J. (2001) Chronic Mercury Exposure Examined with a Computer-Based Tremor System. Journal of Occupational & Environmental Medicine, 43, 295-300. https://doi.org/10.1097/00043764-200103000-00022
- 34. Biernat, H., Ellias, S., Wermuth, L., Cleary, D., de Oliveira Santos, E., Jorgensen, P., et al. (1999) Tremor Frequency Patterns in Mercury Vapor Exposure, Compared with Early Parkinson’s Disease and Essential Tremor. Neurotoxicology, 20, 945-952.
NOTES
1Newspaper quote from Dr B Glass, occupational physician who tested the women.
2Maximale Arbeitsplatz-Konzentration or maximum concentration in air.