World Journal of Nano Science and Engineering
Vol.3 No.4(2013), Article ID:40512,7 pages DOI:10.4236/wjnse.2013.34018
Effective Photocatalytic Reduction of Cr(VI) by Carbon Modified (CM)-n-TiO2 Nanoparticles under Solar Irradiation
1Marine Chemistry Department, Faculty of Marine Sciences, King Abdulaziz University, Jeddah, KSA
2National Institute of Oceanography & Fisheries, Qayet Bay, Alexandria, Egypt
Email: yasrsh@yahoo.com
Copyright © 2013 Yasser A. Shaban. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received August 23, 2013; revised September 20, 2013; accepted September 27, 2013
Keywords: Photocatalytic reduction; Chromium; Titanium oxide; Carbon modification
ABSTRACT
Photocatalytic reduction of toxic Cr(VI) was successfully achieved using carbon modified titanium oxide (CM-n-TiO2) nanoparticles under natural sunlight illumination. Modification of titanium oxide by carbon significantly enhanced the photocatalytic reduction of Cr(VI) under natural sunlight irradiation. The effects of various experimental parameters such as catalyst dose, initial concentration of Cr(VI), and solution pH on the reduction rate of Cr(VI) were investigated. The highest reduction rate of Cr(VI) was obtained at the optimal conditions of pH 5 and 2.0 g·L−1 of CM-n-TiO2. Interestingly, in the presence of phenol, as a sacrificial electron donor, the rate of Cr(VI) reduction was nearly 1.7 times higher than in its absence. The solar photoreduction of Cr(VI) in aqueous solution using CM-n-TiO2 obeyed a pseudo-first order kinetics according to the Langmuir-Hinshelwood model.
1. Introduction
The discharge of toxic heavy metals into aquatic environment has been known to cause serious pollution problems. Among these pollutants, chromium possesses the most severe environmental concern due to its high toxicity, potential carcinogenicity, and high mobility in water [1,2]. The major sources of chromium pollution are electrochemical, steel manufacturing industries and leather tanning [3,4]. In aquatic environments, chromium exists in hexavalent (Cr(VI)) and trivalent (Cr(III)) forms, of which hexavalent form is more toxic than the trivalent one, and is known to be human carcinogen [5]. Different techniques have been reported for the treatments of Cr (VI), such as ion exchange [6], membrane separation [7], physical and biological adsorption [8-10], and electrocoagulation [11,12]. However, most of these techniques have several limitations and drawbacks, and they require either high energy or massive use of reducing agents.
Semiconductor photocatalysis has attracted considerable interest as an effective and economical technique for detoxification of polluted waters [13-22]. It can effectively reduce highly toxic Cr(VI) into the less harmful Cr(III), which can then be precipitated as Cr(OH)3 in neutral or alkaline solutions [23]. Titanium dioxide photocatalyst was considered as one of the most practical candidates due to its optical and electronic properties, low cost, high level of photocatalytic activity, chemical stability and non-toxicity. However, its wide band gap (3.0 - 3.2 eV) limits its photoresponse in the ultraviolet region which is only a small fraction (~5%). Therefore, much attention has been devoted to enhancing its catalytic efficiency or expanding its applicability under solar irradiation. Recently, carbon modification of n-TiO2 has been proved to be an effective approach to enhance its catalytic efficiency [21,22,24-26].
Most of the studies on heterogeneous photocatalytic reduction of Cr(VI) using n-TiO2 were performed under illumination of UV light. Therefore, in this study, visible light active carbon-modified (CM)-n-TiO2 nanoparticles were prepared via sol-gel method. The photocatalytic performance of CM-n-TiO2 was examined for the photoreduction of Cr(VI) in aqueous solution under illuminetion of natural sunlight. The photocatalytic activity of CM-n-TiO2 was compared with regular n-TiO2. The effects of various experimental parameters such as photocatalyst loading, Cr(VI) concentration, and pH on the photocatalytic removal rate of Cr(VI) were studied. Additionally, the effect of presence of phenol, as a sacrificial electron donor, on the photocatalytic reduction of Cr(VI) was also investigated.
2. Experimental
2.1. Synthesis and Characterization of n-TiO2 and CM-n-TiO2 Nanoparticles
Visible light active carbon modified titanium dioxide (CM-n-TiO2) nanoparticals were synthesized by a sol-gel method using titanium butoxide (Ti[O(CH2)3CH3]4, Fluka, 97%), a carbon-containing precursor, as a molecular precursor of TiO2 as well as a carbon source. Regular (unmodified) titanium dioxide (n-TiO2) nanoparticles were synthesized by hydrolysis and oxidation of titanium trichloride (TiCl3 12% in hydrochloric acid (5% - 12%), Sigma-Aldrich) in an aqueous medium. Details on the procedures used for catalysts preparation and characterization can be found elsewhere [22].
2.2. Photocatalytic Experiments
All solar photocatalytic experiments were carried out at the Faculty of Marine sciences, Obhur, Jeddah, KSA, in the daytime between 11:00 am to 15:00 pm, during MayJune, 2013. Experimental set up consisted of a magnetically stirred 500 mL glass photoreactor loaded with the aqueous solution containing different concentrations of Cr(VI) ranging from 1 to 10 ppm, then the synthesized photocatalyst (n-TiO2 or CM-n-TiO2) was added. The photocatalytic reactor was then directly exposed to natural sunlight. The average solar intensity was about 1200 W m−2, measured by Field Scout Light Sensor Reader (Spectrum Technologies, Inc.) equipped with 3670i Silicon Pyranometer Sensor. Prior to analysis, aliquots of treated samples were regularly withdrawn from the reactor and centrifuged immediately to remove the catalyst. The Cr(VI) was determined colorimetrically at 540 nm using a Shimadzu UV-VIS Spectrophotometer (Model PharmaSpec UV-1700) according to the diphenylcarbazide colorimetric method [27].
3. Results and Discussion
3.1. Photocatalytic Activity of n-TiO2 and CM-n-TiO2
Figure 1 compares the photoreduction efficiency of 3.0 ppm of Cr(VI) under illumination of real sunlight in the presence of 2.0 g·L−1 of CM-n-TiO2 and n-TiO2, respectively. It is clearly observed that the photocatalytic activity of CM-n-TiO2 is higher than that of n-TiO2 under sunlight irradiation. Complete photoreduction of 3.0 ppm of Cr (VI) was achieved after only 10 min using 2.0 g/L
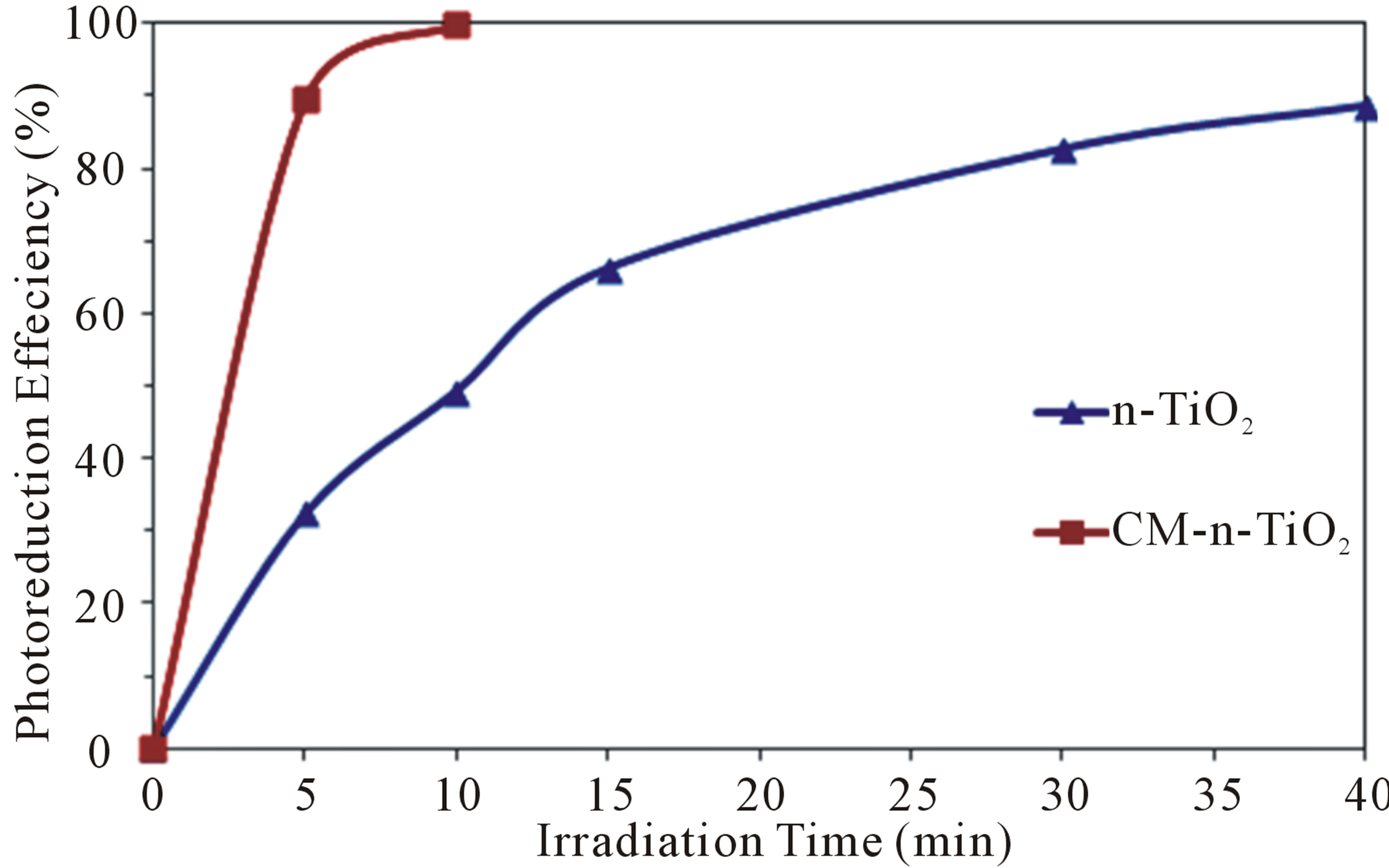
Figure 1. Photoreduction of 3 ppm of Cr(VI) in the presence of 2.0 g·L−1 of CM-n-TiO2 and n-TiO2 under illumination of natural sunlight.
CM-n-TiO2. After the same irradiation time, only 49.25% of the same concentration of Cr was removed using regular n-TiO2 under the same experimental conditions. The observed enhanced photocatalytic activity of carbon modified CM-n-TiO2 nanoparticles, can be attributed to carbon modification of TiO2 during the synthesis process [21,22,24-26].
3.2. Effect of Solution pH
pH of the solution plays a major role in the photocatalytic reaction as it is known to influence the surface charge of the semiconductor thereby affecting the interfacial electron transfer and the photoredox process [28]. The possible functional groups on TiO2 surface in water are , TiOH, and TiO−. The point of zero charge (pHpzc) of TiO2 is an important factor determining the distribution of the surface groups. The surface of TiO2 is negatively charged with the species TiO− when pH is higher than pHpzc. On the contrary, it is positively charged with the species
, TiOH, and TiO−. The point of zero charge (pHpzc) of TiO2 is an important factor determining the distribution of the surface groups. The surface of TiO2 is negatively charged with the species TiO− when pH is higher than pHpzc. On the contrary, it is positively charged with the species . Figure 2 depicts the effect of pH on the photocatalytic reduction rate of Cr(VI). It is worth noting that, the removal rate of Cr(VI) decreased with increasing the initial pH value from 5 to 9 (Figure 2(b)). The higher removal efficiency at pH 5, which is lower than the point of zero charge (pHpzc) of TiO2, can be attributed to the strong electrostatic attraction between the anionic chromate species (
. Figure 2 depicts the effect of pH on the photocatalytic reduction rate of Cr(VI). It is worth noting that, the removal rate of Cr(VI) decreased with increasing the initial pH value from 5 to 9 (Figure 2(b)). The higher removal efficiency at pH 5, which is lower than the point of zero charge (pHpzc) of TiO2, can be attributed to the strong electrostatic attraction between the anionic chromate species (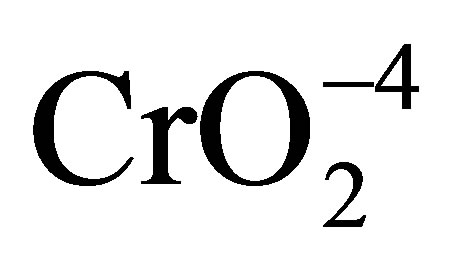 and/or HCrO−4) and the positively charged
and/or HCrO−4) and the positively charged  surface [29]. On the other hand, at higher pH value the TiO2 surface is negatively charged in a state of TiO− and
surface [29]. On the other hand, at higher pH value the TiO2 surface is negatively charged in a state of TiO− and  becomes dominate species, which may be repelled away from the TiO2 surface which subsequently leads to inhibited adsorption of Cr(VI). Furthermore, Cr(III) will be precipitated on the surface of TiO2 as Cr(OH)3 at increased solution pH, covering the activity sites of the catalyst [30]. As a result, Cr(VI) the photoreduction efficiency decreases in alkaline medium.
becomes dominate species, which may be repelled away from the TiO2 surface which subsequently leads to inhibited adsorption of Cr(VI). Furthermore, Cr(III) will be precipitated on the surface of TiO2 as Cr(OH)3 at increased solution pH, covering the activity sites of the catalyst [30]. As a result, Cr(VI) the photoreduction efficiency decreases in alkaline medium.
 (a)
(a)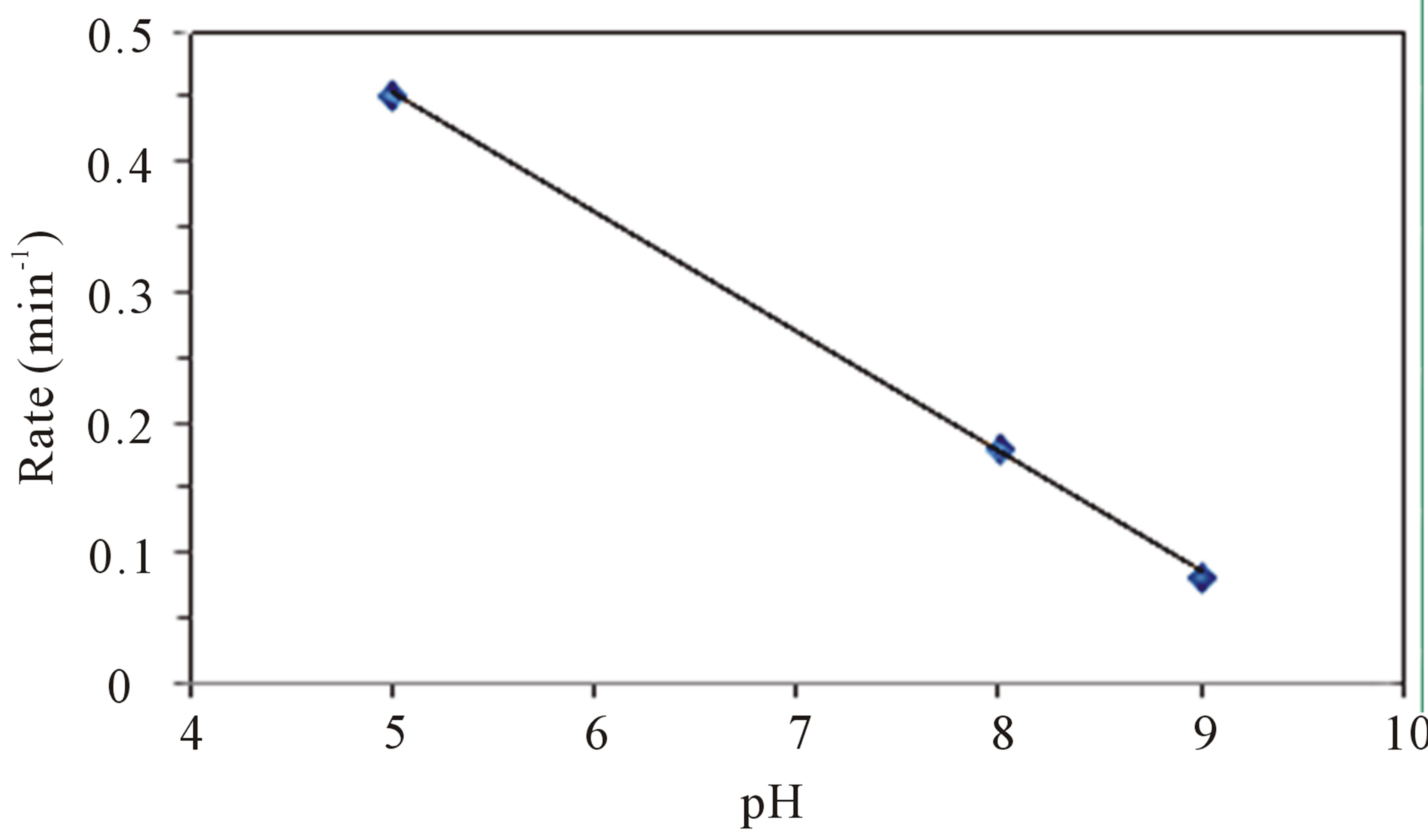 (b)
(b)
Figure 2. (a) Effect of pH on the photoreduction of 5 ppm of Cr(VI) in the presence of 2.0 g·L−1 of CM-n-TiO2 under illumination of natural sunlight; (b) Rate of photoreduction of 5 ppm of Cr(VI) as a function of pH in the presence of 2.0 g·L−1 of CM-n-TiO2.
3.3. Effect of Catalyst Loading
In a typical photocatalytic experiment, it is essential to determine the minimum amount of catalyst required to remove the maximum amount of pollutants. Therefore, the influence of CM-n-TiO2 dose on photoreduction of Cr (VI) was investigated in order to attain the maximum absorption of efficient solar light photons as well as to avoid an ineffective excess amount of the photocatalyst. Figure 3" target="_self"> Figure 3 shows the photoreduction rate of 3 ppm of Cr (VI) at the optimum pH 5 using various amounts of CM-n-TiO2 under illumination of natural sunlight. The photocatalytic reduction rate of Cr(VI) increased with the increase in catalyst dose from 0.5 to 2.0 g·L−1. Further increment in catalyst loading to 2.5 g·L−1 leads to decrease in photoreduction efficiency. The observed increment in the photoreduction efficiency at the catalyst concentration of 2.0 g·L−1 is due to the increase of the total number of active sites on the photocatalyst surface thus causing an increase in the number of e− which can take part in the photoreduction of Cr(VI). At catalyst loading beyond the optimum, the tendency toward particles aggregation increases, resulting in a reduction in
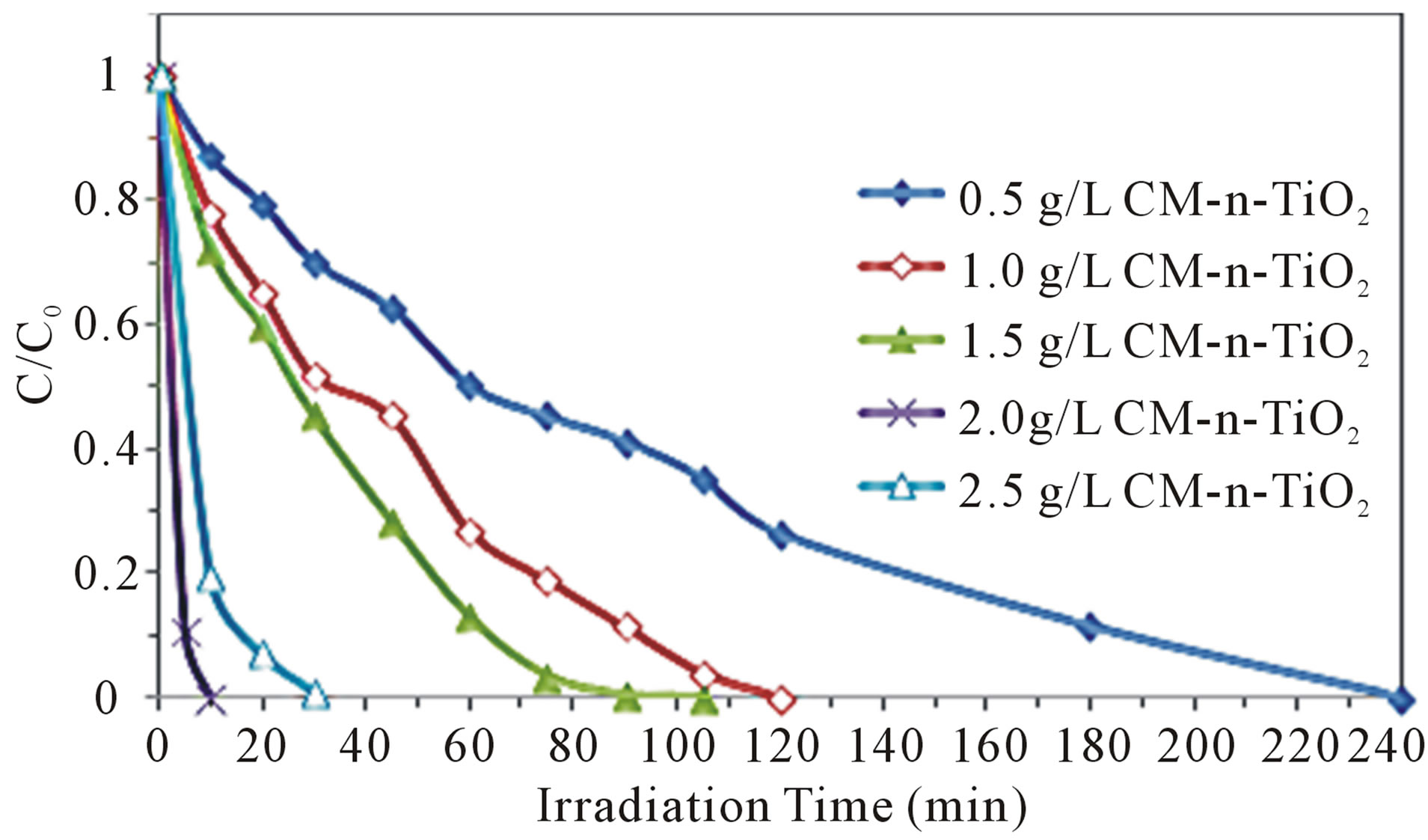
Figure 3. Effect of CM-n-TiO2 loading on photoreduction of 3 ppm of Cr(VI) under illumination of natural sunlight.
surface area available for light absorption and hence a drop in photocatalytic degradation rate [31]. Furthermore, the increase of the turbidity of the suspension reduces light penetration due to the enhancement of light scattering; the result is the decrease of the number of activated sites on the TiO2 surface and shrinking of the effective photoactivated volume of suspension. The interaction of these two processes resulted in a reduced performance of photocatalytic performance with the overloaded catalyst [32].
3.4. Effect of Initial Cr(VI) Concentration
The effect of initial Cr(VI) concentration on its photoreduction rate was investigated over the range of 1 to 10 ppm at the optimal conditions of pH 5 and 2.0 g·L−1 of CM-n-TiO2 (Figure 4). It is clearly noted that the irradiation time required for complete removal of Cr(VI) under natural sunlight was extended as the initial Cr(VI) concentration increased. This can be rationalized according to Beer-Lambert’s law, as Cr(VI) concentration increases, the path length of photons entering into the reaction mixture decreases, and a fewer photons reach the catalyst surface. As the incident intensity, catalyst amount and irradiation time are constant, therefore, the availability of active sites will be reduced. Consequently, the photoreduction rate of Cr(VI) decreases as the concentration increases [33,34]. Moreover, an increase in Cr(VI) concentration can lead to the saturation of the limited number of accessible active sites on the photocatalyst surface and/or deactivation of the active sites of the catalyst, resulting in a reduction in the photoreduction rate.
3.5. Kinetic Studies
Typically, the Langmuir-Hinshelwood (L-H) model is usually used to describe the photocatalytic removal of inorganic and organic pollutants [35-38]. It basically relates the degradation rate (r) and reactant concentration in water at time t (C), which is expressed as follows:
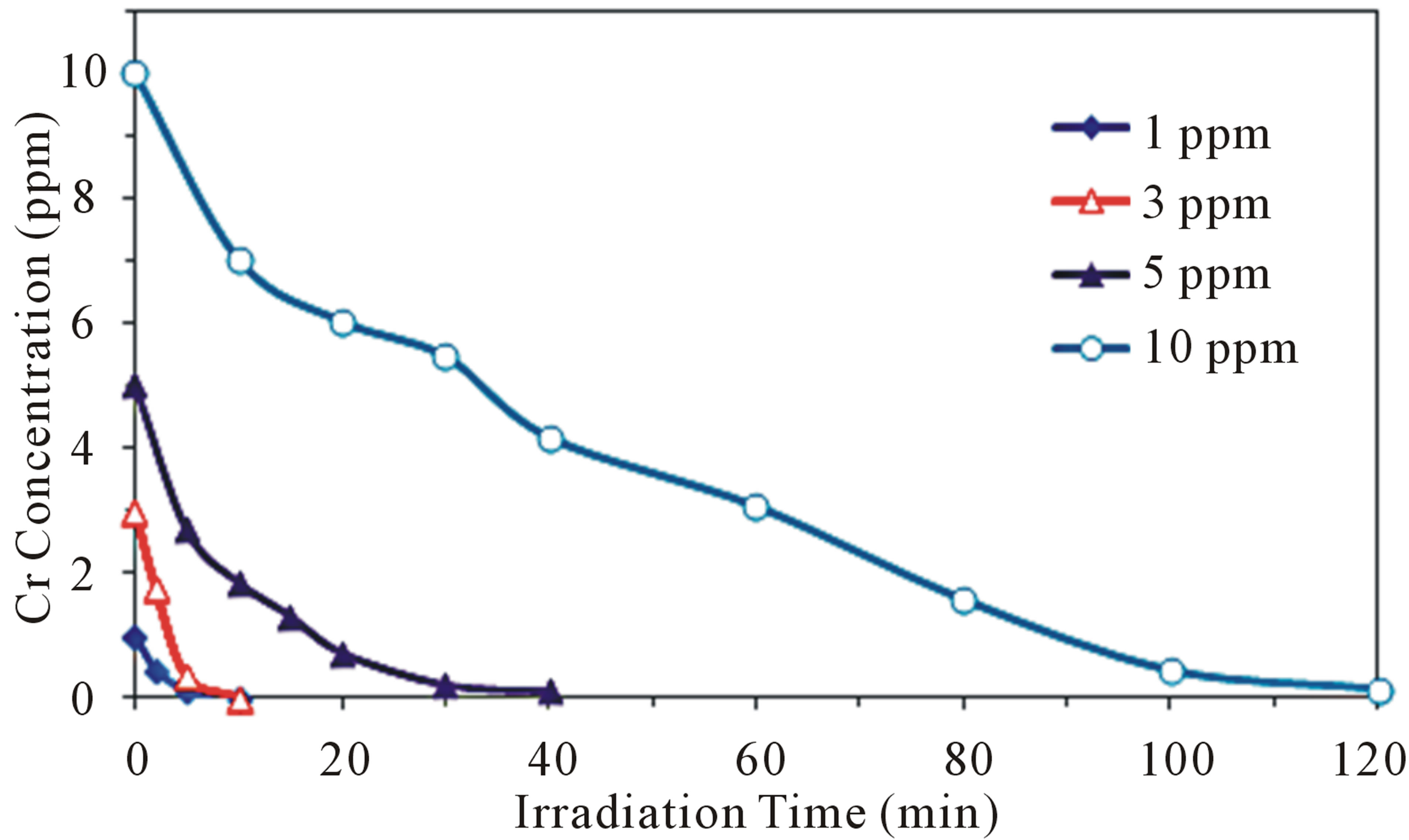
Figure 4. Effect of initial concentration of Cr(VI) on its photoreduction at optimal conditions of pH 5 and 2.0 g·L−1 CM-n-TiO2 under natural sunlight.
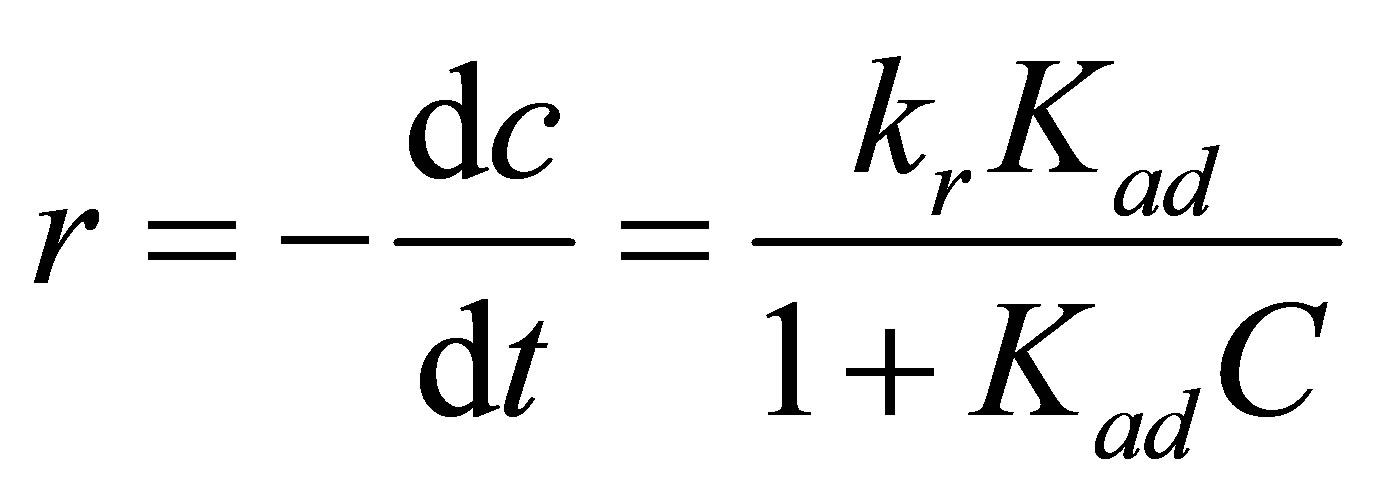 (1)
(1)
where kr is the rate constant and Kad is the adsorption equilibrium constant. When the adsorption is relatively weak and/or the reactant concentration is low, Equation (1) can be simplified to the pseudo-first order kinetics with an apparent first-order rate constant kapp:
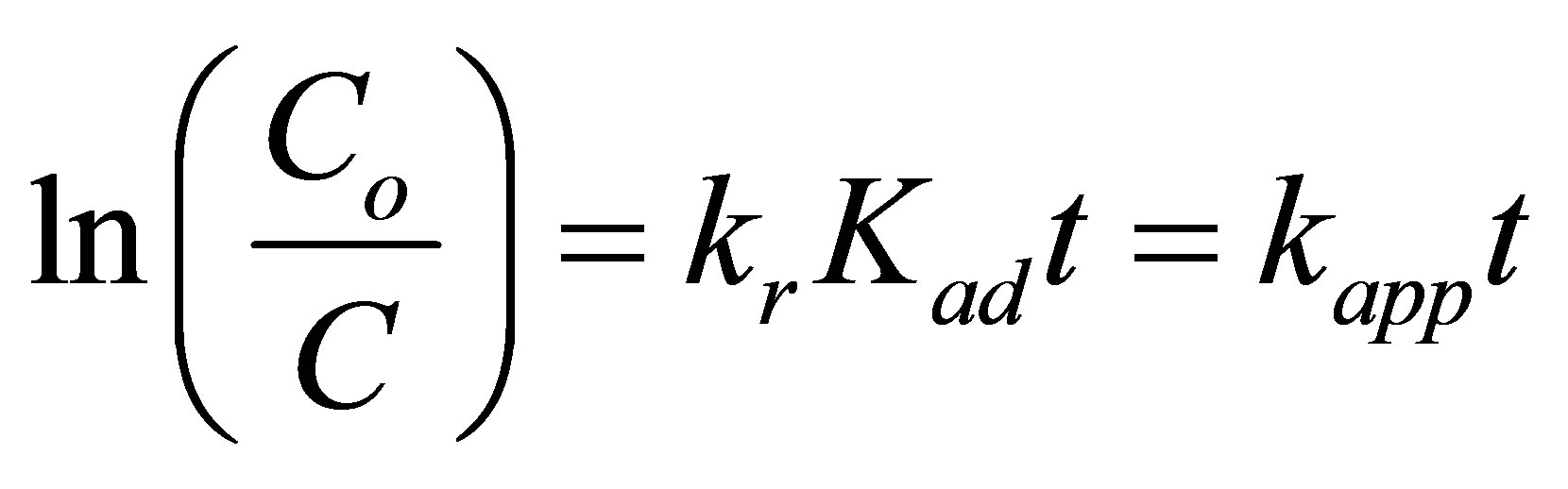 (2)
(2)
where Co denotes the initial concentration. Plotting ln (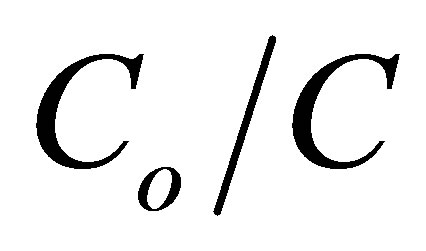 ) versus illumination time (t) yields a straight line, and the slope is the apparent rate constant kapp. The kinetic experiments for the photoreduction of various concentrations of Cr(VI) ranging between 1 and 10 ppm were conducted at the optimal conditions of pH 5 and 2.0 g·L−1 of CM-n-TiO2 under illumination of sunlight. The photocatalytic reduction of Cr(VI) was successfully fitted using L-H model, and can be described by the pseudofirst order kinetic, as confirmed by the obtained straight line (Figure 5). Similar observation was reported for the photoreduction of Cr(VI) by Ku and Jung [39].
) versus illumination time (t) yields a straight line, and the slope is the apparent rate constant kapp. The kinetic experiments for the photoreduction of various concentrations of Cr(VI) ranging between 1 and 10 ppm were conducted at the optimal conditions of pH 5 and 2.0 g·L−1 of CM-n-TiO2 under illumination of sunlight. The photocatalytic reduction of Cr(VI) was successfully fitted using L-H model, and can be described by the pseudofirst order kinetic, as confirmed by the obtained straight line (Figure 5). Similar observation was reported for the photoreduction of Cr(VI) by Ku and Jung [39].
3.6. Effect of Phenol
Figure 6 shows the kinetic analysis of Cr(VI) reduction in the absence and presence of phenol. Interestingly, the presence of phenol (1 ppm) significantly promoted the reduction rate of 5 ppm of Cr(VI) by nearly 1.75 times higher than in its absence. This synergism is based on the photogenerated electrons and holes on the surface of n-TiO2 semiconductor. Illumination of TiO2 by light with energy (hυ) greater than or equal to the bandgap energy (Eg) of TiO2 elevates electron in the valence band (VB) to the conduction band (CB), and a positive hole is formed in the valence band (Equation (3)). At the external TiO2 surface, the positive hole and the excited electron either recombine or become involved in redox reactions with ad-
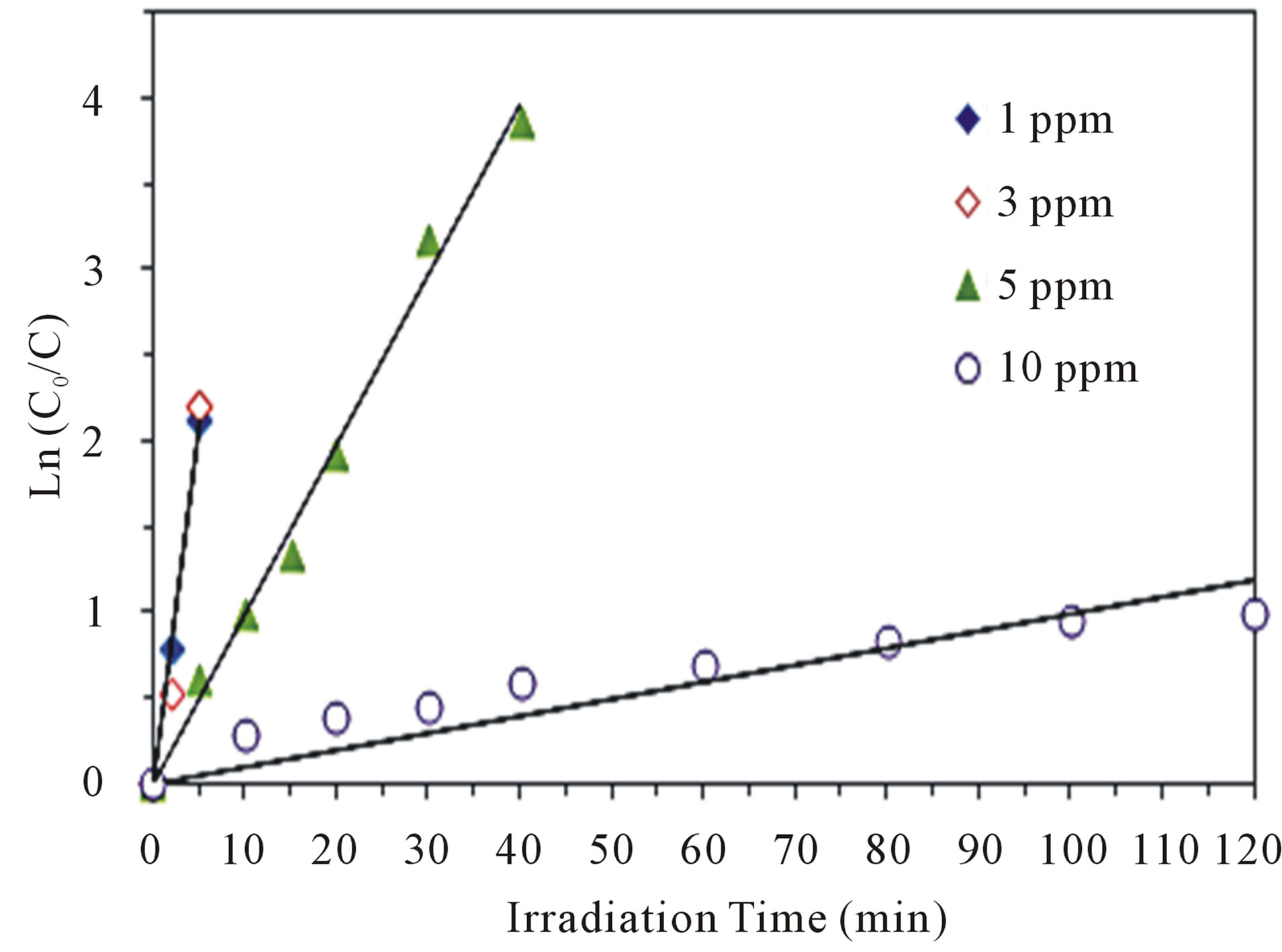
Figure 5. Plot of ln(Co/C) versus illumination time for the photoreduction of Cr(VI) at optimal conditions of pH 5 and 2.0 g·L−1 CM-n-TiO2 under natural sunlight.
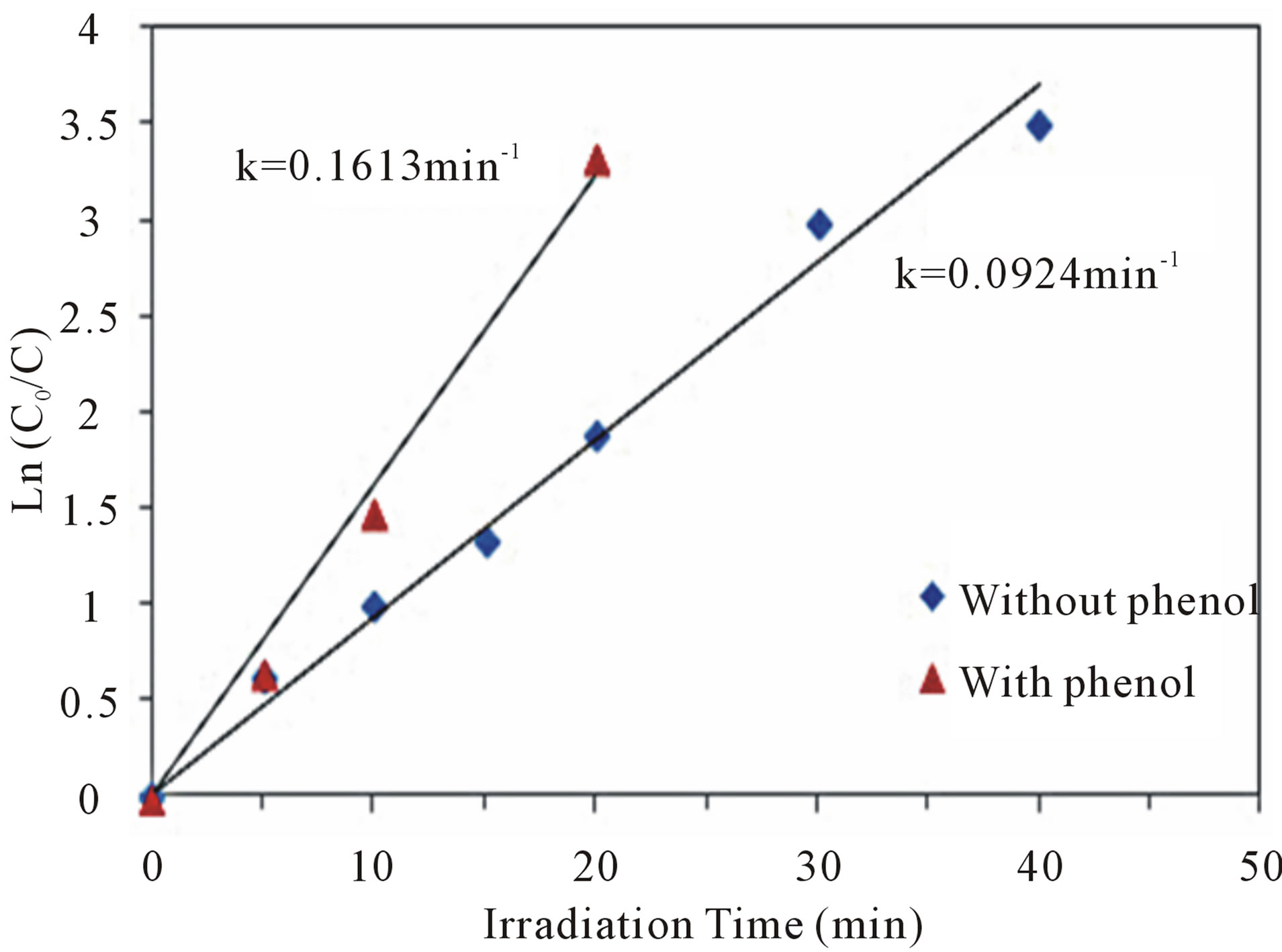
Figure 6. Kinetics of photocatalytic reduction of Cr(VI) in absence and presence of phenol at optimal conditions of pH 5 and 2.0 g·L−1 CM-n-TiO2 under natural sunlight.
sorbed groups.
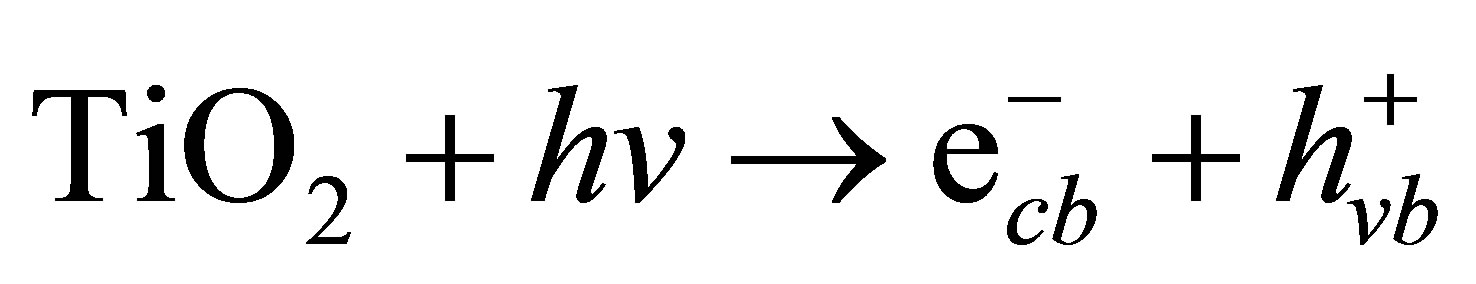 (3)
(3)
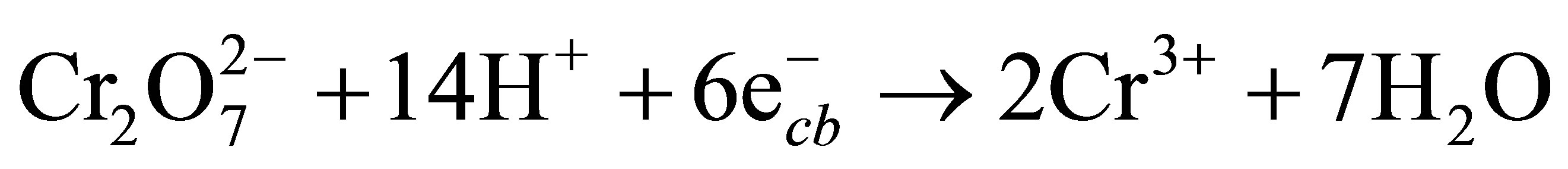 (4)
(4)
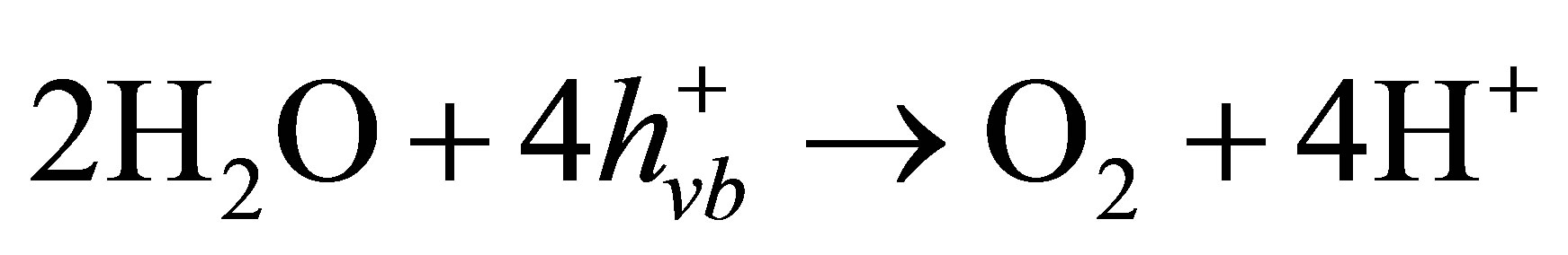 (5)
(5)
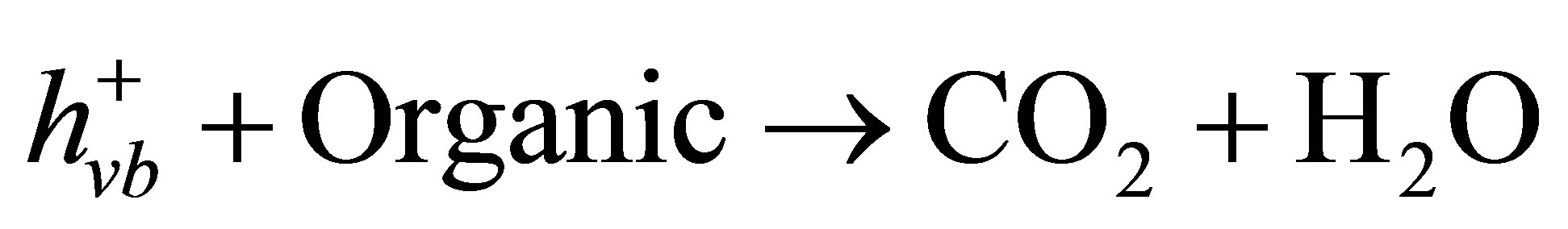 (6)
(6)
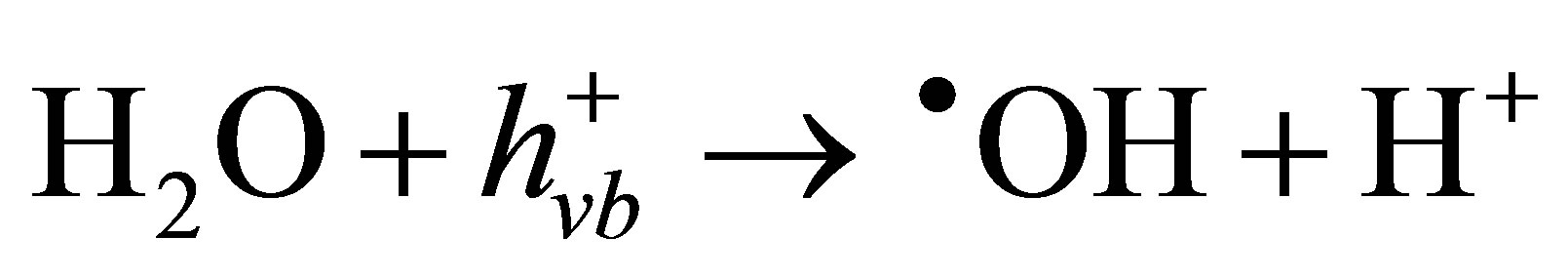 (7)
(7)
 (8)
(8)
The conduction band electrons 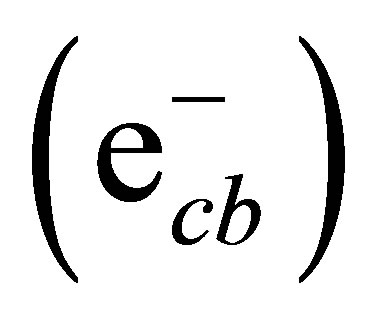 can reduce Cr(VI) to Cr(III) (Equation (4)) [40,41], and the valence band hole (
can reduce Cr(VI) to Cr(III) (Equation (4)) [40,41], and the valence band hole (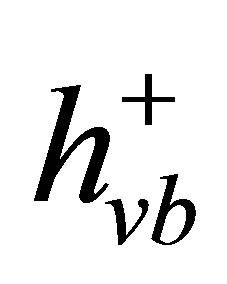 ) can oxidize water to generate O2 (Equation (5)). Therefore, the net photocatalytic reaction is the three-electron-reduction of Cr(VI) to Cr(III) with oxidation of water to oxygen, which is a kinetically slow fourelectron process [40,42]. Accordingly, the photocatalytic reduction of Cr(VI) alone is quite slow. Furthermore, the photo-generated electrons and holes can recombine in bulk or on surface of the semiconductor within a very short time, resulting in a reduction in the photocatalytic efficiency. Therefore, adding electron donors (sacrificial reagents or hole scavengers) to react irreversibly with the photo-generated VB holes (
) can oxidize water to generate O2 (Equation (5)). Therefore, the net photocatalytic reaction is the three-electron-reduction of Cr(VI) to Cr(III) with oxidation of water to oxygen, which is a kinetically slow fourelectron process [40,42]. Accordingly, the photocatalytic reduction of Cr(VI) alone is quite slow. Furthermore, the photo-generated electrons and holes can recombine in bulk or on surface of the semiconductor within a very short time, resulting in a reduction in the photocatalytic efficiency. Therefore, adding electron donors (sacrificial reagents or hole scavengers) to react irreversibly with the photo-generated VB holes (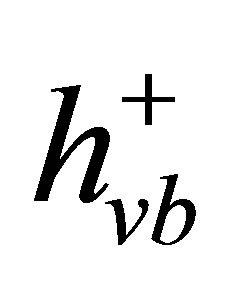 ), thereby suppressing the electron-hole recombination [43,44]. If this sacrificial agent is an organic pollutant present in water or wastewater, the positive hole (
), thereby suppressing the electron-hole recombination [43,44]. If this sacrificial agent is an organic pollutant present in water or wastewater, the positive hole (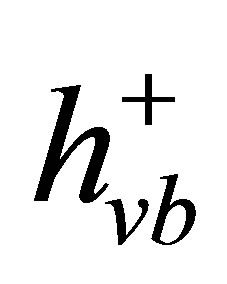 ) would oxidize either pollutant directly (Equation (6)) or water to produce hydroxyl radicals (•OH) which are strong oxidizing agents (Equation (7)). Therefore, in the presence of phenol, as a sacrificial electron donor, the photogenerated holes are rapidly scavenged from the TiO2 particles, suppressing electron-hole recombination on CM-n-TiO2 and accelerating the reduction of Cr(VI) by photogenerated electron [45]. It has been reported that the presence of organic species as sacrificial electron donor can accelerate the photocatalytic reduction of Cr(VI) [4,46,47]. A simplified diagram illustrating the simultaneous photocatalytic reduction of Cr(VI) and degradation of phenol using CM-n-TiO2 under illumination of sunlight is presented in Figure 7.
) would oxidize either pollutant directly (Equation (6)) or water to produce hydroxyl radicals (•OH) which are strong oxidizing agents (Equation (7)). Therefore, in the presence of phenol, as a sacrificial electron donor, the photogenerated holes are rapidly scavenged from the TiO2 particles, suppressing electron-hole recombination on CM-n-TiO2 and accelerating the reduction of Cr(VI) by photogenerated electron [45]. It has been reported that the presence of organic species as sacrificial electron donor can accelerate the photocatalytic reduction of Cr(VI) [4,46,47]. A simplified diagram illustrating the simultaneous photocatalytic reduction of Cr(VI) and degradation of phenol using CM-n-TiO2 under illumination of sunlight is presented in Figure 7.
4. Conclusion
The photocatalytic reduction of Cr(VI) in aqueous solution was successfully achieved using carbon-modified (CM)-n-TiO2 nanoparticles under irradiation of natural sunlight. CM-n-TiO2 nanoparticles exhibited significantly enhanced photocatalytic efficiency compared to unmodified n-TiO2. This observed enhancement in the photoactivity can be attributed to the carbon modification. The photoreduction of Cr(VI) was favorable at pH 5 and optimal catalyst dose of 2.0 g·L−1. The presence of phenol significantly promoted the reduction rate of Cr(VI) by nearly 1.75 times higher than in its absence. The solar photocatalytic removal of Cr(VI) in aqueous solution

Figure 7. Simultaneous photocatalytic reduction of Cr(VI) and degradation of phenol using CM-n-TiO2 under illumination of sunlight.
using CM-n-TiO2 obeyed a pseudo-first order kinetics according to the Langmuir-Hinshelwood model.
5. Acknowledgements
The author is thankful to Mr. Mousa Al Zobidi for his valuable help in the experimental analysis.
REFERENCES
- C. Lee and P. Linneman, S. Rengaraj, S. Venkataraj, J. Yeon, Y. Kim, X. Z. Li and G. K. H. Pang, “Preparation, Characterization and Application of Nd-TiO2 Photocatalyst for the Reduction of Cr(VI) under UV Light Illumination,” Applied Catalysis B: Environmental, Vol. 77, No. 1-2, 2007, pp. 157-165. http://dx.doi.org/10.1016/j.apcatb.2007.07.016
- S. Deng and R. Bai, “Removal of Trivalent and Hexavalent Chromium with Aminated Polyacrylonitrile Fibers: Performance and Mechanisms,” Water Research, Vol. 38, No. 9, 2004, pp. 2424-2432. http://dx.doi.org/10.1016/j.watres.2004.02.024
- R. J. Kieber, J. D. Willey and S. D. Zvalaren, “Chromium Speciation in Rainwater: Temporal Variability and Atmospheric Deposition,” Environmental Science and Technology, Vol. 36, No. 24, 2002, pp. 5321-5327. http://dx.doi.org/10.1021/es020777n
- J. J. Testa, M. A. Grela and M. I. Litter, “Heterogeneous Photocatalytic Reduction of Chromium(VI) over TiO2 Particles in the Presence of Oxalate: Involvement of Cr(V) Species,” Environmental Science and Technology, Vol. 38, No. 5, 2004, pp. 1589-1594. http://dx.doi.org/10.1021/es0346532
- H. J. Gibb, P. S. Lecs, P. F. Pinsky and B. C. Rooney, “Lung Cancer among Workers in Chromium Chemical Production,” American Journal of Industrial Medicine, Vol. 38, No. 2, 2000, pp. 115-126. http://dx.doi.org/10.1002/1097-0274(200008)38:2<115::AID-AJIM1>3.0.CO;2-Y
- Y. Xing, X. Chen and D. Wang, “Electrically Regenerated Ion Exchange for Removal and Recovery of Cr(VI) from Wastewater,” Environmental Science and Technology, Vol. 41, No. 4, 2007, pp. 1439-1443. http://dx.doi.org/10.1021/es061499l
- R. Guell, E. Antico, V. Salvado and C. Fontas, “Efficient Hollow Fiber Supported Liquid Membrane System for the Removal and Preconcentration of Cr(VI) at Trace Levels,” Separation and Purification Technology, Vol. 62, No. 2, 2008, pp. 389-393. http://dx.doi.org/10.1016/j.seppur.2008.02.015
- S. Mor, K. Ravindra and N. R. Bishnoi, “Adsorption of Chromium from Aqueous Solution by Activated Alumina and Activated Charcoal,” Bioresource Technology, Vol. 98, No. 4, 2007, pp. 954-957. http://dx.doi.org/10.1016/j.biortech.2006.03.018
- M. E. R. Carmona, M. A. P. da Silva and S. G. F. Leite, “Biosorption of Chromium Using Factorial Experimental Design,” Process Biochemistry, Vol. 40, No. 2, 2005, pp. 779-788. http://dx.doi.org/10.1016/j.procbio.2004.02.024
- M. Kobya, “Removal of Cr(VI) from Aqueous Solutions by Adsorption onto Hazelnut Shell Activated Carbon: Kinetic and Equilibrium Studies,” Bioresource Technology, Vol. 91, No. 3, 2004, pp. 317-321. http://dx.doi.org/10.1016/j.biortech.2003.07.001
- I. Heidmann and W. Calmano, “Removal of Zn(II), Cu(II), Ni(II), Ag(I) and Cr(VI) Present in Aqueous Solutions by Aluminium Electrocoagulation,” Journal of Hazardous Materials, Vol. 152, No. 3, 2008, pp. 934-941. http://dx.doi.org/10.1016/j.jhazmat.2007.07.068
- P. Gao, X. Chen, F. Shen and G. Chen, “Removal of Chromium (VI) from Wastewater by Combined Electrocoagulation-Electroflotation without a Filter,” Separation and Purification Technology, Vol. 43, No. 2, 2005, pp. 117-123. http://dx.doi.org/10.1016/j.seppur.2004.10.008
- J.-K. Yang and S.-M. Lee, “Removal of Cr(VI) and Humic Acid by Using TiO2 Photocatalysis,” Chemosphere, Vol. 63, No.10, 2006, pp. 1677-1684. http://dx.doi.org/10.1016/j.chemosphere.2005.10.005
- C. Burda, Y. Lou, X. Chen, A. C. S. Samia, J. Stout and J. L. Gole, “Enhanced Nitrogen Doping in TiO2 Nanoparticles,” Nano Letters, Vol. 3, No. 8, 2003, pp. 1049-1051. http://dx.doi.org/10.1021/nl034332o
- T. Oppenlander, “Photochemical Purification of Water and Air,” Wiley-VCH, Weinheim, 2003.
- A. A. C. Magalhães, D. L. Nunes, P. A. Robles-Dutenhefner and E. M. B. De Sousa, “Catalytic Activity of Porous TiO2 Obtained by Sol-Gel Process in the Degradation of Phenol,” Journal of Non-Crystalline Solids, Vol. 348, No. 1-2, 2004, pp. 185-189. http://dx.doi.org/10.1016/j.jnoncrysol.2004.08.166
- S. Parsons, “Advanced Oxidation Processes for Water and Wastewater Treatment,” IWA Publishing, Cornwall, 2004.
- R. M. Mohamed, A. A. Ismail, I. Othman and I. A. Ibrahim, “Preparation of TiO2-ZSM-5 Zeolite for Photodegradation of EDTA,” Journal of Molecular Catalysis A: Chemical, Vol. 238, No. 1-2, 2005, pp. 151-157. http://dx.doi.org/10.1016/j.molcata.2005.05.023
- K. Demeestere, J. Dewulf, T. Ohno, P. H. Salgado and H. Van Langenhove, “Visible Light Mediated Photocatalytic Degradation of Gaseous Trichloroethylene and Dimethyl Sulfide on Modified Titanium Dioxide,” Applied Catalysis B: Environmental, Vol. 61, No. 1-2, 2005, pp. 140- 149. http://dx.doi.org/10.1016/j.apcatb.2005.04.017
- H. Park and W. Choi, “Photocatalytic Reactivities of Nafion-Coated TiO2 for the Degradation of Charged Organic Compounds under UV or Visible Light,” The Journal of Physical Chemistry B, Vol. 109, No. 23, 2005, pp. 11667-11674. http://dx.doi.org/10.1021/jp051222s
- C. Xu, R. Killmeyer, M. L. Gray and S. U. M. Khan, “Photocatalytic Effect of Carbon-Modified n-TiO2 Nanoparticles under Visible Light Illumination,” Applied Catalysis B: Environmental, Vol. 64, No. 3-4, 2006, pp. 312- 317. http://dx.doi.org/10.1016/j.apcatb.2005.11.008
- Y. A. Shaban, M. A. El Sayed, A. A. El Maradny, R. Kh. Al Farawati and M. I. Al Zobidi, “Photocatalytic Degradation of Phenol in Natural Seawater Using Visible Light Active Carbon Modified (CM)-n-TiO2 Nanoparticles under UV Light and Natural Sunlight Illuminations,” Chemosphere, Vol. 91, No. 3, 2013, pp. 307-313. http://dx.doi.org/10.1016/j.chemosphere.2012.11.035
- D. P. Das, K. Parida and B. R. De, “Photocatalytic Reduction of Hexavalent Chromium in Aqueous Solution over Titania Pillared Zirconium Phosphate and Titanium Phosphate under Solar Radiation,” Journal of Molecular Catalysis A: Chemical, Vol. 245, No. 1-2, 2006, pp. 217- 224. http://dx.doi.org/10.1016/j.molcata.2005.10.001
- S. U. M. Khan, M. Al-Shahry and W. B. Ingler Jr., “Efficient Photochemical Water Splitting by a Chemically Modified n-TiO2,” Science, Vol. 297, No. 5590, 2002, pp. 2243-2245. http://dx.doi.org/10.1126/science.1075035
- Y. A. Shaban and S. U. M. Khan, “Carbon Modified (CM)-n-TiO2 Thin Films for Efficient Water Splitting to H2 and O2 under Xenon Lamp Light and Natural Sunlight Illuminations,” Journal of Solid State Electrochemistry, Vol. 13, No. 7, 2009, pp. 1025-1036. http://dx.doi.org/10.1007/s10008-009-0823-4
- Y. A. Shaban and S. U. M. Khan, “Efficient Photoelectrochemical Splitting of Water to H2 and O2 at Nanocrystalline Carbon Modified (CM)-n-TiO2 and (CM)-n-Fe2O3 Thin Films,” International Journal of Nanotechnology, Vol. 7, No. 1, 2010, pp. 69-98. http://dx.doi.org/10.1504/IJNT.2010.029549
- APHA, “Standard Methods for the Examination of Water and Wastewater,” 20th Edition, American Public Health Association, Washington DC, 1998.
- M. C. Lu, G. D. Roam, J. N. Chen and C. P. Huang, “Factors Affecting the Photocatalytic Degradation of Dichlorovos over Titanium Dioxide Supported on Glass,” Journal of Photochemistry and Photobiology A: Chemistry, Vol. 76, No. 1-2, 1993, pp. 103-109. http://dx.doi.org/10.1016/1010-6030(93)80180-H
- N. Wang, Z. Chen, L. Zhu, X. Jiang, B. Lv and H. Tang, “Synergistic Effects of Cupric and Fluoride Ions on Photocatalytic Degradation of Phenol,” Journal of Photochemistry and Photobiology A: Chemistry, Vol. 191, No. 2-3, 2007, pp. 193-200. http://dx.doi.org/10.1016/j.jphotochem.2007.04.023
- S. Chen and G. Cao, “Study on the Photocatalytic Reduction of Dichromate and Photocatalytic Oxidation of Dichlorvos,” Chemosphere, Vol. 60, No. 9, 2005, pp. 1308- 1315. http://dx.doi.org/10.1016/j.chemosphere.2005.01.056
- R. Wang, D. Ren, S. Xia, Y. Zhang and J. Zhao, “Photocatalytic Degradation of Bisphenol A (BPA) Using Immobilized TiO2 and UV Illumination in a Horizontal Circulating Bed Photocatalytic Reactor (HCBPR),” Journal of Hazardous Materials, Vol. 169, No. 1-3, 2009, pp. 926- 932. http://dx.doi.org/10.1016/j.jhazmat.2009.04.036
- M. L. Chin, A. R. Mohamed and S. Bhatia, “Performance of Photocatalytic Reactors Using Immobilized TiO2 Film for the Degradation of Phenol and Methylene Blue Dye Present in Water Stream,” Chemosphere, Vol. 57, No. 7, 2004, pp. 547-554. http://dx.doi.org/10.1016/j.chemosphere.2004.07.011
- H. Chen, Y. Shao, Z. Xu, H. Wan, Y. Wan, S. Zheng and D. Zhu, “Effective Catalytic Reduction of Cr(VI) over TiO2 Nanotube Supported Pd Catalysts,” Applied Catalysis B: Environmental, Vol. 105, No. 3-4, 2011, pp. 255- 262. http://dx.doi.org/10.1016/j.apcatb.2011.04.004
- H. Mekatel, S. Amokran, B. Bellal, M. Trari and D. Nibou, “Photocatalytic Reduction of Cr(VI) on Nanosized Fe2O3 Supported on Natural Algerian Clay: Characteristics, Kinetic and Thermodynamic Study,” Chemical Engineering Journal, Vol. 200-202, 2012, pp. 611-618. http://dx.doi.org/10.1016/j.cej.2012.06.121
- M. A. Fox and M. T. Dulay, “Heterogeneous Photocatalysis,” Chemical Reviews, Vol. 93, No. 1, 1993, pp. 341- 357. http://dx.doi.org/10.1021/cr00017a016
- P. Zamostny and Z. Belohlav, “Identification of Kinetic Models of Heterogeneously Catalyzed Reactions,” Applied Catalysis A: General, Vol. 225, No. 1-2, 2002, pp. 291-299. http://dx.doi.org/10.1016/S0926-860X(01)00875-4
- N. J. Peill and M. R. Hoffmann, “Mathematical Model of a Photocatalytic Fiber-Optic Cable Reactor for Heterogeneous Photocatalysis,” Environmental Science and Technology, Vol. 32, No. 3, 1998, pp. 398-404. http://dx.doi.org/10.1021/es960874e
- A. Houas, H. Lachheb, M. Ksibi, E. Elaloui, C. Guillard and J.-M. Hermann, “Photocatalytic Degradation Pathway of Methylene Blue in Water,” Applied Catalysis B: Environmental, Vol. 31, No. 2, 2001, pp. 145-157. http://dx.doi.org/10.1016/S0926-3373(00)00276-9
- Y. Ku and I. Jung, “Photocatalytic Reduction of Cr(VI) in Aqueous Solutions by UV Irradiation with the Presence of Titanium Dioxide,” Water Research, Vol. 35, No. 1, 2001, pp. 135-142. http://dx.doi.org/10.1016/S0043-1354(00)00098-1
- J. J. Testa, M. A. Grela and M. I. Litter, “Experimental Evidence in Favor of an Initial One-Electron-Transfer Process in the Heterogeneous Photocatalytic Reduction of Chromium(VI) over TiO2,” Langmuir, Vol. 17, No. 12, 2001, pp. 3515-3517. http://dx.doi.org/10.1021/la010100y
- L. A. G. Rodenas, A. D. Weisz, G. E. Magaz and M. A. Blesa, “Effect of Light on the Electrokinetic Behavior of TiO2 Particles in Contact with Cr(VI) Aqueous Solutions,” Journal of Colloid Interface Science, Vol. 230, No. 1, 2000, pp. 181-185. http://dx.doi.org/10.1006/jcis.2000.7053
- S. G. Schrank, H. J. José and R. F. P. M. Moreira, “Simultaneous Photocatalytic Cr(VI) Reduction and Dye Oxidation in a TiO2 Slurry Reactor,” Journal of Photochemistry and Photobiology A: Chemistry, Vol. 147, No. 1, 2002, pp. 71-76. http://dx.doi.org/10.1016/S1010-6030(01)00626-8
- J. R. Bolton, “Solar Photoproduction of Hydrogen: A Review,” Solar Energy, Vol. 57, No. 1, 1996, pp. 37-50. http://dx.doi.org/10.1016/0038-092X(96)00032-1
- Y. Li, G. Lu and S. Li, “Photocatalytic Hydrogen Generation and Decomposition of Oxalic Acid over Platinized TiO2,” Applied Catalysis A: General, Vol. 214, No. 2, 2001, pp. 179-185. http://dx.doi.org/10.1016/S0926-860X(01)00491-4
- S. Mu, Y. Z. Long and S. Z. Kang. “Surface Modification of TiO2 Nanoparticles with a C60 Derivative and Enhanced Photocatalytic Activity for the Reduction of Aqueous Cr(VI) Ions,” Catalysis Communications, Vol. 11, No. 8, 2010, pp. 741-744. http://dx.doi.org/10.1016/j.catcom.2010.02.006
- B. Sun, E. P. Reddy and P. G. Smirniotis, “Visible Light Cr(VI) Reduction and Organic Chemical Oxidation by TiO2 Photocatalysis,” Environmental Science and Technology, Vol. 39, No. 16, 2005, pp. 6251-6259. http://dx.doi.org/10.1021/es0480872
- H. Fu, G. Lu and S. Li, “Adsorption and Photo-Induced Reduction of Cr(VI) Ion in Cr(VI)-4CP (4-Chlorophenol) Aqueous System in the Presence of TiO2 as Photocatalyst,” Journal of Photochemistry and Photobiology A: Chemistry, Vol. 114, No. 1, 1998, pp. 81-88. http://dx.doi.org/10.1016/S1010-6030(98)00205-6

