Journal of Environmental Protection
Vol. 3 No. 3 (2012) , Article ID: 17939 , 8 pages DOI:10.4236/jep.2012.33032
Mercury and Methyl Mercury in Sediments of Northern Lakes-Egypt
![]()
1National Institute of Oceanography and Fisheries, Alexandria, Egypt; 2Chemistry Department, Faculty of Science, Alexandria University, Alexandria, Egypt.
Email: niof@hotmail.com
Received December 1st, 2011; revised January 16th, 2012; accepted February 18th, 2012
Keywords: Mercury; Methyl mercury; Northern Lakes; Egypt
ABSTRACT
Fifty-four sediment samples of the five Northern Egyptian lakes, (Mariout, Edku, El-Burullus, El-Manzallah, and ElBardaweel) were analyzed to investigate the pollution status of mercury (Hg). The total mercury (T-Hg) content in sediment samples ranged from 15.33 to 171.29 ng·g–1 dry wt). The results showed that T-Hg were lower than the back ground values reported and also lower than the ranges of uncontaminated sediments. Moreover, the T-Hg concentrations in all sediments were under the upper chemical Exceedance level (1 µg·g–1). The concentrations of Methyl mercury (MeHg) in surface sediments of the Northern lakes ranged from 0.002 - 0.023 ng·g–1 dry wt. The contribution of MeHg was less than 0.1% of total mercury concentration with index values from 0.08 - 1.37 ng·g–1; dry wt). MeHg showed insignificant correlation with T-Hg. This suggested that MeHg contents were not controlled by the T-Hg in sediments. The T-Hg and MeHg concentrations were insignificantly correlated with TOC content which indicates that the concentration of T-Hg and MeHg in sediments of Northern lakes were not influence by TOC. The average T-Hg concentration was found in the following order; Mariout > El-Manzallah > El-Burullus > Edku > El-Bardaweel. While the MeHg was found in the order; El-Bardaweel > El-Burullus ≥ El-Manzallah > Mariout > Edku.
1. Introduction
Mercury (Hg) is listed as a priority pollutant by many international agencies because of its persistence, bioaccumulation, and toxicity (PBT) in the environment. With the development of agriculture and industry, Hg has been extensively used in the manufacture of pesticides, fungicides, electrical goods, paper, batteries and other items, which has caused large amounts of Hg to be emitted to the environment [1].
In aquatic system, sediment is an important sink and source of mercury (Hg) and is also considered as the main production site of methyl mercury (MeHg). Now, the concentration of total mercury (T-Hg) is not by far the most important because various chemical species of mercury behave distinctly, thereby affecting its biogeochemical behavior and toxicity to organisms [2]. As the most toxic mercury species, MeHg is neurotoxic and can cause blockage of binding sites of enzymes, interfere protein synthesis and impede thymidine incorporation into DNA [3]. Furthermore, MeHg could be accumulated in the animal and human food chain more easily than the inorganic forms; thus, it was given particular concern all the time.
The Egyptian Mediterranean coast exhibits five lakes or lagoons, namely, Northern Delta Lakes which located along the Nile Delta coast and to the west and east of Suez Canal (Figure 1). These lakes are, namely, from west to east: Mariout, Edku, El-Burullus, El-Manzallah and ElBardaweel. All of them, with the exception of Lake Mariout are directly connected to the Sea. The northern lakes are economically the most important fishing ground. They are providing a rich and vital habitat for estuarine and marine fish and their regeneration, and have always been major areas of fish production in Egypt, since more than 75% of the Egyptian lakes production are harvesting from them. They are also important sites for wintering water birds providing valuable habitats for several hundred thousand of birds. Unfortunately many challenges are facing these takes i.e. degradation and decreasing the lakes area in addition to pollution problems which are increasing due to the expansion of human activities; fish farming activities and aquatic plants which reduce the water exchange through the lakes. Since the construction of the High Dam and the almost complete cessation of sedimentation, the coasts of the eastern Delta have altered from predominantly accretion and erosion processes. In addition construction of Aswan High Dam accompanied by considerable increase in population and consequently in man’s activities constitutes the main reason of pollution in the Nile Delta Lakes, mainly eutrophication, as well as occurrence of heavy metals and pesticide contaminants constituted in these lakes problems of increasing concern. These conditions deteriorate the aquatic habitats, harm the living organisms, and change of aquatic biota causing less diverse system. In spite of increasing human activities, it is the first effort of investigations on “Organomercury” in sediments of the five northern lakes.
The present study aims to determine the current levels of T-Hg and (MeHg) in surface sediments of the five northern lakes to provide adequate information about the impact on human health and the environment by using recent advanced hyphenated techniques. A comparative study of the Hg and MeHg pollution levels among studied lakes was also evaluated.
2. Materials and Methods
2.1. Sampling
During summer 2009, Fifty four sediment samples were collected from Lakes Mariout (n = 10), Edku (n = 9), ElBurullus (n = 12), El-Manzallah (n = 11), and El-Bardaweel (n = 12) using a Van-Veen grab sampler (Figure 1). The locations were selected taking into consideration the expected polluted area due to industrial and human activities. The samples were frozen in polyethylene plastic bags and stored at –20˚C until further analysis.
2.2. Analyses
2.2.1. Total Mercury
In the laboratory, the sediment samples were freeze-dried by using Freeze-dried (Labconco, England), grinding with agate mortar and stored at room temperature. One gram of homogenized dry samples was weighed into a previously pre-cleaned Teflon vial, 8 ml of nitric acid and 4 ml of sulfuric acid (Merck, Germany) were added, and the mixture was heated at 90˚C for 5 hours on an aluminum block hot plate. After cooling to room temperature, the volume was diluted using deionized water, filtered and made up to 100 ml, and then subjected to total Hg determination [4]. Atomic Absorption Spectrophotometer (Shimadzu Atomic Absorption Spectrophotometer-AA- 6800 with the auto sampler Shimadzu ASC 6100, Japan) equipped with mercury cold vaporizer unit (MVU-1A) was used for total mercury determination.
2.2.2. Methyl Mercury
1) Preparation of a spiked sediment About 200 g of dried marine sediment (previously checked for methylmercury content) was accurately weighed and mixed with 50 ml of methanol. About 50 ml of methanol containing a known amount of methylmercury was added drop by drop to the sediment under mechanical shaking in about 3 h. Then, the spiked sediment was left overnight to equilibrate under mechanical shaking. The sediment was completely dried in oven at 45˚C under vacuum for about 8 h. Finally, the spiked sediment was stored in a glass container at 4˚C in the darkness. The nominal concentration of the spiked sediment was 42.8 ng/g dry weight.
2) Methyl mercury was determined according to A.M. Caricchia et al. [5]. About 2 g of sediment sample were accurately weighed and extracted by 5 ml KOH/CH3OH (25%) in ultrasonic bath (45 min). After cooling, 5 ml of H2SO4 (4 M, saturated CuSO4), 5 ml of 4 M KBr and 4 ml of toluene were carefully added and the sample was manually shaken for about 3 min. After centrifugation (2200 rpm for 10 min), the supernatant organic phase was collected. The solvent extraction was repeated three times by 2 ml of toluene. The collected organic extract (10 ml) was subjected twice to a back-extraction by 1 ml cysteine solution (1%). The two cysteine solutions (1/1 ml) were collected in the same vial and extracted by a mixture of benzene (0.5 ml), CuSO4 saturated solution (0.5 ml), and 4 M KBr (1 ml). After manual shaking, an adequate volume of the solution was added, as internal injection standard. Finally, the organic phase was separated from the aqueous phase and 2 µl were injected in the Gas Chromatography Mass Spectrometer (GC-MS): Thermo Electron Corporation, Trace GCUltra coupled to DSQ II-Mass Spectrometer. S/N (DSQ II MS 220- 5695), column: TR-35 (35% PhenylpolysilphenyleneSiloxane), 30 m × 0.25 mm × 0.25, P/N 260C 142P, S.No. 10924B13. Model, K-0734-B-3010000, S.No.:3200804, Italy. Operating condition: initial temperature 50˚C, hold time 2 min, ramp 10˚C/min, to final temperature 120˚C, hold time 1 min., injection temperature 160˚C, He carrier flow 1 ml/min., splitless, injection 2 µl.
2.2.3. Total Organic Carbon (TOC %)
Organic carbon was determined using acid/dichromate titration method as described by Gaudette et al. [6]. About 0.3 g was dried and sieved sediment sample was placed in a 500 ml Erlenmeyer flask. Exactly 10 ml of 1N K2Cr2O7 solution was added to the sediment and the two were mixed by swirling the flask. 20 ml of concentrated H2SO4 were added by burette and mixed by gentle rotation of the flask for about one minute. This should be done carefully to insure complete mixing of the reagents with the sediment, avoiding throwing the sediment onto the sides of the flask out of contact with the reagents. The mixture was allowed to stand for 30 min. A standardization blank for sediments was run with each new batch of samples. After 30 min, the solution was diluted to 200 ml volume with distilled water, and 10 ml (85% H3PO4), 0.2 g NaF and 15 drops of diphenylamine indicator were added to the sample flask. The solution was back titrated with 0.5N ferrous ammonium sulfate solution.
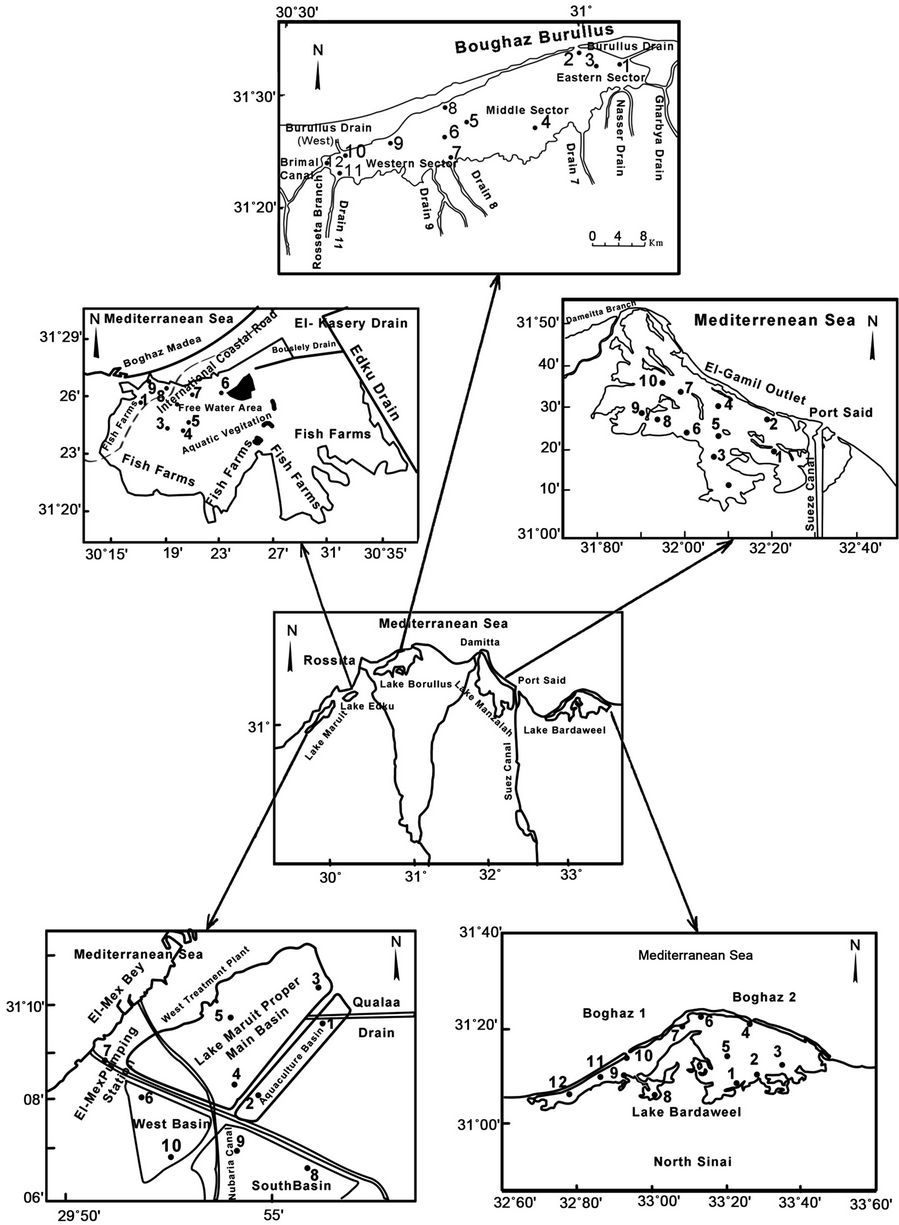
Figure 1. Map showing the positions of sampling stations of the five Egyptian Northern lakes.
2.3. Method Validation and Quality Control Studies
For quality control (QC) and quality assurance (QA), replicates, method blanks, matrix spikes and standard reference material (IAEA-356) (marine sediment; Analytical Quality Control Services, Austria) were analyzed under the same procedures mentioned above. Analytical results of the quality control samples indicated a satisfactory performance of mercury and Methyl mercury determination within the range of certified values with 90.4% - 97.5% and 90% - 95.3% recovery for Hg and MeHg respectively.
3. Results and Discussion
The present T-Hg and MeHg concentrations of sediments of the five Northern lakes have been determined to investigate the pollution status of mercury. The concentration of T-Hg and MeHg in sediments of the Northern lakes are presented in (Table 1).The concentrations of T-Hg scattered in the ranges (31.05 - 171.29 ng·g–1; dry wt), (26.78 - 38.91 ng·g–1; dry wt), (23.29 - 66.22 ng·g–1; dry wt), (23.29 - 122.65 ng·g–1; dry wt), (15.33 - 39.13 ng·g–1; dry wt) for lakes Mariout, Edku, El-Brullus, ElManzallah and Bardweel respectively. Generally, the highest absolute concentration values in the five Northern lakes were observed at stations which are located near the drains and affected by the industrial and sewage discharge. These are observed at stations (6) and (5) and (1) for lakes Mariout and El-Manzallah respectively (Figure 2) due to the impact of sewage and industrial wastes coming from El-Nubaria, El-Umoum and El-Kalla drains in lake Mariout and from Bahr El-Baqar, El-Hadous and Ramsis drains in lake El-Manzallah.
The inorganic compounds such as iron and manganese hydroxides can have a significant effect on mercury transportation, they provide an excellent surface for adsorption of organic matter and they are alone able to bind this toxic element [7]. The importance of iron and manganese hydroxides derives from their high sorption capacity, the ability to co-precipitate with mercury compounds, and the ability of mercury release from iron and manganese hydroxides [8]. Many authors have noted a correlation between the concentration of mercury and that of iron and manganese [7]. High concentrations of Fe (18062 µg·g–1; dry wt) and Mn (818 µg·g–1; dry wt) were recorded in sediments of lake Mariout [9] which create a suitable environment for deposition of Hg leading to increasing T-Hg concentrations at these stations. Also high Fe concentration (27723 µg·g–1; dry wt) and relatively high Mn value (538 µg·g–1; dry wt) were recorded at station (1) in lake El-Manzallah.
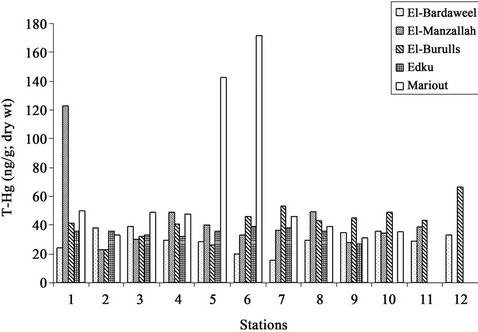
Figure 2. The distribution of T-Hg (ng·g–1; dry wt) in sediments of Egyptian Northern lakes during summer 2009.
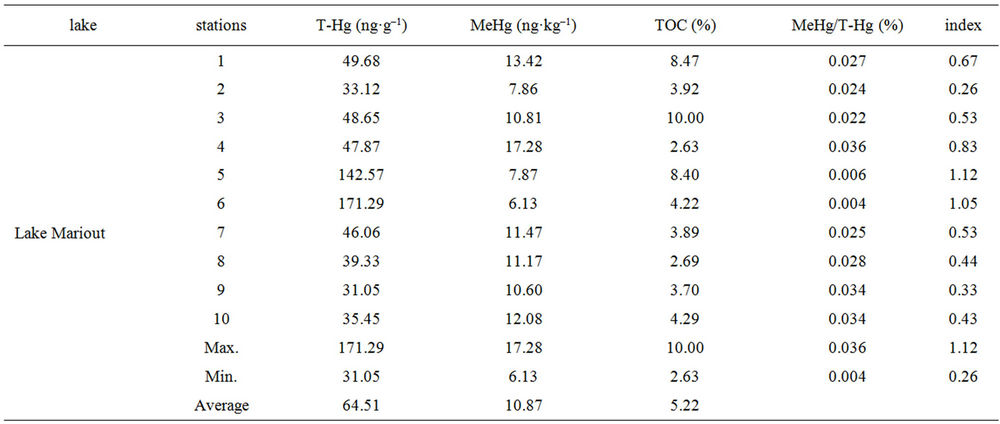
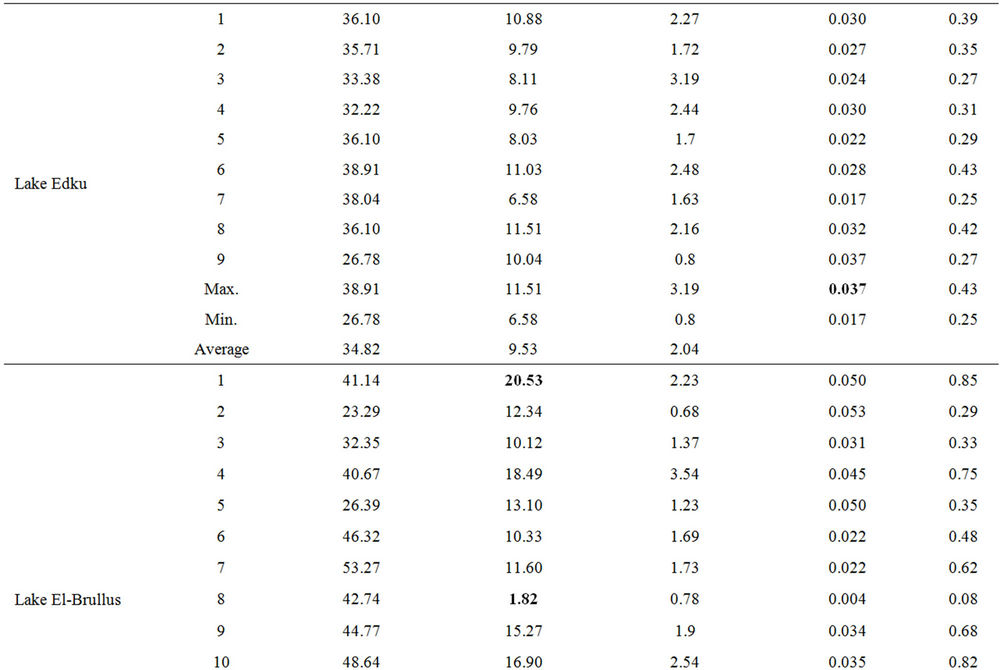
Table 1. Concentrations of T-Hg, MeHg and TOC % (dry wt) in sediments of the Egyptian Northern lakes during summer 2009.
Craig [10] reported concentration ranges of 0.2 to 0.4 µg·g–1 total mercury for uncontaminated sediments where as sediments in urban, industrial or mineralized areas can contain up to 100 µg·g–1 T-Hg. In the other hand, Fujii [11] reported back ground levels of 0.05 µg·g–1 in river sediments, 0.1 to 0.3 µg·g–1 in lake sediments and 0.05 to 0.08 µg·g–1 in sea sediments. Therefore, the T-Hg content in sediments collected from five Northern lakes (15.33 - 171.29 µg·g–1; dry wt) were lower than the back ground values reported and also lower than the ranges of uncontaminated sediments. Moreover, the T-Hg concentrations in all sediments were under the upper chemical Exceedance level (UCEL, 1 µg·g–1) [12]. Therefore, we can conclude that the five Northern lakes sediments are not polluted with mercury.
Prior to the present study no detailed studies of the extent of contamination of these lakes with MeHg have been reported. The concentrations of MeHg in surface sediments of the Northern lakes scattered in the ranges (0.006 - 0.017 ng·g–1; dry wt), (0.006 - 0.011 ng·g–1; dry wt), (0.001 - 0.02 ng·g–1; dry wt), (0.007 - 0.023 ng·g–1; dry wt) and (0.009 - 0.017 ng·g–1; dry wt) for lakes Mariout, Edku, El-Brullus, El-Manzallah and Bardweel respectively (Table 1).
The MeHg contents in sediments account for normally 1% to 1.5% of T-Hg except for some lakes and wetlands where the percentage of MeHg can reach 10% [13,14] while it makes less than 0.5% in the estuaries and seas [15,16].
Kannan and Falandysz [16] suggested that the product of total mercury concentration and the MeHg concentration could be a useful index describing the degree of pollution in given sediment. According to these authors, in non-polluted areas this index lakes values lower than 1.
In the surface sediments of the northern lakes, the contribution of MeHg was less than 0.1% of total mercury concentration with this index values from 0.08 - 1.37 ng·g–1; dry wt). From among the samples studied, the highest index value was found for the sediments of station (5) of lake Mariout (1.12 ng·g–1; dry wt) and station (1) of lake El-Manzallah (1.37 ng·g–1; dry wt). The distribution of MeHg in sediments is shown in (Figure 3).
The relationship between MeHg and T-Hg was studied and MeHg showed insignificant correlation with T-Hg. This suggested that MeHg contents were not controlled by the T-Hg in sediments and sources of T-Hg and MeHg pollution are different and giving clear evidence that biomethylation process is the main source of MeHg.
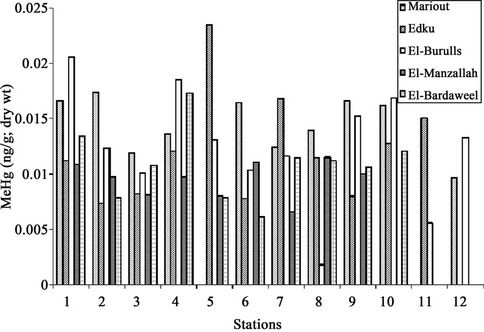
Figure 3. The distribution of MeHg (ng·g–1; dry wt) in sediments of Egyptian Northern lakes during summer 2009.
Organic carbon plays an important role in the bioavailability and methylation of inorganic mercury although its role has not been well understood [17]. Ullrich et al. [14] argued that, organic carbon enhance methylation by stimulating the activity of heterotrophic microorganism, or through direct abiotic methylation of Hg by humic or fulvic substances. On the other hand, Hg methylation may be inhibited at high organic carbon concentrations due to increased complexation of Hg with organic ligands, reducing Hg bioavailability to bacterias, particularly in the neutral pH rang.
The total organic carbon concentrations in surface sediments of the Northern lakes were also investigated (Table 1). The contents of TOC in sediment samples scattered in the ranges (2.62% - 10%), (0.78% - 3.54%), (0.68% - 3.54%), (1.25% - 4.68%) and from (0.29% - 2.15%) for lakes Mariout, Edku, El-Brullus, El-Manzallah and Bardweel respectively.
The T-Hg and MeHg concentrations were insignificantly correlated with TOC content which indicates that the concentration of T-Hg and MeHg in sediments of Northern lakes were not influence by TOC. This result was in agreement with other study [18].
Figure 4 shows that concentrations of T-Hg in the Northern lakes decreased in the following order: Mariout (64.51 ng·g–1; dry wt) > El-Manzallah (44.06 ng·g–1; dry wt) > El-Burullus (42.44 ng·g–1; dry wt) > Edku (34.82 ng·g–1; dry wt) > El-Bardaweel (29.73 ng·g–1; dry wt. The highest Hg concentrations were recorded among stations of Lake Mariout, while, the lowest Hg concentrations were measured in Lake El-Bardaweel due to lacking sources of pollution with Hg compounds. Comparing the present data with other results from previous studies (Table 2) reveals that, our results are comparable to those from Lake Shihwa, Korea,but still lower than those from Victoria Harbour, Hong Kong and Haihe River, China.
Concentrations of MeHg compounds in the Northern lakes decreased in the following order: El-Bardaweel > El-Burullus ≥ El-Manzallah > Mariout > Edku (0.009 ng·g–1; dry wt) (Figure 5). Lake El-Bardaweel maintained the highest MeHg concentrations reflecting physical and chemical conditions that enhanced mercury methylation in the surface sediments. Growth of different genera of biota, algae and other organic material especially in Lake El-Bardaweel which is a shallow water lagoon stimulate biomethylation processes and bioconversion of Hg into MeHg. Lake Edku maintained the lowest values due to decrease sources of pollution with Hg, besides lacking of methylation conditions.
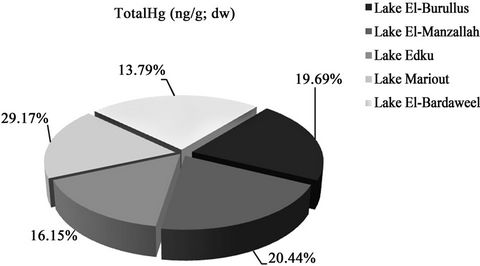
Figure 4. Comparison between concentrations of T-Hg (ng·g–1; dw) in sediments of Egyptian Northern lakes during summer 2009.
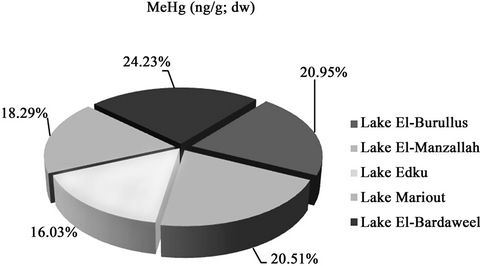
Figure 5. Comparison between concentrations of MeHg (ng·g–1; dw) in sediments of Egyptian Northern lakes during summer 2009.
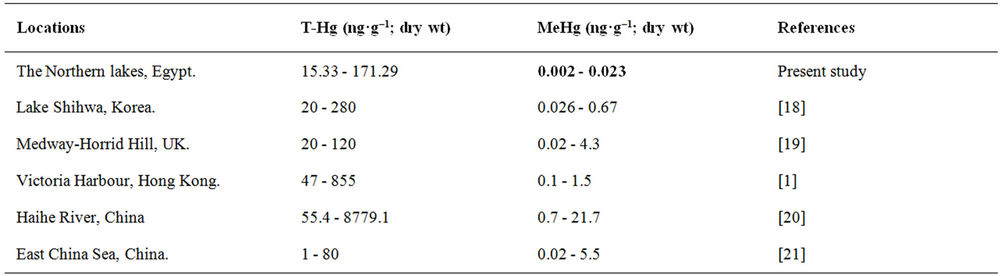
Table 2. Concentrations of T-Hg and Me-Hg (ng·g–1; dry wt) in sediments of the Egyptian Northern lakes during summer 2009 compared to other areas.
Comparing MeHg concentrations in the Northern lakes with other different areas cleared out that Lake Shihwa; Korea, Medway-Horrid Hill; UK, Victoria Harbour; Hong Kong, Haihe River, China and East China Sea; China showed attained high MeHg concentrations than those observed in sediments of Egyptian Northern lakes (Table 2).
REFERENCES
- J.-B. Shi, C. M. Carman, C. W. Y. Tang, G. Zhang, S. S. Rudolf and X. Dong, “Spatial and Temporal Variations of Mercury in Sediments from Victoria Harbour, Hong Kong,” Marine Pollution Bulletin, Vol. 54, 2007, pp. 464-488.
- Y. Cai, J. Raffe and R. Jones, “Ethylmercury in the Soils and Sediments of the Florida Everglades,” Environmental Science and Technology, Vol. 31, No. 1, 1997, pp. 302- 305. doi:10.1021/es960587a
- J. S. Thayer, “Organometallic Compounds and Living Organisms,” Academic Press, Orlando, 1984.
- IAEA, “Standard Operating Procedure for Determination of Trace Metals,” A Training Course on the Analysis of Trace Metals in Biological and Sediment Samples for MEDPol, 1997, p. 46.
- A. M. Caricchia, G. Minervini, P. Soldati, S. Chiavarini, C. Ubaldi and R. Morabito, “GC-ECD Determination of Methylmercury in Sediment Samples Using a SPB-608 Capillary Column after Alkaline Digestion,” Microchemical Journal, Vol. 55, No. 1, 1997, pp. 44-55. doi:10.1006/mchj.1996.1357
- E. Gaudette, R. Flight, L. Toner and W. Folger, “An Inexpensive Titration Method for the Determination of Organic Carbon in Recent Sediments,” Journal of Sedimentary Petrology, Vol. 44, 1974, pp. 249-253.
- J. L. Guentzel, R. T. Powell, W. M. Landing and R. P. Mason, “Mercury, Association with Colloidal Material in Estuarine and Open-Ocean Environment,” Marine Chemistry, Vol. 55, No. 1-2, 1996, pp. 177-188. doi:10.1016/S0304-4203(96)00055-2
- C. Gagnon, É. Pelletier, A. Mucci and W. F. Fitzgerald, “Diagenetic Behavior of Methylmercury in Organic-Rich Coastal Sediments,” Limnologyical Oceanography, Vol. 41, No. 3, 1996, pp. 428-434. doi:10.4319/lo.1996.41.3.0428
- Egyptian Environmental Affairs Agency/National Institute of Oceanography and Fisheries (EEAA/NIOF), “Annual Report on Data Information and Monitoring Programme for the Northern Lakes during 2010,” 2010.
- P. J. Craig and P. A. Moreton, “Total Mercury, Methylmercury and Sulphide Levels in British Estuarine Sediments-III,” Water Researches, Vol. 20, No. 9, 1986, pp. 1111-1118. doi:10.1016/0043-1354(86)90057-6
- M. Fujii, “Mercury Distribution in Lithosphere and Atmosphere,” In: S. Kitamura, M. Kondo, Y. Takizawa, M. Fujii and M. Fujiki, Eds., Kodansha Scientific, Mercury, Tokyo, 1976.
- HK ETWB, “Management of Dredged/Excavated Sediment,” Appendix A of ETWB (W) No. 34, Environment, Transport and Works Bureau, Hong Kong, 2002, p. A1.
- C. C. Gilmour and N. S. Bloom, “A Case Study of Mercury and Methylmercury Dynamics in a Hg-Contaminated Municipal Waste-Water Treatment Plant,” Water, Air, and Soil Pollution, Vol. 80, No. 1-4, 1995, pp. 799- 803. doi:10.1007/BF01189731
- S. M. Ullrich, T. W. Tanton and S. A. Abdrashitova, “Mercury in the Aquatic Environment, a Review of Factors Affecting Methylation, Critical Reviews,” Environmental Science and Technology, Vol. 31, No. 3, 2001, pp. 241-293. doi:10.1080/20016491089226
- T. Hamasaki, H. Nagase, Y. Yoshioka and T. Sato, “Formation, Distribution, and Ecotoxicology of Methy Lmetals of Tin, Mercury, and Arsenic in the Environment,” Critical Review in Environmental Science and Technology, Vol. 25, No. 1, 1995, pp. 45-91.
- K. Kannan and J. Falandysz, “Speciation and Concentrations of Mercury in Certain Coastal Marine Sediment,” Water, Air, and Soil Pollution, Vol. 103, No. 1-4, 1998, pp. 129-136. doi:10.1023/A:1004967112178
- I. Andersson, H. Parkman and A. Jernelov, “The Role of Sediments as Sink or Source for Environmental Contamination, a Case Study of Mercury and Chlorinated Organic Compounds,” Limnologica, Vol. 20, 1990, pp. 347-359.
- O. Sehee, K. Moon-Kyung, Y. Seung-Muk and Z. Kyung-Duk, “Distributions of Total Mercury and Methylmercury in Surface Sediments and Fishes in Lake Shihwa, Korea,” Science of the Total Environment, Vol. 408, No. 5, 2010, pp. 1059-1068. doi:10.1016/j.scitotenv.2009.11.007
- B. Ouddane, N. Mikac, A. B. Cundy, L. Quillet and J. C. Fischer, “A Comparative Study of Mercury Distribution and Methylation in Mudflats from Two Macrotidal Estuaries: The Seine (France) and the Medway (United Kingdom,” Applied Geochemistry, Vol. 23, No. 4, 2008, pp. 618-631. doi:10.1016/j.apgeochem.2007.11.001
- J. B. Shi, L. N Liang, G. B. Jiang and X. L. Jin, “The Speciation and Bioavailability of Mercury in Sediments of Haihe River,” China Environment International, Vol. 31, No. 3, 2005, pp. 357-365.
- J. B. Shi, L. N. Liang, C. G. Yuan, B. He and G. B. Jiang, “Methylmercury and Total Mercury in Sediment Collected from the East China Sea,” Bulletin of Environmental Contamination and Toxicology, Vol. 74, No. 5, 2005, pp. 980-987. doi:10.1007/s00128-005-0676-1

