Journal of Environmental Protection
Vol.08 No.04(2017), Article ID:75672,10 pages
10.4236/jep.2017.84030
Adsorption of Naphthalene on Modified Zeolite from Aqueous Solution
Ning Li1,2, Wang-Yu Cheng1,2, Yong-Zhang Pan1,2*
1School of Environment, Jinan University, Guangzhou, China
2Key Laboratory of Water/Soil Toxic Pollutants Control and Bioremediation of Guangdong Higher Education Institutes, Jinan University, Guangzhou, China

Copyright © 2017 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY 4.0).
http://creativecommons.org/licenses/by/4.0/



Received: March 14, 2017; Accepted: April 23, 2017; Published: April 26, 2017
ABSTRACT
In this study, the modified zeolite with certain hydrophobicity was prepared by modifying natural zeolite by the silane coupling agents such as vinyltrimethoxysilane (VTMO), trimethoxysilane (TMS) and trimethylchlorosilane (TMCS). The modified zeolite has a higher hydrophobicity and adsorption capacity than natural zeolite while ensuring the advantages of natural zeolite, which is more conducive to the adsorption of organic pollutants from aqueous solution. The adsorption experiment on naphthalene in aqueous solution shows that the modified zeolite has a stronger adsorption capacity. In the adsorption thermodynamics experiment, the isothermal adsorption models such as Freundlich and Langmuir can better describe the adsorption of naphthalene on modified zeolite, but the isothermal adsorption model Freundlich has a higher correlation. At 303K, the static adsorption capacity is 339μg/g. The kinetic analysis shows that the adsorption of naphthalene on modified zeolite conforms to the quasi-second order kinetic model.
Keywords:
Silane Coupling Agent, Modified Zeolite, Naphthalene Adsorption

1. Introduction
Polycyclic aromatic hydrocarbons (PAHs) belong to a kind of typical persistent organic pollutants in the environment because of its strong teratogenic, carcinogenic and mutagenic effects [1] , and once they enter the environment and ecology, they may be spread to the city’s domestic water supply system through natural circulation so that the residents’ physical safety may be harmed [2] , therefore, it is greatly significant for pollution prevention in the water environment in China to effectively reduce the content of PAHs in wastewater. The solubility of PAHs in water decreases with the increase of molecular weight, and relative to PAHs with heavier molecular weight, PAHs with lighter molecular weight is more common in the environment. Naphthalene molecule, containing two benzene rings, is a kind of white solid with pungent odor, which is widely used in the preparation of fuel, solvent, resin, etc. as raw material in the industrial field, and relative to other types of PAHs, naphthalene is more easily found in the environment, therefore a certain method and ideas can be provided for naphthalene removal in the environment if the naphthalene is taken as the target of the study.
At present, the common methods for removing PAHs in aqueous solution at home and abroad include biodegradation, advanced oxidation and adsorption [3] , among which the adsorption method, with the advantages of simple operation, high efficiency and low cost, has been one removal method [4] of polycyclic aromatic hydrocarbons in aqueous solution concerned by researchers recently. Natural zeolites are aluminosilicate minerals; it has rich reserve and low price in
In this study, the modified zeolite was obtained by modifying natural zeolite by using silane coupling agents such as trimethylchlorosilane (TMCS), trimethoxysilane (TMS) and vinyltrimethoxysilane (VTMO) and the removal effect of modified zeolite on naphthalene in aqueous solution was investigated by batch adsorption experiments. The experiments show that the modified zeolite does not only retain the structure of natural zeolite, but also enhances its ability to adsorb organic pollutants by enhancing its hydrophobicity, which is very helpful for the treatment of wastewater containing organic pollutants.
2. Materials and Methods
2.1. Preparation of Adsorbents
The natural mordenite used in the experiments was purchased from Gongyi, Henan, with a particle size of less than
2.2. Preparation of Adsorbates
Because the naphthalene (purity ≥99.7%, the United States) has a lower water solubility but is soluble in methanol, the required concentration of naphthalene solution was prepared after mixing and constant volume of an appropriate volume of stock solution from 400 mg/L stock solution prepared after dissolving the naphthalene in the methanol (chromatographically pure, Tianjin) and a certain volume of deionized water in the study. Because the methanol content in the solution is about 0.1% (v/v), the increased solubility of naphthalene in methanol is negligible [13] . The naphthalene aqueous solution with a concentration of 900 μg/L was prepared according to the water solubility of naphthalene in the study.
2.3. Analytical Instruments
The main instruments used in the experiments include HH-2 constant temperature water bath kettle (Yuyao, Zhejiang), BS-1E constant temperature culture oscillator (Changzhou, Jiangsu), CP
2.4. Analytical Methods
The concentration of naphthalene in aqueous solution was determined by Agilent 1100 high performance liquid chromatographic system (HPLC), and acetonitrile-water was used as the mobile phase. The optimized detection conditions were shown in Table 1.
2.5. Adsorption Experiment
The naphthalene water solution was prepared at different concentrations and gradients, and then a certain amount of natural zeolite or modified zeolite and 50 mL naphthalene solution were added to the adsorption bottle according to a solid-liquid ratio in the adsorption kinetics. The adsorption bottle was placed in a constant temperature culture oscillator and then the oscillation and adsorption were carried out at 25˚C and 180 r/min. After a certain period of time, the con-
Table 1. HPLC analysis method of naphthalene.
centration of naphthalene in the solution was measured after being sampled, and then the curve that the adsorption capacity of naphthalene was changed with time and the unit adsorption capacity was changed with initial concentration of naphthalene was drawn. The adsorption capacity of naphthalene on natural zeolite and modified zeolite was calculated according to Formula (1). In order to avoid the case of naphthalene solution, the naphthalene solution was placed in a brown bottle and the adsorption experiments were carried out in a dark environment.
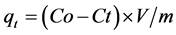 (1)
(1)
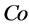 is the initial concentration of naphthalene in the solution (μg/L);
is the initial concentration of naphthalene in the solution (μg/L); 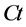 is the concentration of naphthalene in the solution after adsorption (μg/L);
is the concentration of naphthalene in the solution after adsorption (μg/L); 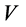 is the volume of the solution (L); m is the dosage of zeolite (g);
is the volume of the solution (L); m is the dosage of zeolite (g); 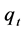 is the adsorption capacity of naphthalene on zeolite after adsorption (μg/L).
is the adsorption capacity of naphthalene on zeolite after adsorption (μg/L).
3. Results and Discussion
3.1. Structural Characteristics of Modified Zeolite
The XRD patterns of natural zeolite and modified zeolite were shown in Figure 1. It can be seen that both of natural zeolite and modified zeolite had stronger characteristic diffraction peaks of SiO2 and Al2O3 and also contained some impurity peaks, but the diffraction patterns of modified zeolite and natural zeolite were basically similar, indicating that the crystal structure of modified zeolite was unchanged compared with the natural zeolite.
Figure 2 showed the infrared spectra of natural zeolite and modified zeolite. It can be seen from the figure that the characteristic peaks of natural zeolite andmodified zeolite were basically the same and the bending, oscillation and ab-
Figure 1. XRD patterns of natural zeolite and modified zeolite: (1) natural zeolite; (2) zeolite (VTMO); (3) zeolite (TMCS); (4) zeolite (TMS).
Figure 2. IR spectra of natural zeolite and modified zeolite: (1) natural zeolite; (2) zeolite (VTMO); (3) zeolite (TMCS); (4) zeolite (TMS).
sorption band of Si(Al)-O gene group was presented in the range of 400 - 550 cm−1 and 950 -
The contact angle measurement of modified zeolite was shown in Figure 3. It can be seen from the figure that the contact angle of TMCS modified zeolite was the largest among the three organic modified zeolites. Natural zeolite can quickly absorb water droplets because of its good surface hydrophobicity; therefore, its contact angle was set to zero.
TG diagrams of natural zeolite and modified zeolite were shown in Figure 4, their situations of heat loss were basically the same, both of them began to lose weight from 40˚C, with the temperature rise, the more the loss of quality would be, and the loss rate was basically the same and kept flat, but the weight loss rates of four zeolites were almost the same, the natural zeolite had the lowest value 29.13%, and 31.75% for TMCS modified zeolite, 31.20% for TMS modified zeolite and 30.07% for VTMO modified zeolite. The weight loss of natural zeolite was mainly for the evaporation and escape of the absorbed water, crystal water, structural water and zeolite water within the zeolite with different temperatures, and for three organic modified zeolites, in addition to moisture evaporation, a part of modifiers would also be lost, the results showed that the modified zeolite had been successfully loaded on the modifier.
3.2. Adsorption Capacity of Naphthalene on Different Modified Zeolites from Aqueous Solution
The adsorption capacity of naphthalene on natural zeolite and three kinds of

Figure 3. Contact angles of modified zeolites: (a) zeolite (TMCS); (b) zeolite (TMS); (c) zeolite (VTMO). (1) 29.6˚; (2) 17.8˚; (3) 24.3˚.
Figure 4. Thermogravimetric curves of natural zeolite and modified zeolites: (1) natural zeolite; (2) zeolite (VTMO); (3) zeolite (TMCS); (4) zeolite (TMS).
organic modified zeolites was as shown in Figure 5, the figure shows that when C0 was 900 μg/L, the volume fraction was 20%, m/V was
From the molecular structure of three modifiers in Table 2, VTMO and TMS modified zeolites reacted with the hydroxyl group (−CH3O) in the molecule and the hydroxyl group (-OH) on the zeolite surface during the modified reaction so that VTMO molecules were loaded on the zeolite surface. The TMCS modified zeolite was a reactant between the Cl atom in the molecule and the hydroxyl group (−OH) on the surface of the zeolite so that the TMCS molecules were loaded on the zeolite surface. The reaction scheme was shown in Figure 6, the three kinds of silane coupling agents contained the hydroxyl group (−CH3O), vinyl (−CH=CH2) or methyl (−CH3) which can adsorb organic matters, and all
Figure 5. Sorption of naphthalene by natural zeolite and zeolites modified by different modifiers: (1) natural zeolite; (2) zeolite (VTMO); (3) zeolite (TMCS); (4) zeolite (TMS) Values represent the mean ± SD, n = 3. Significantly different from control: * = p < 0.05.
Figure 6. Effect of initial concentration on adsorption capacity of naphthalene on VTMO modified zeolite and natural zeolite.
Table 2. Molecular structures of three modifiers.
of them can improve the adsorption capacity of naphthalene on zeolite after modification, and the solution of VTMO modified zeolite contained more gene groups with adsorption function, while the carbon chain of vinyl was longer than methyl, which was helpful for enriching the organic matters by VTMO modified zeolite. Therefore, among the three silane coupling agents, the adsorption effect of naphthalene on VTMO modified zeolite was best.
3.3. Adsorption Isotherm
The effect of the initial concentration on the adsorption of naphthalene on VTMO modified zeolite and natural zeolite was shown in Figure 6. It can be seen from Figure 6 that when the volume fraction was 10%, m/V was
The isothermal adsorption curves of naphthalene on VTMO modified zeolite and natural zeolite were fitted by Langmuir and Freundlich models [14] .The fitting parameters were shown in Table 3. It can be seen from Table 3 that the adsorption capacity of naphthalene on VTMO modified zeolite and natural zeolite can well meet the Langmuir model.
On the basis of a large number of the experimental data in the previous adsorption experiments, the length of the carbon chain indicated a close relationship with the enrichment mechanism of the nonionic organic substance on the adsorbent. When the modifier was a long chain alkane with alkane group such as hexadecyl, the nonionic organic matter would be enriched in a distributed manner and the adsorption isotherm would be linear, and when the modifier was a short chain with alkyl group such as methyl or ethyl, the nonionic organic would be enriched in an adsorption manner, the adsorption isotherm would be non-linear, and Langmuir isothermal would be applicable for non-linear adsorption characteristics, which were consistent with the experimental results.
3.4. Adsorption Kinetics
The effect of contact time on naphthalene sorption by VTMO modified zeolite and natural zeolite was shown in Figure 7. In the experiment, the adsorption process of naphthalene on VTMO modified zeolite and natural zeolite was fitted by quasi-second order kinetic model. The fitting curve and kinetic parameters were shown in Table 4 respectively.
From Figure 7 and Table 4, it can be seen that the calculated equilibrium unit adsorption capacity 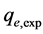 was close to the experimental measured value
was close to the experimental measured value
Table 3. Parameters of the Langmuir and Freundlich adsorption isotherms of naphthalene on modified zeolite and natural zeolite.
Table 4. Kinetic parameters for sorption data fitted to Pseudo-second-order model.
Figure 7. Effect of contact time on naphthalene sorption by VTMO modified zeolite and natural zeolite.
i.e. about 0.339 mg/g, the quasi-second-order kinetic model had a better fittingness for experimental data, therefore, the quasi-second-order kinetic model can describe the adsorption kinetics process of naphthalene on HAMO modified zeolite from aquatic solution.
4. Conclusions
1) The three kinds of silane coupling agents had been successfully loaded on the surface of acidified zeolite, the hydrophobicity of zeolite was improved to some extent, the structure of zeolite itself was not destroyed, and the heat resistance was consistent with that of natural zeolite;
2) Among the three kinds of silane coupling agent modified zeolites, VTMO modified zeolite had the best adsorption capacity of naphthalene, reaching 339 μg/g, 32% higher than that of natural zeolite. Compared with the results of other literature, the naphthalene sorption capacity of modified peat increased by more than 33% and the adsorption of phenanthrene by CPB modified zeolite increased by more than 30% [13] [15] . As known VTMO modified zeolite was an effective modifier, the adsorption behavior can be Langmuir isothermal adsorption model; the quasi-second order kinetic model can be used to represent the adsorption kinetics process;
3) The organic modification enhanced the hydrophobicity of the zeolite and its ability to adsorb organic pollutants and they can be used to treat wastewater containing organic contaminants.
Acknowledgements
This study was funded by Scientific Research Foundation of Environmental Protection Bureau of Guangzhou City, China (No. 2014).
Cite this paper
Li, N., Cheng, W.-Y. and Pan, Y.-Z. (2017) Adsorption of Naphthalene on Modified Zeolite from Aqueous Solution. Journal of Environmental Protection, 8, 416-425. https://doi.org/10.4236/jep.2017.84030
References
- 1. Krupadam, R.J., Khan, M.S. and Wate, S.R. (2010) Removal of Probable Human Carcinogenic Polycyclic Aromatic Hydrocarbons from Contaminated Water Using Molecularly Imprinted Polymer. Water Research, 44, 681-688.
- 2. Wen, F., Yin, H., Fan, L., et al. (2005) Analysis of Current Situation of Polycyclic Aromatic Hydrocarbons Contamination in Water of Chengdu Section of Minjiang River. Sichuan Environment, 6, 77-79. (In Chinese)
- 3. Mesquita, S.R., L. van Drooge B., Barata, C., et al. (2014) Toxicity of Atmospheric particle-Bound Pahs: An Environmental Perspective. Environmental Science and Pollution Research, 21, 11623-11633. https://doi.org/10.1007/s11356-014-2628-y
- 4. Jiang, R.L., Xiao, B.C., Yu, N., et al. (2014) Research Advance in Toxic Effects of PAHs on Aquatic Animals. Marine Fisheries, 4, 2-384. (In Chinese)
- 5. Xiao, X.M. (2014) Preparation of Active Carbon from Coal by Microwave Method and Its Absoption Capacity of Naphthalene, Phenanthrene and Pyrene. Ph.D. Thesis, Shihezi University, Shihezi. (In Chinese)
- 6. Yi, X., Yuan, J.H., Gu, S.D., et al. (2013) Kinetic Characteristics of Naphthalene, Phenanthrene and Pyrene Uptake by Wheat Roots. Journal of Environmental Sciences, 33, 1135-1140. (In Chinese)
- 7. Qin, Y. and Le, Y.H. (1997) Hydrophobic Research on Mordenite. Chinese Journal of Applied Chemistry, 14, 9-11. (In Chinese)
- 8. Yang, SH.D. (2004) Reseach on Hydrophobic Modification and Adsorption Performance of Mordenite. Ph.D. Thesis, Dalian University of Technology, Dalian. (In Chinese)
- 9. Qin, F., Wen, B., Shan, X.-Q., et al. (2006) Mechanisms of Competitive Adsorption of Pb, Cu, and Cd on Peat. Environmental Pollution, 144, 669-680.
- 10. Huang, H., Hao, SH. SH., Zhu, J.L., et al. (2013) Adsorption Characteristic of Hg 2+ on Natural and CPB Modified Zeolite. Chinese Journal of Environmental Engineering, 7, 579-584. (In Chinese)
- 11. Hou, L., Xu, R.L., Yu, S.Y., et al. (2016) Hydrophobilized Modification of Calcium Oxide with Stearic Acid and Silane Coupling Agent KH570. Acta Scientiae Circumstantiae, 36, 960-965.(In Chinese)
- 12. Shi, J., Zhang, N., Zhang, C., et al. (2016) Study on the Effect of Different Modified Zeolite to Phosphorus Activation in Red Soil. Journal of Environmental Protection, 7, 2036-2046. https://doi.org/10.4236/jep.2016.713158
- 13. Taang, X.Y. (2010) Sorption of Hydrophobic Organic Contaminants from Water by Modified Peat and Its Mechanism Study. Ph.D. Thesis, East China University of Science and Technology, Shanghai. (In Chinese)
- 14. Chang, C.F., Chang, C.Y., Chen, K.H., et al. (2004) Adsorption of Naphthalene on Zeolite from Aqueous Solution. Journal of colloid and Interface Science, 277, 9-34.
- 15. Li, J., Lin J.-W., Zhan, Y.-H., et al. (2014) Adsorption of Phenanthrene from Aqueous Solution on Cetylpyridinium Bromide (CPB)—Modified Zeolite. Environmental Science, 35, 611-618. (In Chinese)










