Green and Sustainable Chemistry
Vol.07 No.03(2017), Article ID:78256,14 pages
10.4236/gsc.2017.73016
CO2 Absorption Performance of “Dry Matter” Prepared with Amino Acid-Based Ionic Liquids
Masaya Miyake, Mitsuru Satoh*
Department of Chemical Science and Engineering, Tokyo Institute of Technology, Tokyo, Japan

Copyright © 2017 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY 4.0).
http://creativecommons.org/licenses/by/4.0/



Received: July 3, 2017; Accepted: August 6, 2017; Published: August 9, 2017
ABSTRACT
Dry Matter (DM) is a powdery substance which is composed of micro droplets and surrounding hydrophobic silica nanoparticles. Because of the much larger surface area than that of the corresponding bulk liquid, DM, which contains amino-functionalized ionic liquids (ILs), is a promising CO2 absorption material provided with quick absorption speed. In the present study, we successfully prepared powdery DMs by utilizing aqueous solutions of amino acid-based ILs (tetraethylammonium glycine [N2222][Gly], and tetraethylammonium alanine [N2222][Ala]). Although a DM with lysine-based IL (N2222) [Lys]) was also prepared, only a soufflé-like material was obtained. We measured CO2 absorption performance for the DMs to find that the mass-base absorption ability (mass-base A.A.) (CO2 mol/DM kg) and the mol-base one (CO2 mol/IL mol) of [N2222][Lys] were ca. two times of [N2222][Gly] and [N2222][Ala], while the absorption speed of the former was inferior to the latter two, i.e., ca.15 min vs. 5 min for 90% absorption. In order to improve the mass-base A.A. of [N2222][Gly], we used 10% of aqueous poly(allylamine) (PAlAm) solution instead of water. The resultant mass-base A.A. proved to be significantly larger (1.9) than either of those of the respective single component systems (1.1 and 0.75 for the bulk IL and aq. PAlAm, respectively), and comparable to the A.A. (1.6 - 2.5) of 20% - 30% monoethanolamine solution which is commonly used in industrial application.
Keywords:
Dry Matter, Ionic Liquid, CO2 Absorption, Amino Acid, Polyallylamine

1. Introduction
CO2 capture from flue gases is now vital to reduce the green-house effect. As a main process to remove CO2 from the industrially emitted gases, the chemical absorption to aqueous solutions of low molecular weight amines, such as monoethanolamine (MEA) and N-methyldiethanolamine (MDEA), has been traditionally utilized. However, this conventional procedure has some serious drawbacks, e.g., inevitable loss and emission of those corrosive amines and thermal degradation during the energy-demanding regeneration process of the absorbents [1] . As a promising candidate for CO2-absorbing materials, amine-func- tionalized ionic liquids have recently attracted much attention [1] - [10] . Since ionic liquids (ILs) have favorable properties such as negligible volatility and excellent thermal stability, they are free from some of the above drawbacks. A prominent example for the application of ILs for the CO2 absorption has been reported by Romanos et al. [11] who employed the “inverse supported ionic liquid phase materials” (inverse SILPs). SILP is a material in which ILs are supported or adsorbed on the surface of porous substrate. In the “inverse” SILP, which is a powder-like substance, the phases are inversed and the IL is surrounded by solid materials. For example, the inversed SILP was prepared by a two-step method; in the first step an amino acid-derived IL such as N, N, N-tri- methyl-N-ethyl ammonium propionate was mixed with fumed silica nanoparticles. Then the mixture was slurried with ethanol and the suspension thus obtained was dried to obtain the final powdery substance. This substance may be called Dry Ionic Liquid (D-IL) according to our original naming [12] . However, the D-IL can be prepared, in principle, with a much easier procedure, i.e., by simply mechanically mixing an appropriate IL with a hydrophobized fumed silica.
D-IL is a member of Dry Matter (DM), which is a collective name of powdery materials that are composed of micro droplets as an inner core phase and surrounding hydrophobic silica nanoparticles. According to the inner phase, DM may be called Dry Water, Dry Polymer Solution (D-PS), Dry gel and Dry Ionic Liquid (D-IL), etc. In our previous studies [12] [13] , we first reported the successful preparation of powdery D-ILs. Since the liquid droplet diameter of the D-IL particles is as small as 10 μm [12] , the large surface area enabled quick absorption [13] . Due to the relatively low surface tension of ILs, however, it proved to be rather difficult to obtain powdery D-ILs by employing pure ILs. As an expedient procedure we added water to ILs to increase their surface tensions and found that this method was usable to prepare powdery DMs containing ILs (aqueous IL-type of DM or aqIL-DM) for most ILs tested. For example, 1-butyl- 3-methylimidazolium acetate ([bmim][ace]), which is an excellent CO2-absorb- ing IL [14] , provided only a paste-like substance when the bulk liquid was used, while a powdery DM could be obtained by adding 60 wt% of water. Then we examined the aqIL-DM on its CO2 absorption ability and only found a much less absorption than that of the bulk IL. The failure of CO2 absorption in the presence of water was interpreted in terms of the unique CO2 absorption mechanism of [bmim][ace] [15] ; C2 proton of the imidazolium ring is abstracted by an acetic acid counter anion to form a site where CO2 reacts. Namely, stable hydration of the carboxyl anions may interfere with the proton abstraction.
In the present study we prepared aqIL-DMs by using some amino acid-based ILs, because their CO2 absorption abilities may be rather enhanced in the presence of water [16] . The performance as CO2-absorption materials was checked in terms of the absorption speed and the capacity. Since water absorbs CO2 only slightly, the addition of water to ILs would reduce the total mass-base CO2 absorption ability even if the mol-base ability of IL employed were enhanced by the presence of water. Thus, we also tested an aqIL-DM in which water was replaced by aqueous solution of poly(allyl amine) (PAlAm) because the polyamine should have a substantial CO2 absorption ability [13] [17] . The resultant total CO2 absorption ability, in fact, proved to become higher than either of the respective bulk absorptions.
2. Materials and Methods
2.1. Materials
Tetraethyl ammonium hydroxide ([N2222][OH]) and three kinds of amino acids, i.e., glycine [Gly], L-alanine [Ala] and L-lysine [Lys], were purchased from Sigma Aldrich as 35 wt% aqueous solution and powder samples, respectively, and used as received. Poly(allyl amine)hydrochloride (PAlAm∙HCl) (MW:15000, Sigma Aldrich) was neutralized by NaOH and then filtrated through an Ultrafilter UP-10 (Advantec) for several times with pure water to remove inorganic ions and low molecular weight polymers. Concentration of PAlAm thus purified was estimated by conductometric titration to be ca. 24 wt%.
Hydrophobic fumed silica particle (HDK-H18, primary particle size: 5 - 30 nm), the surface OH groups of which are methylated with poly(dimethyl siloxane) by 75%, was purchased from Wacker Asahikasei Silicone Co. and used as received.
2.2. Methods
2.2.1. Preparation of Amino Acid-Based AqILs
[N2222][Gly], [N2222][Ala], and [N2222][Lys] ILs were prepared by neutralizing the respective amino acids with the equimolar [N2222][OH]. For example, in the case of [N2222][Gly] 1.0 g of glycine was neutralized with 5.6 g of the 35 wt% of [N2222] [OH] solution to obtain 6.6 g of [N2222][Gly] containing 55.2 wt% of water (i.e., 45 wt% aqueous [N2222][Gly] solution). In a similar way we prepared [N2222] [Ala] and [N2222][Lys] ILs containing 54 wt% and 48 wt% of water, respectively.
2.2.2. Preparation of D-IL
AqIL-DMs were prepared with a blender machine (Waring J-SPEC blender, container volume: 50 mL) by mixing 6.0 g of an aqueous IL solution and 1.05 g of the silica at a fixed speed (22,500 rpm) for 90 s (30 s × 3 with an interval of 10 s each) at a room temperature. Partial remove of water from the prepared DM samples was performed with an infrared humidity meter (FD-720, Kett Co. Ltd.) at 40˚C or 80˚C. AqIL-DM containing PAlAm was also prepared with the same preparation procedure except for using mixed solutions of aq. ILs and aq. PAlAm solution (9.9 wt%).
2.2.3. CO2 Absorption Measurements
Absorption performance of aqIL-DM and bulk liquids (aq. solution of IL, aq. solution of PAlAm and their mixture) for CO2 was estimated by measuring pressure depression within connected glass cylinders (Hyper Glass Cylinder HPG-96-3 (90 mL), Taiatsu Garasu Kogyo Co. Ltd.) due to the absorption of CO2 into 2 g of DM or bulk sample. The experimental set up which was assembled within a thermostat incubator (FMU-133I, Fukushima Kogyo Co. Ltd.) and the detailed description on the measurements were given in our previous report [13] . The absorption ability (A.A.) was measured or expressed by three kinds of ways; mol-base A.A. calculated by (mol of absorbed CO2)/(mol of IL), mass-base A.A. (CO2 mol/absorbent kg), and pressure-reduced A.A. (mol/mol/final partial pressure (bar) of CO2). In order to evaluate the recyclability of the aqIL-DM ([N2222][Gly] + PAlAm), the sample was regenerated by heating in the infrared humidity meter at 50˚C, 80˚C or 100˚C for 1 hour. The reduced water content was almost recovered by keeping the sample under 100% humidity for 6 hrs.
3. Results and Discussion
3.1. Preparation of DMs
AqIL-DMs were prepared by mixing aqueous solutions of [N2222][Gly] (water content: 55%), [N2222][Ala] (54%) and [N2222][Lys] (48%), with the hydrophobic silica nano particles. The first two ILs provided powdery DMs, while the last one resulted in only a soufflé-like substance. This failure was first ascribed to the lesser amount of water than those of the others. Thus, another [N2222][Lys] sample containing 57% of water was tested but the result was the same; a soufllé-like substance was obtained. Since one lysine molecule has two amino groups, it was expected that the surface of the aqueous [N2222][Lys] solution may be more polar than those of the other two. In fact, however, the long side chain, i.e., four methylene groups, seemed to lower the surface tension of the aqIL.
Aqueous [N2222][Gly] containing 10 wt% of PAlAm (1.8 g of [N2222][Gly], 0.6 g of PAlAm, 3.6 g of water) proved to provide a powdery DM, while only a paste- like D-PS was obtained with the 10 wt% polymer solution. The CO2 absorption performance of the former DM was investigated.
3.2. CO2 Absorption Performance
3.2.1. Comparison among the ILs
CO2 absorption behaviors for the three kinds of aqIL-DMs are shown in Figure 1 as the time course of the partial pressure. The figure also shows the result for the bulk aq. [N2222][Gly] to see how the absorption speed was enhanced by using the aq.IL in the DM form. In the case of the DM, it took ca.5 min to reach 90% of the final absorption level, which was much shorter than that of the bulk; ca.270 min. This absorption acceleration more than 50 times is comparable to that reported in our previous study on DMs prepared with [bmim][ace] [13] . As for [N2222][Lys], it took ca.15 min. This slower absorption than those of DMs prepared from [N2222][Gly] and [N2222][Ala] may be because the [N2222][Lys] DM was not powdery but soufllé-like.
Figure 1. CO2 absorption profiles of the amino acid-based ILs.
The experimental results on the absorption abilities are summarized on Table 1. As for the absorption ability measured by (mol of absorbed CO2)/(mol of IL) (A.A. (mol/mol)) given in the 6th column, it was highest for the [N2222][Lys]DM, ca. two times of those of the other DMs. This means that either of the two amino groups of lysine worked as an equivalent reaction site to accommodate one CO2 molecule. The A.A. (mol/kg), which was given in the 7th column, is a measure of CO2 absorption ability which was calculated by (mol of absorbed CO2)/(kg of DM or bulk liquid). Since the aqILs contained water of more than 50%, the mass-base A.A. would be useful when one compares CO2 absorption materials for actual application in industry. In the case of 20% - 30% monoethanolamine (MEA) solution, which is often used to remove CO2 from natural gas in the industrial scale, the A.A. (mol/kg) is ca. 1.6 - 2.5 [9] . Although the A.A. values obtained for the aqIL-DMs were lower than or at most comparable to the MEA system, the former values may be improved by reducing the water content, as shown in the following section. The A.A. (mol/mol/bar) in the last column is a measure of CO2 absorption at the same equilibrium partial pressure. Since the present CO2 absorption mechanism is not physisorption but via the chemical reaction with the amino group, the mol-base A.A. may not be simply proportional to the CO2 partial pressure at equilibrium. However, the pressure-reduced
Table 1. Experimental condition and three kinds of absorption abilities for CO2 absorption by aqIL-DMs.
*Soufllé-like material.
A.A. may be utilized as a qualitative measure for CO2 absorption material that is efficient even at a low pressure <1 bar. With this measure, the lysine-based aqIL- DM proved to be more efficient than the others.
3.2.2. Effects of Water Content
It has been known that A.A. (mol/mol) of the amino acid-based ILs may be improved by addition of substantial amount of water. For example, Zhang et al. [16] reported that the mol-base absorption ability of [N1111][Gly] (0.97 bar, 298 K) increased by adding water; from ca.0.17 for pure IL to 0.60 at 70% water. Further, according to Anderson et al. [10] , several amino acid-based ILs containing tetrabutylphosphonium cation ([P4444]) showed maximum CO2 absorption when the water/IL mol ratio was around 1.5 (water content: ca. 6% - 8%) while the A.A. (mol/mol) of [P4444][Ala] decreased with increasing water content up to water/IL ratio = 2.06, at least. These two studies, which were performed under different water content conditions, suggest that the A.A. of amino acid- based ILs may largely depend on the water content. In fact, the present [N2222] [amino acid] IL systems (aqIL-DMs) also showed similar water-dependency. Figure 2 shows the results for [N2222][Gly] of three different water contents as the time course of the partial pressure of CO2. The most significant mol-base CO2 absorption was obtained with the aqIL containing 55% of water (the same plot as that in Figure 1), while the second one and the least one were observed for systems containing 16% and 30% of water, respectively. Similar non-mono- tonic water content dependencies were also observed for [N2222][Ala] and [N2222] [Lys] systems. The results are shown on Table 2. In the alanine-IL systems, the highest A.A. (mol/mol) was obtained in the absence of water, which is contrastive to the other two systems which showed significant CO2 absorption in the presence of large amounts of water. As for the alanine system, some literature data are available in addition to that by Anderson et al. [10] ; according to Jiang et al. [3] , the mol-base A.A. of the bulk [N2222][Ala] was ca. 0.43˚C at 40˚C and ambient pressure. Although the constituting cation was different, aq. [P4444][Ala] solutions containing 1% or less of water have been reported to absorb almost equimolar CO2 [2] [10] . The latter results may be compared with the present one for [N2222][Ala] in the absence of water. Since the DMs were treated in air, the
Figure 2. Effect of water content on the CO2 absorption by aqIL-DMs prepared with [N2222][Gly].
Table 2. Experimental condition and the water content dependencies for CO2 absorption by aqIL-DMs.
*These row data are the same as those given on Table 1.
0% water sample possibly contained small amount of water after the heating at 80˚C. Although some CO2 absorption mechanisms of amine-functionalized absorbents in the presence and absence of water have been proposed by different researchers [2] [6] [16] , the detailed examination is out of scope of the present study. We just note here that the CO2 absorption performance on the mass base, which must be important from an application point of view, was most significant for [N2222][Ala] in the absence of water and [N2222][Lys] in the presence of both large and small amount of water.
3.2.3. PAlAm-Containing AqIL-DM
CO2 absorption for the powdery aqIL([N2222][Gly])-DM was measured three times. As shown later, each measurement was carried out as a first absorption measurement before the CO2 desorption by the heat treatment which was done to evaluate the recyclability of the DM. Figure 3 and Table 3 show only a typical
Figure 3. Comparison of CO2 absorption profile of the PAlAm-containing aqIL-DM with those of related systems.
Table 3. Experimental condition and absorption abilities for CO2 absorption by [N2222] [Gly] and PAlAm.
*Average of three measurements (0.70, 0.65 and 0.61).
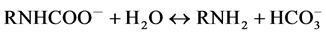
In order to figure out what caused the higher performance, we first examine the A.A. values of the respective bulk systems. As for the bulk PAlAm solution, Nagai et al. [17] reported that the A.A. (mol/kg) for the 10 wt% solution reached a saturated value, 0.86, after ca.120 min of CO2 flow through the sample at 25˚C and under the ambient pressure. The A.A. value and the absorption time are both superior to those obtained in the present study; 0.75 mol/kg and ca.500 min. However, the present A.A. value, 0.75, may be comparable to the literature value if the lower pressure condition (pfinal = 0.864 bar) was taken into account. Further, the several times longer absorption time may be ascribed to the difference in the CO2 absorption method. On the other hand, the 0.43 as the mol-base A.A. is significantly less than ca. 0.65 of a typical CO2 absorbent, monoethanolamine (MEA) obtained at 40˚C under the corresponding pressure (~0.85 bar) [18] [19] . According the proposed mechanism for the CO2 absorption into aqueous MEA solution,
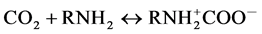

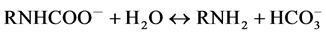
CO2 may be absorbed via the zwitterion formation, the carbamate formation and the recovering free amino group via the dissociation of the protonated MEA. Although the above three reactions comprise just a part of the complicated reaction mechanism [18] , they suggest that MEA may absorb CO2 in a molar ratio larger than 0.5, which is in fact consistent with their experimental observations. Thus, the 0.43 as the mole-base A.A. value obtained for the present aqueous PAlAm solution was an unexpected result because the polymer also contains primary amino groups as MEA does. As a possible cause for the poor result, we note the phase separation and/or the gel formation due to the electrostatic cross linking formation among the carbamate anions and the protonated amino groups, which has been reported by several authors [13] [17] [19] [20] . In fact, the much slower absorption into the bulk polymer solution compared with the bulk aqIL, which is easily seen from the respective time courses of the CO2 pressure shown in Figure 3, may be ascribed to the gel formation or phase separation due to CO2 absorption in the former system. Thus, the lower A.A. performance of the PAlAm compared with that of the small amines may be because the gel formation or phase separation partially secluded amino groups in the separated phase, keeping them intact.
This crosslinking reaction of PAlAm via CO2 absorption should also occur in the bulk IL + PAlAm system, and in fact quite a distinguishing absorption behavior, apparently two-step absorption, was observed. Comparing the first and the second absorption “width” (decrements in the CO2 partial pressure) with those of the respective bulk systems (the bulk aqIL and the bulk aq. PAlAm), the first one seems to be almost corresponding to the absorption by the IL and followed by the second one, i.e., absorption by the polymer. Needless to say, since both absorption modes should occur simultaneously, the existence of such a marked kink in the CO2 absorption suggests that the absorption by the polymer was largely retarded in the IL + PAlAm system. As a possible mechanism to effect such retardation, a “preferential reaction” with the IL rather than that with the polymer may be considered. As illustrated in a schema for the CO2 diffusion through the IL + PAlAm system (Figure 4), most CO2 molecules may be “trapped” with the IL amino groups before the reaction with the polymer amino groups. Further, CO2 molecules in the polymer coil, which was depicted as a circle in the figure, would take a much longer time to diffuse through than that through the IL region, because of the crosslinking produced due to the reaction with CO2. Thus, it seems that in the first step (t < ca.1000 min), PAlAm, which was near the surface of the bulk IL + PAlAm solution, could only react with CO2, while most IL molecules reacted with CO2 rather freely, and after the first step period, free access of CO2 to the other polymer amino groups became substantial.
Are the above speculations on the bulk systems consistent with the absorption behavior of the DM consisting of the IL and PAlAm? First of all, one must note that the time course profile of the CO2 partial pressure was smooth and no kink was observed. This may be because the maximum diffusion length of CO2 in the
Figure 4. CO2 diffusion schema for the bulk aqIL containing PAlAm.
liquid phase to reach the innermost amino groups from the surface was much shorter than those in the respective bulk systems; order of 100 μm for the former [12] and ~1 cm for the latter. Thus, the reaction of CO2 with the respective amino groups, i.e., IL and PAlAm, could almost simultaneously proceed in the DM system. In fact, the higher performance of the DM compared with both bulk systems may also be explained by the same reasoning. Namely, the small size (~100 μm) of the DM droplets enabled quick access of CO2 to all the amino groups before the gel formation effectively retarded the CO2 diffusion. Then, the total A.A. in molar base should become higher than that of the bulk system, in which the polymer amino groups were partially inaccessible to CO2 due to the gel formation. Thus, the present experimental results strongly suggest that the shortcoming involved in the bulk (IL + PAlAm) system may be overcome by employing the same system in the DM form.
As for the mass-base A.A., Table 3 tells us the DM was much superior to either of the bulk systems. This comes from the efficient use of water; although water was necessary to improve the CO2 absorption ability of [N2222][Gly], water itself only scarcely absorb the gas. Thus, the large amount of water in the DM just reduced the mass-base A.A. However, the incorporation of the PAlAm into the DM could significantly improve the mass-base A.A. (1.9 vs. 0.95) because the water phase including PAlAm could also work as a substantial CO2 absorbent.
3.3. Recyclability of the DM
Recyclability is one of the prerequisites that should be met by CO2 absorbents for industrial application. In the case of CO2 capture by aq. MEA [10] [21] , the regeneration of the absorbent has been performed by stripping with water vapor at 100˚C - 120˚C. In the present study, CO2 removal was carried out simply by heating CO2-saturated aqIL ([N2222][Gly])-DM samples, which contained 10% PAlA maq. solution, at 50˚C, 80˚C or 100˚C. Then, the regenerated materials were subject to the second CO2 absorption, and the ratio of the resultant A.A. in the molar base to that of the original one obtained before the first heat treatment was used as a measure for the recyclability. The results are summarized on Table 4. Unfortunately all the trials to recycle the DM material proved to be unsuccessful; although the recovery of water content was satisfying to some extent, the A.A. recovery was far less from ca. 82% of aq. MEA solution which was regenerated at 120˚C [10] . As for the reason for the miserably bad results, it may be appropriate to refer to the study by Nagai et al. again [17] . The authors successfully prepared PAlAm hydrogel by crosslinking the polymer amino groups via urea bond under high pressure of CO2 (3.5 MPa) at 170˚C. The same reaction might occur in the DM particles by the heat treatment. Namely, many free amino groups of the polymer were consumed by the irreversible urea bond formation and the resultant gel formation also made the free IL inaccessible to CO2. In fact, a heat treatment at 50˚C for 10 wt% PAlAm solution resulted in a glassy substance as shown in Figure 5 and the solid was swollen but insoluble in water. This observation strongly suggested that some irreversible crosslinking via cova-
Figure 5. Glassy PAlAm after a heat treatment at 50˚C for 1 h.
Table 4. Experimental conditions, absorption abilities, and recoveries after heat treatment at 50˚C, 80˚C and 100˚C for CO2 absorption by PAlAm-containing [N2222][Gly] systems.
*Same data as those given on Table 3.
lent bond formation occurred between the polymer chains. Thus, in order to apply the PAlAm-containing aqIL-DM for industrial use, the irreversible urea group formation due to the heat treatment must be inhibited.
4. Conclusions
In the present study we examined the CO2 absorption performance of several kinds of DMs to find the following results:
1) Powdery DMs were successfully prepared with amino acid-based IL/water mixtures, i.e., [N2222][Gly], [N2222][Ala] as aqueous solutions of ca. 50%, while only soufflé type of DM was obtained for [N2222][Lys] even when the water content was as high as ca.60%;
2) The mol-base CO2 absorption ability was largely dependent on the water content and the IL species;
3) Whereas only a paste-like D-PS was obtained with the 10 wt% PAlAm solution, powdery DM was successfully prepared with [N2222][Gly] containing 10 wt% of the polymer and 60 wt% of water;
4) The aqIL-DM ([N2222][Gly] + PAlAm) showed higher CO2 absorption abilities (especially the mass-base A.A.) than those of DM containing only the IL;
5) The recyclability of the PAlAm-containing aqIL-DM proved to be rather poor, probably because the heat treatment, which was necessary to remove the absorbed CO2 from the absorbent, irreversibly crosslinked the polymer, leading to gelation of the inner phase of the DM particles.
Thus, the present study demonstrated that the amino acid-based aqIL-DM containing PAlAm is a promising absorbent material for CO2. In order to overcome the shortcoming found for the recyclability, it may be effective to use a Dry Gel system in which PAlAm constitutes the gel phase [17] swollen with the aqIL. The results will be soon reported in a forthcoming paper.
Acknowledgements
This work was supported by JSPS KAKENHI grant number JP15K05584.
Compliance with Ethical Standards
Conflict of interest the authors declare that they have no conflict of interest.
Cite this paper
Miyake, M. and Satoh, M. (2017) CO2 Absorption Performance of “Dry Matter” Prepared with Amino Acid-Based Ionic Liquids. Green and Sustainable Chemistry, 7, 203-216. https://doi.org/10.4236/gsc.2017.73016
References
- 1. Yang, Z.-Z., Zhao, Y.-N. and He, L.-N. (2011) CO2 Chemistry: Task-Specific Ionic Liquids for CO2 Capture/Activation and Subsequent Conversion. RSC Advances, 1, 545-567. https://doi.org/10.1039/c1ra00307k
- 2. Zhang, J., Zhang, S., Dong, K., Zhang, Y., Shen, Y. and Lv, X. (2006) Supported Absorption of CO2 by Tetrabutylphosphonium Amino Acid Ionic Liquids. Chemistry: A European Journal, 12, 4021-4026. https://doi.org/10.1002/chem.200501015
- 3. Jiang, Y.-Y., Wang, G.-N., Zhou, Z., Wu, Y.-T., Geng, J. and Zhang, Z.-B. (2008) Tetraalkylammonium Amino Acids as Functionalized Ionic Liquids of Low Viscosity. Chemical Communications, No. 4, 505-507. https://doi.org/10.1039/B713648J
- 4. Yokozeki, A., Shiflett, M.B., Junk, C.P., Grieco, L.M. and Foo, T. (2008) Physical and Chemical Absorption of Carbon Dioxide in Room-Temperature Ionic Liquids. Jounal of Physical Chemistry B, 112, 16654-16663. https://doi.org/10.1021/jp805784u
- 5. Yu, H., Wu, Y.T., Jiang, Y.-Y., Zhou, Z.Z. and Zhang, Z.-B. (2009) Low Viscosity Amino Acid Liquids with Asymmetric Tetraalkylammonium Cations for Fast Absorption of CO2. New Journal of Chemistry, 33, 2385-2390. https://doi.org/10.1039/b9nj00330d
- 6. Goodrich, B.F., de la Fuente, J.C., Gurkan, B.E., Lopez, Z.K., Price, E.A., Huang, Y. and Brennecke, J.F. (2011) Effect of Water and Temperature on Absorption of CO2 by Amine-Functionalized Anion-Tethered Ionic Liquids. Journal of Physical Chemistry B, 115, 9140-9150. https://doi.org/10.1021/jp2015534
- 7. Zhang, X., Zhang, X, Dong, H., Zhao, Z., Zhang, S. and Huang, Y. (2012) Carbon Capture with Ionic Liquids: Overview and Progress. Energy and Environmental Science, 5, 6668-6681. https://doi.org/10.1039/c2ee21152a
- 8. Ramdin, M., de Loos, T.W. and Vlugt, T.J.H. (2012) State-of-the-Art of CO2 Capture with Ionic Liquids. Industrial and Engineering Chemistry Research, 51, 8149-8177. https://doi.org/10.1021/ie3003705
- 9. Niedermaier, I., Bahlmann, M., Papp, C., Kolbeck, C., Wei, W., Calderón, S.K., Grabau, M., Schulz, P.S., Wasserscheid, P., Steinrück, H-P. and Maier, F. (2014) Carbon Dioxide Capture by an Amine Functionalized Ionic Liquid: Fundamental Differences of Surface and Bulk Behavior. Journal of American Chemical Society, 136, 436-441. https://doi.org/10.1021/ja410745a
- 10. Anderson, K., Atkins, M.P., Estager, J., Kuah, Y., Ng, S., Oliferenko, A.A., Plechkova, N.V., Puga, A.V., Seddon, K.R. and Wassell, D.F. (2015) Carbon Dioxide Uptake from Natural Gas by Binary Ionic Liquid-Water Mixtures. Green Chemistry, 17, 4340-4354. https://doi.org/10.1039/C5GC00720H
- 11. Romanos, G.E., Shulz, P.S., Bahlmann, M., Wasserscheid, P., Sapalidis, A., Katsaros, F.K., Athanasekou, C.P., Beltsios, K. and Kanellopoulos, N.K. (2014) CO2 Capture by Novel Supported Ionic Liquid Phase Systems Consisting of Silica Nanoparticles Encapsulating Amine-Functionalized Ionic Liquids. Journal of Physical Chemistry C, 118, 24437-24451. https://doi.org/10.1021/jp5062946
- 12. Shirato, K. and Satoh, M. (2011) “Dry Ionic Liquid” as a New Comer to “Dry Matter”. Soft Matter, 7, 7191-7193. https://doi.org/10.1039/c1sm05999h
- 13. Ishihara, M., Miyake, M. and Satoh, M. (2016) CO2 Absorption by “Dry Ionic Liquids”. Green and Sustainable Chemistry, 6, 167-181. https://doi.org/10.4236/gsc.2016.64016
- 14. Shiflett, M.B., Drew, D.W., Cantini, R.A. and Yokozeki, A. (2010) Carbon Dioxide Capture Using Ionic Liquid 1-Butyl-3-Methylimidazolium Acetate. Energy Fuels, 24, 5781-5789. https://doi.org/10.1021/ef100868a
- 15. Cadena, C., Anthony, J.L., Shah, J.K., Morrow, T.I., Brennecke, J.F. and Maginn, E.J. (2004) Why Is CO2 So Soluble in Imidazolium-Based Ionic Liquids? Journal of American Chemical Society, 126, 5300-5308. https://doi.org/10.1021/ja039615x
- 16. Zhang, F., Fang, C.-G., Wu, Y.-T., Wang, Y.-T., Li, A.-M. and Zhang, Z.-B. (2010) Absorption of CO2 on the Aqueous Solutions of Functionalized Ionic Liquids and MDEA. Chemical Engineering Journal, 160, 691-697. https://doi.org/10.1016/j.cej.2010.04.013
- 17. Nagai, D., Suzuki, A. and Kuribayashi, A. (2011) Synthesis of Hydrogels from Polyallylamine with Carbon Dioxide as Gallant: Development of Reversible CO2 Absorbent. Macromolecular Rapid Communications, 32, 404-410. https://doi.org/10.1002/marc.201000601
- 18. Aboudheir, A., Tontiwachwuthikul, P., Chakma, A. and Idem, R. (2003) Kinetics of the Reactive Absorption of Carbon Dioxide in High CO2-Loaded, Concentrated Aqueous Monoethanolamine Solutions. Chemical Engineering Science, 58, 5195-5210. https://doi.org/10.1016/j.ces.2003.08.014
- 19. Portugal, A.F., Sousa, J.M., Magalhães, F.D. and Mendes, A. (2009) Solubility of Carbon Dioxide in Aqueous Solutions of Amino Acid Salts. Chemical Engineering Science, 64, 1993-2002. https://doi.org/10.1016/j.ces.2009.01.036
- 20. George, M. and Weiss, R.G. (2003) Primary Alkyl Amines as Latent Gelators and Their Organogel Adducts with Neutral Triatomic Molecules. Langmuir, 19, 1017-1025. https://doi.org/10.1021/la026639t
- 21. Rochelle, G.T. (2009) Amine Scrubbing for CO2 Capture. Science, 325, 1652-1654. https://doi.org/10.1126/science.1176731







