Advances in Chemical Engineering and Science
Vol.05 No.02(2015), Article ID:54557,4 pages
10.4236/aces.2015.52014
Basic Engineering of a Two-Stage Process for Co-Upgrading Natural Gas and Petroleum Coke
Jorge Laine, Maria Tosta
Centro de Química, Instituto Venezolano de Investigaciones Científicas, Caracas, Venezuela
Email: jlaine@ivic.gob.ve
Copyright © 2015 by author and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/



Received 20 February 2015; accepted 8 March 2015; published 11 March 2015
ABSTRACT
This communication highlights the possibility of using a novel two-stage process for the co-up- grading of natural gas and petroleum coke into liquid hydrocarbons. The first stage consists of the catalytic dehydroaromatization of methane characterized by producing hydrogen and aromatics: benzene, naphtalene, toluene, etc. The non-reacted methane plus hydrogen and aromatics pro- duced in the first stage are directed to the second stage to react with the petroleum coke. Basic engineering analysis of proposed two-stage process suggests light petroleum production of 160,000 bbl/day from 20,000 ton/day of petroleum coke actually by-produced from Venezuelan Orinoco’s heavy oil belt. Residual coke should be volatiles free therefore useful as a calcined coke.
Keywords:
Dehydroaromatization, Natural Gas, Petroleum Coke, Co-Upgrading

1. Introduction: The Two-Stage Process
The increasing demand for liquid fuels and the existing large world reserves of natural gas make attractive the direct transformation of it into more desirable feedstocks. On the other hand, the increasing by-production of petroleum coke due to the processing of heavy oils (e.g., Orinoco bitumen, Alberta tar sands, etc.) opens the perspective of reacting natural gas with coke to obtain liquid hydrocarbons.
The possibility of joining these two processes: The direct transformation of natural gas and the reaction of natural gas with coke, both together into a two-stage process are presented in this communication.
Regarding the first stage, the process hereby considered is methane deshydroaromatization (MDA), which would transform natural gas into valuable high-octane number aromatic fuels beside of hydrogen [1] .
In connection with the second stage: methane reaction with coke (MRC), some reports deal with the reaction of methane with coal [2] - [6] . However, to the author’s knowledge, reports regarding reaction of methane with coke are not found in the relevant literature related to hydrocarbon processing.
It should be remarked that processes most widely employed for upgrading separately either natural gas or coal raw materials, are referred to as indirect liquefaction, involving steam reforming of the raw material to produce syngas (CO + H2) for Fischer-Tropsch synthesis [7] .
In the case of the others coal upgrading processes referred to as direct liquefaction [8] , most attention has been paid to H2 as the main gas reactant, using solvents in order to improve H2 diffusion to the carbon matrix, including hydrogen-donor solvents such as tetralin, cyclohexane, etc. Nevertheless, these direct coal liquefactions require the costly and highly CO2 footprint technology for producing H2 reactant. The use of natural gas instead of H2 for liquefaction, as hereby proposed, would save this obstacle.
Within the above scope, this communication reviews some information available in order to carry an analysis of the basic engineering of the proposed two stages co-upgrading of natural gas and petroleum coke.
2. Reactions Involved
First approximation is to assume that natural gas is CH4, and coke is pure C.
For MDA stage, it is assumed to involve the production of benzene [1] :
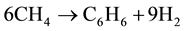 (I)
(I)
Notice that other products expected in MDA, e.g., toluene and naphthalene, imply reaction ratio H2/CH4 (1.4 and 1.6 respectively) similar than that of benzene (1.5)
For the sake of simplicity, MRC stage is assumed to involve the production of alkanes:
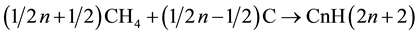 (II)
(II)
Similarly, for the reaction of coke with hydrogen coming from MDA stage:
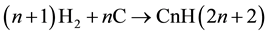 (III)
(III)
Curiously, solid carbon is not only a reactant for gasification and liquefaction, the solid carbon can also behave as a catalyst decomposing methane to produce hydrogen [9] [10] and mixtures of coke and unsaturated hydrocarbons such as C2H2, C2H4 and benzene.
Hydrogen is reported to synergistically promote liquefaction function of CH4 [3] ; a key factor for using the first stage MDA gas outlet (CH4 + H2) to feed MRC stage.
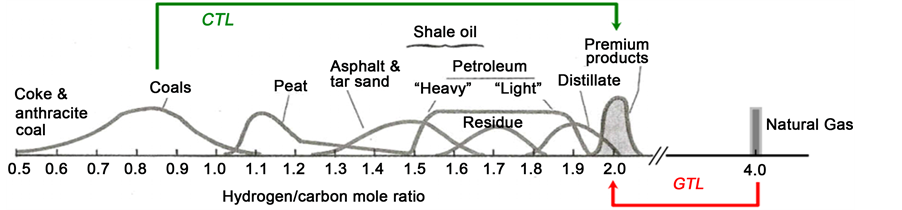
Figure 1 shows atomic ratios H/C for the existing types of fossil hydrocarbos. Notice GTL (gas-to-liquid) and CTL (coals (or coke)-to-liquids) routes are in fact connected in the present two-stage proposal.
The basic engineering analysis has been carried out taking into account the reported literature data for the two individual reactions: MDA and MRC. It is hereby remarked the limitation of assuming coke behaves in a similar manner as coal. Certainly, coal, particularly those with a high H/C ratio should be liquified easier than low H/C ratio raw materials like coke or anthracite coal (see Figure 1). However, high Ni and V contents, an important characteristic of petroleum coke differentiating it from coals, might act as hydrogenation catalysts favouring liquefaction, but this is a matter requiring more scientific evidence.
Figure 1. Atomic ratios H/C of fossil fuels. The routes GTL: natural gas to liquids, and CTL: coals (or coke) to liquids are combined in the present co-upgrading process.
3. Basic Engineering Analysis
3.1. MDA Stage
Previous works on MDA [1] [11] have shown that most promising catalysts results from the combination of a transition metal with a zeolite. Particularly, molybdenum impregnated on a HZSM-5 zeolite support is one of the catalysts most studied. The unusual resistance to deactivation by carbon deposition of that zeolite [12] could probably be one of the key factors for functioning in MDA. Furthermore, making-up methane feed with a small concentration of hydrogen plus steam, is reported to stabilize MDA suppressing deactivation effectively [13] . Nevertheless, previous reports [1] [11] [13] [14] suggest low methane conversions (~10%) for steady state MDA.
For MDA, a trickled bed reactor is recommended where both natural gas and the liquid products flow down across a bed of pelletized Mo/HZSM-5 catalyst. It should be anticipated the necessity for catalyst regeneration in the case that deactivation by carbon deposition is not stabilized. One alternative is to stop MDA stage by- passing methane directly to MRC stage while catalyst regenerating. Another alternative is to employ two parallel reactors to allow catalyst regeneration in one reactor while carrying MDA in the other.
Two alternatives are possible for connecting MDA to MRC: one is to separate the liquid MDA products leaving non-reacted CH4 plus H2 product to be directed as the gaseous reactants for MRC. The other is to direct all MDA output to MRC. This latter is backed by recent report suggesting a synergistic effect between coal pyrolysis and methane aromatization [15] . Certainly, the aromatics produced by MDA may improve diffusion of the gas reactants to the carbonaceous surface enhancing liquefaction.
3.2. MRC Stage
Catalytic agents and/or solvents should be introduced for MRC. It is well known that the use of solvents improves coal liquefaction. Previous experiments on the liquefaction with H2 of a Venezuelan coal [16] demonstrated the advantage of using H-donor solvents. Therefore, in addition to the aromatics coming from MDA, certain fraction of liquids produced in MRC stage should be pre-treated with H2 and recycled to function as H-donor solvent for the liquefaction.
Early experiments using batch reactor yield high conversions (~50%) when reacting CH4 and H2 mixtures with coal [3] [5] , as well as in the case of Venezuelan heavy oil [17] . The possibility of adapting two parallel batch reactors operating similarly as in delayed coking processes may be one alternative to consider for MRC.
Several other alternatives may be investigated for continues flow MRC process reactor: A fluidized-bed or ebullated-bed as employed in the H-coal direct-liquefaction process [8] should be taken into account. The use of small coke particle size is recommended in order to ensure more solid surface area that should improve reactant gas diffusion phenomena to favor coke reaction kinetics. Tubular reactors where a slurry of the carbon powder plus a solvent containing dissolved gases (CH4 + H2) flowing continuously have also been experimented [4] , as well as microwave plasma reactor [6] and flash pyrolysis [2] both under CH4 supply. A novel reactor experimented using Venezuelan petroleum coke under steam gasification employing solar energy [18] could also be considered introducing the variance of employing CH4 instead of steam.
Coke liquefaction residue extracted from MRC may be an appropriate raw material for activated carbon manufacture as proposed else where [19] . In addition, it could have properties similar to calcined coke, a commercial material useful for application in metallurgy and other carbon consuming industries. The possible application of the residual coke for agriculture and desert greening as proposed earlier [20] should also be investigated further.
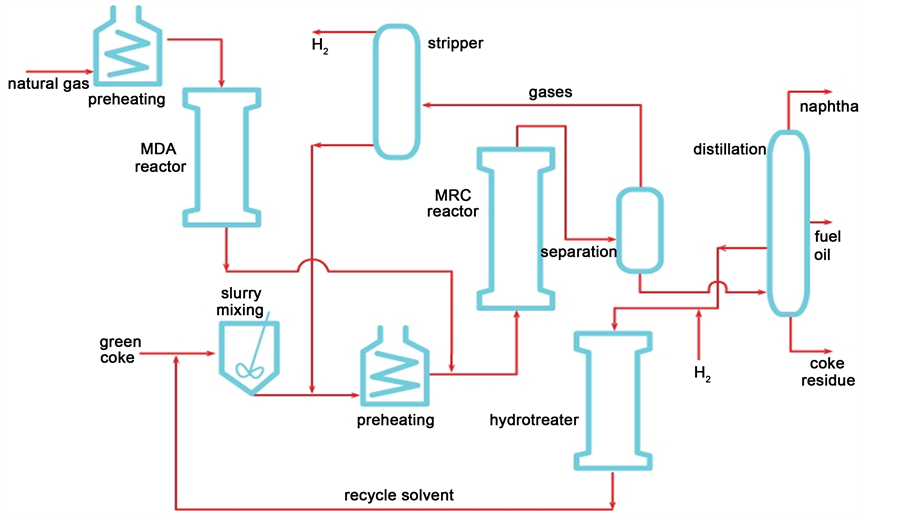
Figure 2 shows a basic flow diagram for the proposed two-stage process for co-upgrading natural gas and petroleum coke into liquid hydrocarbons. Notice that non-reacted gases are recycled to MRC stage after stripping of H2. In addition, certain distillate fraction is recycled to MRC reactor to function as solvent to enhance reactant gas diffusion to solid. Therefore, H2 stripping from MRC output gases may also be available for the hydrotreating reactor as show in Figure 2, an operation intended to produce required hydrogen donor solvent.
Basic engineering analysis employing reactions I and II (n = 8), assuming coke is the limiting reactant employing excess methane, and discrete values of MDA conversion (10%) and coke conversion (30%) to light petroleum, derived from the literature investigated; therefore, it is estimated that 1.500 m3 (1 atm, 25˚C) of natural gas is required to produce 8 bbl of light petroleum for each ton of petroleum coke processed. Venezuelan petroleum coke produced today (20,000 ton/day) would be equivalent to 160.000 bbl/day of light petroleum using this process.
Figure 2. Flow diagram of the two-stage co-upgrading process, showing the two reactors (MDA and MRC) and other operational units.
4. Conclusion
Analysis of the presently proposed co-upgrading of natural gas and petroleum coke precedes a more detailed research in order to optimize operational conditions and equipment specifications. As a result of the Venezuelan large off-shore natural gas reserves and petroleum coke by produced from the Orinoco belt, development of detailed engineering design for pilot plant should be furthermore investigated.
References
- Ma, S.Q., Guo, X.G., Zhao, L.X., Scottc, S. and Bao, X.H. (2013) Recent Progress in Methane Dehydroaromatization: From Laboratory Curiosities to Promising Technology. Review. Journal of Energy Chemistry, 22, 1-20. http://dx.doi.org/10.1016/S2095-4956(13)60001-7
- Calkins, W.H. and Bonifaz, C. (1984) Coal Flash Pyrolysis: 5. Pyrolysis in an Atmosphere of Methane. Fuel, 63, 1716- 1719. http://dx.doi.org/10.1016/0016-2361(84)90106-6
- Voigtmann, M.F., Chen, M. and Batts, B.D. (1995) Coal Pyrolysis with Methane in a Reducing Environment. Coal Science and Technology, 24, 1399-1402. http://dx.doi.org/10.1016/S0167-9449(06)80066-2
- Cai, J.Q., Wang, Y.P. and Huang, Q.W. (2008) Rapid Liquefaction of Longkou Lignite Coal by Using a Tubular Reactor under Methane Atmosphere. Fuel, 87, 3388-3392. http://dx.doi.org/10.1016/j.fuel.2008.06.001
- Yang, K., Batts, B.D., Wilson, M.A., Gorbaty, M.L., Maa, P.S., Long, M.A., He, S.X.J. and Attalla, M.I. (1997) Reaction of Methane with Coal. Fuel, 76, 1105-1115. http://dx.doi.org/10.1016/S0016-2361(97)00134-8
- Kamei, O., Onoe, K., Marushima, W. and Yamaguchi, T. (1998) Brown Coal Conversion by Microwave Plasma Reactions under Successive Supply of Methane. Fuel, 77, 1503-1506. http://dx.doi.org/10.1016/S0016-2361(98)00055-6
- Dry, M.E. (1981) The Fischer-Tropsch synthesis. In: Anderson, J.R. and Boudart, M., Eds., Catalysis Science and Technology, Springer-Verlag, Berlin, Vol. 1, 159-255.
- Winslow, J. and Schmetz, E. (2009) Direct Coal Liquefaction Overview Presented to NETL. http://bellona.org/filearchive/fil_Direct_Coal_Liquefaction_Overview.pdf
- Muradov, N., Smith, F. and Raissi, A. (2005) Catalytic Activity of Carbons for Methane Decomposition Reaction. Catalysis Today, 102, 225-233. http://dx.doi.org/10.1016/j.cattod.2005.02.018
- Bai, Z.Q., Chen, H.K., Li, W. and Li, B.Q. (2006) Hydrogen Production by Methane Decomposition over Coal Char. International Journal of Hydrogen Energy, 31, 899-905. http://dx.doi.org/10.1016/j.ijhydene.2005.08.001
- Quintero, D., Padilla, D., Labady, M. and Laine, J. (2007) A Transient Behavior in the Initial Production of Aromatic Compounds from Methane Catalyzed by Mo/HZSM-5. Catalysis Letters, 118, 244-247. http://dx.doi.org/10.1007/s10562-007-9177-7
- Heinemann, H. (1981) A Brief History of Industrial Catalysis. In: Anderson, J.R. and Boudart, M., Eds., Catalysis Science and Technology, Vol. 1, Springer-Verlag, Berlin, 1-41.
- Ma, H.T., Kojima, R., Kikuchi, S. and Ichikawa, M. (2005) Effective Coke Removal in Methane to Benzene (MTB) Reaction on Mo/HZSM-5 Catalyst by H2 and H2O Co-Addition to Methane. Catalysis Letters, 104, 63-66. http://dx.doi.org/10.1007/s10562-005-7437-y
- Shu, Y.Y., Ma, D., Xu, L.Y., Xu, Y.D. and Bao, X.H. (2000) Methane Dehydro-Aromatization over Mo/MCM-22 Catalysts: A Highly Selective Catalyst for the Formation of Benzene. Catalysis Letters, 70, 67-73. http://dx.doi.org/10.1023/A:1019079603279
- Jin, L.J., Zhou, X., He, X.F. and Hu, H.Q. (2013) Integrated Coal Pyrolysis with Methane Aromatization over Mo/HZSM-5 for Improving Tar Yield. Fuel, 114, 187-190. http://dx.doi.org/10.1016/j.fuel.2012.01.024
- Laine, J. and Becerra, O. (1985) A Semi-Continuous Flow Reactor Technique for Coal Liquefaction Studies. Fuel Processing Technology, 11, 127-132. http://dx.doi.org/10.1016/0378-3820(85)90023-2
- Ovalles, C., Hamana, A., Rojas, I. and Bolívar, R.A. (1995) Upgrading of Extra-Heavy Crude Oil by Direct Use of Methane in the Presence of Water: Deuterium-Labelled Experiments and Mechanistic Considerations. Fuel, 74, 1162-1168. http://dx.doi.org/10.1016/0016-2361(95)00071-C
- Z’Graggen, A., Haueter, P., Trommer, D., Romero, M., de Jesus, J.C. and Steinfeld, A. (2006) Hydrogen Production by Steam-Gasification of Petroleum Coke Using Concentrated Solar Power―II Reactor Design, Testing, and Modeling. International Journal of Hydrogen Energy, 31, 797-811. http://dx.doi.org/10.1016/j.ijhydene.2005.06.011
- Zhang, J.B., Jin, L.J., He, X.F., Liu, S.B. and Hu, H.Q. (2011) Catalytic Methane Decomposition over Activated Carbons Prepared from Direct Coal Liquefaction Residue by KOH Activation with Addition of SiO2 or SBA-15. International Journal of Hydrogen Energy, 36, 8978-8984. http://dx.doi.org/10.1016/j.ijhydene.2011.04.205
- Laine, J. (2012) Perspective of the Preparation of Agrichars Using Fossil Hydrocarbon Coke. Renewable and Sustainable Energy Reviews, 16, 5597- 5602. http://dx.doi.org/10.1016/j.rser.2012.06.009