Open Journal of Yangtze Oil and Gas
Vol.02 No.01(2017), Article ID:74942,18 pages
10.4236/ojogas.2017.21003
Gas Hydrate―Properties, Formation and Benefits
Azeez G. Aregbe
Institute of Oil and Gas Engineering, University of Lagos, Lagos, Nigeria

Copyright © 2017 by author and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY 4.0).
http://creativecommons.org/licenses/by/4.0/



Received: December 26, 2016; Accepted: January 19, 2017; Published: January 23, 2017
ABSTRACT
There are numerous gas hydrate reserves all over the world, especially in permafrost regions and ocean environments. The abundance of gas hydrate reserves is estimated to be more than twice of the combined carbon of coal, conventional gas and petroleum reserves. These hydrate deposits hold significant amount of energy which can make hydrate a sustainable energy resource. The comprehensive research on the properties and formation of methane hydrates is paramount to ensure efficient and effective exploration and development of hydrate reserves. Natural gas is mostly distributed for different purposes through pipelines or pressure vessels such as dry gas, compressed gas or liquefied gas, which means transporting natural gas creates serious safety concerns because methane is highly flammable and almost impossible to detect any leak without using odorant. Alternatively, natural gas can be stored and transported as gas hydrate turns solid or slurry. Gas hydrate can be stored at equilibrium conditions with either saturation temperature or pressure. The equilibrium conditions are influenced by the cost and weight of storage vessel. Hydrate can be transported either as slurry or solid depending on the location of target or destination. The slurry form is usually a better option for distance of approximately 2500 mile or less while the solid form can be used for distances of roughly 3500 miles or more. The paper examines the properties, formation and benefits of gas hydrate. The suitability of gas hydrate as a sustainable energy resource and the possibility of using gas hydrate for the transportation and storage of natural gas (methane) are also stated. Natural gas transportation and storage as gas hydrate will create effectively and efficiently alternative bulk gas transportation and storage for future use of the gas.
Keywords:
Natural Gas, Hydrate, Storage, Energy, Transportation

1. Introduction
The term “gas hydrates” refers to crystalline compounds that are composed of water and any of the following light molecules: methane, ethane, propane, iso-butane, normal butane, nitrogen, carbon dioxide, and hydrogen sulfide. It is known that some polar components between the sizes of argon (0.35 nm) and ethyl cyclohexane (0.9 - 1) can also form hydrates. Hydrate formation usually occurs when water molecule exists in the vicinity of these molecules at temperatures above or below the ice point and relatively high pressure. The water molecules enclose these host molecules and form cage-like structures which are stable at these conditions.
Natural gas hydrates commonly called gas hydrates the crystalline compounds formed when water molecule and methane gas exist together at the right temperature and pressure. Methane hydrate is stable at a temperature slightly above or below 0˚C with high pressure. The pressure and temperature of most ocean environments offer appropriate conditions for methane hydrate stability but considerable amounts of hydrate are formed at the continental shelf due to the effect of geothermal gradient [1] . Geothermal gradient makes it possible for the temperature at some depths to be more than the equilibrium temperature at the in situ pressure, which creates stability for methane hydrate. The cage-like structure formed is stabilized by the interactions that exist between the water molecules and the methane gas. These interactions create a relatively strong and stable lattice. Gas hydrates have high capacity to store methane gas―1 cubic metre of a typical hydrate contains approximately 180 standard cubic metres of methane gas at standard temperature and pressure [1] .
Natural gas which is mainly methane is used as fuel because the technologies for handling it directly. As fuel for heating and indirectly through energy conversion, technologies are readily available and they also cost money effective. In addition to that, methane gas contains more hydrogen atoms for each carbon atom than the other hydrocarbon fuels. This implies less production of carbon dioxide during combustion of methane. Methane gas is also pure and easy to purify, which makes its use as fuel more environmentally friendly than other hydrocarbon fuels such as oil and coal. Methane gas is also surprisingly be found to produce much less carbon dioxide than alcohols, and even much lesser than in liquid petroleum and oil based fuels.
Natural gas (Methane) produced from conventional oil and gas reserves is enormous, which is easily distributed to the consumers through pipeline, and also has abundant heat content. The exploration and development of gas hydrate reserves, with a prospect of almost indefinite supply of methane will definitely influence the growth of the gas-based energy economy to overcome the oil-based energy economy. Figure 1 shows the comparison between hydrate deposits and other sources of fuel, using a unit of 1015 g of carbon.
Natural gas is mostly distributed for different purposes through pipeline system or pressure vessels either as dry gas, compressed gas or liquefied gas. These means of transporting of natural gas poses serious safety concerns due to the fact
Figure 1. Gas hydrates deposits compared with other fuel resources, units = 1015 g of carbon, [2] .
that methane is highly flammable and almost impossible to detect any leak without using odorant.
Hydrates have been one of the flow assurance problems in gas production and transportation. Different models and approaches have been adopted to solve the gas hydrate problems. Hydrates block the conduit of oil and gas pipelines and transportation systems with significant economic impacts. In recent years, hydrate production has been studied and investigated as potential for sustainable energy resource. The abundance of gas hydrate is estimated to be more than twice the combined carbon of coal, conventional gas and petroleum reserves [3] . Gas hydrate reserves contain enormous source of energy in form of pure methane gas which can serve as sustainable energy resource.
Nature of Gas Hydrate Deposits
The hydrates of CH4 and C3H8 gases occur naturally all over the world. The formation of Arctic hydrates is usually under the permafrost layer. Oceanic hydrates are also discovered to be deposited at the continental shelves. In natural settings, such as the ocean bottom, where organic matter are buried and decomposed to methane, the methane formed is dissolved in water to form methane hydrates at temperatures around 277 K. Biogenic methane usually dissolves slowly in water to form hydrate because of mass transfer limitations.
Over geologic time, the estimated methane hydrate in the ocean is approximately 2.1 × 1016 standard cubic metres―twice the total energy of all other fossil fuels on earth. The amount of methane hydrate in the northern latitude permafrost is approximately 7.4 × 1014 standard cubic metres. Hydrates deposits are usually determined by indirect methods such as the seismic reflection method known as bottom simulating reflectors (BSRs). This method uses seismic signals which are caused by velocity inversion because the presence of gas below some high-velocity barriers such as a hydrate deposit.
Significant hydrate sediments in the ocean beds can jeopardize the foundation of subsea platforms, manifolds or even pipelines. Water molecules form frameworks of large cavities as a result of the hydrogen bond within the water molecule which enclose the guest gas molecule. The gas hydrate is thermodynamicaly stable because of the interaction between the water and the gas molecules [4] . Gases hydrates samples have been discovered from many places―19 or more areas worldwide and are believed to be deposited at roughly 77 areas including the Antarctica and Siberia. Generally, they are discovered in continental shelf sediments and onshore polar areas beneath the permafrost due to the fact that the pressure-temperature conditions in these regions are within the hydrate stability conditions [5] . An artificially produced sample of methane hydrate is shown in Figure 2.
Hydrate deposits are generally found in waters deeper than 300 m―their zones of existence is from seafloor to a depth of few hundred metres and this is also a function of the local thermal gradient. Based on research and investigation, huge amount of methane gas is believed to be trapped in the hydrate deposits as well as the sediments beneath these deposits. Exploration and development of gas hydrate reserves is the key solution to unlimited supply of energy. This source of energy is pure and environmentally friendly. The investigation of the properties and how these hydrate deposits are formed is one of the objectives of this paper. The study of the formation of hydrate is also crucial for the transportation and storage of natural gas as hydrates which is the focus of this paper. A better understanding of the hydrate formation and dissociation mechanism is vital for proper design and optimization of separation processes involving hydrate equilibrium and the understanding of natural gas hydrate formation, accumulation and destabilization in nature.
2. Literatures/Recent Works on Gas Hydrates
There has been several research works on gas hydrate to mitigate its formation and also to explore gas hydrate reserves. Although a significant amount of research on gas hydrates has been carried out in both industrial and academic laboratories, several study areas such as the formation mechanism and the hydrate growth are still poorly understood, especially for complex systems. However, a
Figure 2. Sample of methane hydrate [6] .
better understanding of the hydrate formation and dissociation mechanism is required for both the design and optimization of separation processes involving hydrate equilibrium and the understanding of natural gas hydrate formation, accumulation and destabilization in nature.
Gas Hydrate was discovered by Sir Humphrey Day in 1810. The study and research on hydrate became of interest to the oil and gas industry in 1934 when the first pipeline blockage was observed by Hamrnerschmidt. This was due to the crystalline, non-flowing nature of hydrates. Different experiments have been performed to determine the structures of gas hydrates:
Jin et al. [7] used micro focus X-ray computed tomography (CT) to study the structure of natural gas sediments with and without gas hydrates. A newly developed high pressure vessel was used for the analysis to observe the sediments at temperatures above 273 K and relatively high pressures. They measured the spatial distribution of the free gas, sand particles, liquid water and solid hydrate phases. They also assessed the permeability of the sediments through the correlation between the absolute permeability and the pore networks of the sediment. The results showed that the proportion of horizontal continuous pore channel is a major determinant of the absolute permeability.
Minagawa et al. [8] used proton nuclear magnetic resonance measurements combined with a permeability measurement system to characterize methane hydrate sediments based on their permeability and pore size distribution. They compared the effective permeability of the sediments and different effective porosities, which had been measured by using water flow based on Darcy’s law, with the permeability calculated by NMR spectra based on SDR (Schlumberger-Doll Research) model. The results of the permeability from both methods were similar with a minimal difference of less than factor of 2. The results were used to describe the relationship among pore size distribution, porosity and effective permeability.
Santamarina et al. [9] conducted series of experiments to determine the mechanical, thermal, electrical and electromagnetic properties of hydrate-bearing soils using standardized geotechnical devices and test protocols. They experimented with a range of grain sizes subjected to an effective stress of up to 2 MPa and with well-controlled saturations of synthetic hydrates.
Stoll and Bryan [10] conducted an experimental work on the thermal conductivity and acoustic wave velocity of hydrates as well as sediments containing hydrates. The most significant part of their results was that the formation of hydrates decreases with the thermal conductivity of the sediments. This behavior was found to be different from what should be expected of the behavior of frozen sediments. Their research work was also able to confirm that both pure water and water-bearing sediments are converted to a stiff elastic mass by formation of a sufficiently high amount of hydrate. This led to their conclusion that as a basis for using sharp acoustic impedance contrast at the boundary of sediment containing hydrates to locate hydrate deposits.
Pearson et al. [11] were able to predict the physical properties of sediments containing hydrates in order to include their effects on production models and develop geophysical exploration as well as reservoir characterization techniques. They incorporated empirical relationships between the composition of ice and seismic velocity, electrical resistivity, density and heat capacity for frozen soils to estimate the physical properties of the deposits. They stipulated that the resistivity of laboratory permafrost samples followed a variation of Archie’s equation. They measured the parameters and also calculated these parameters for variety of lithological types. They also concluded that the compressional wave velocities of the partially frozen sediments are related to the velocities of the matrix, liquid and solid phases present in the pores of the sediments.
Winters et al. [12] performed experimental to measure a wide range of acoustic-wave velocities in coarse grain sediments for different pore space occupants. The measured values from the experiments ranged from less than 1000 m/s for gas-charged sediments to 1770 - 1940 m/s for water saturated sediments, 2910 - 4000 m/s for other sediments with varying degree of hydrate saturations and 3880 - 4330 m/s for frozen sediments. The sediments shear strength was increased as a result of the presence of gas hydrate, ice and other solid pore-filling substances. The magnitude of the shear strength increment was found to be a function of the amount of hydrate in the pore spaces and cementation characteristics between the hydrate and grains of the sediments. They also concluded that the presence of free gas in pore spaces influenced pore pressure response and during shearing and also the strengthening effect of gas hydrate in sands.
There has been a significant improvement in characterizing the relationship between hydrates and sediments containing hydrates. Tinivella et al. [13] conducted a research work to quantify the concentrations of gas hydrate in pore spaces of the sediments by travel-time inversion modeling of the acoustic properties of these sediments. Such analysis has created rooms for the identification of free gas distribution in pore spaces, likely patterns of fluid migration and the physical properties of these sediments.
Tohidi et al. [14] performed series of visualization experiments using two- dimensional transparent glass micro-models and stipulated that hydrates can be formed form either free gas or dissolved gas in the system. They concluded that hydrates usually form at the center of pore spaces with a thin film of water covering the grains instead of nucleating around the grains.
Kingston et al. [15] conducted an experimental work by using a specially manufactured laboratory porous medium, gas hydrate resonant column (GHRC) to explore different methods hydrate synthesis and also measure the properties of the sediments formed. The experiments were performed with different water saturation conditions of the porous medium. In low water saturated condition, where there is gas saturation; the growth of the hydrate was on the water location. Thus, the water saturation was considered the limiting factor. In partially saturated sands, water was collected at grain contacts and coated individual sand grains. As the content of hydrate increased, the filling of the pore spaces began but the increased stiffness was produced by the increased quantity of cement at grain contacts.
Moridis and Kowalsky [16] researched on Gas Production from Unconfined Class 2 Hydrate Accumulations in the Oceanic Subsurface. Hydrate accumulations in oceanic sediments are characterized by mobile saline water zones encaging the hydrate deposits and by the absence of impermeable layers to vertical flow. They evaluated the gas production potential of such hydrate deposits using both single-well and five-spot well configurations. Single-well production is based on depressurization-induced dissociation of the hydrates, whereas the five-spot configuration involves both depressurization at the production wells and thermal stimulation at the injection wells. The study showed that unconfined Class 2 hydrate accumulations are among the most challenging targets for gas production because of the absence of confining boundaries, water production with the gas, and thermal stimulation to enhance production requires substantial energy inputs.
Different international R&D programs have been conducted on gas hydrates all over the world. The review on International Gas Hydrate Research conducted by USGS―science for a changing world in 2014 and the reports of the review showed that different countries all over the world conducted R&D programs on gas hydrate exploration and production. Some of the R&D programs are [17] :
I. Japan
・ 2012/13: R&D on both Arctic and Marine projects.
・ 2013: A week deepwater production test.
・ 2014/15: “Extended” deepwater production test.
・ New Japan Sea project.
II. Korea
・ 2007 & 2010: UBGH-1 & UBGH-2 expeditions.
・ 2015: Marine production test.
III. China
・ 2007 & 2013: GMGS-1 & GMGS-2 expeditions.
・ 2007 through 2011: Onshore “tests”.
IV. Norway (Statoil)
・ Onshore long-duration production test.
・ Gas hydrates global screening.
V. Canada
・ Onshore Mallik Project 1998, 2002, 2007-2008.
・ Beaufort Shelf hazard and climate research.
・ Pacific and Atlantic marine gas hydrate studies.
VI. New Zealand
・ Gas hydrates on the Hikurangi Margin, GNS and University of Auckland.
・ Energy focus, marine surveys and drilling.
VII. Germany
・ SUGAR Energy Assessment Project, BGR plus others.
・ GEOMAR marine gas hydrates research, marine surveys.
・ MARUM MeBo (sea floor drilling rig) drilling research.
3. Formation of Gas Hydrate
When water molecules come in contact with gas molecules at low temperature and high pressure, different geometric structures contrary to that of a hexagonal ice are formed. The water molecules serve as host molecules and create cage lattices that can hold gas molecules as guest molecules. These cage-like crystalline structures are less dense than crystalline water structure because of the presence of the gas molecules. The gas hydrate formed is held together by the hydrogen bonds of the water molecules and also stabilized by Vander Waals forces holding the gas and water molecules together. The Vander Waals force is responsible for the stable nature of the gas hydrate and even makes the hydrate more stable than normal ice formed by water. There are different structures of gas hydrate and they are characterized by the shape of their cages. Natural gas composed mainly of methane gas and the complete combustion of methane gas gives water, carbon dioxide, and energy, as shown in the Equation (1.0).
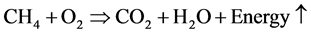 (1.0)
(1.0)
The energy liberated from this process can serve different purposes. This makes natural gas more environmentally friendly than other fossil fuels because more energy is liberated and less CO2 produced. The pictorial view of the lattice structure of gas hydrate is shown in Figure 3. Methane gas is the guest in the middle (Green) while water molecule is the host (Pink).
Gas hydrate can be stored or transported at equilibrium conditions with either its saturation temperature or pressure as shown in Figure 4. At the saturation temperature and pressure, hydrates are usually stable. Some factors affect the saturation pressure and temperature of the hydrate.
Factors such as cost and weight of material for hydrate storage vessel as well as the environment of the sediments containing the hydrate deposits. Hydrates are usually stable at moderate temperatures and pressures when compared to the conditions required for LNG and CNG.
Figure 3. Cage-like structure of gas hydrate [18] .
Figure 4. Phase diagram of gas hydrate stability [18] .
3.1. Classification of Gas Hydrate Structures
For naturally-occurring hydrates, the gases originate either from biogenic or thermogenic sources. Biogenic gases are microbial transformation products which consist mainly of methane. Thermogenic gases usually come from deep petroleum reservoirs and although the methane is overwhelmingly represented in those gases, the fraction of C2 - C5 hydrocarbons is very significant. In the case of hydrates from thermogenic gases, the change in the vapor phase composition during that process of formation is particularly interesting because it provides valuable insight into the mechanism of the reaction.
The different classes of hydrates are formed when methane gas is trapped. They are classified according to the structures of the cages which are characterized by the arrangement of water molecules in the crystals. When the gas (CH4) is trapped, it rearranges the host molecules (H2O) and a cage that traps the gas is formed. Not every water molecule attracts a gas molecule; some remain empty even when a cage appears. But immediately the gas hydrate melts, methane gas is released. The properties of the cages formed by the three structures of gas hydrate crystals are shown in Table 1.
3.1.1. Structure I Gas Hydrates (sI)
These are body-centered cubic structures formed from small gas molecules and are usually found in deep ocean environments. They are formed from two different sizes and shapes of cage; small cages and large cages. The compositions of the two cages are:
I. Small Cages
They are composed of 9 dodecahedrons formed from 12 pentagons (512). They are not in contact with one another but are bonded to the larger cages.
Table 1. Characteristics of the structures I, II, and H gas hydrates.
II. Large Cages
They are made of 12 tetradecahedrons formed from 12 pentagons and 2 hexagons (51262). The small cages are attached to the large cages to form cubic structures.
3.1.2. Structure II Gas Hydrates (sII)
These hydrates have diamond lattices within cubic frameworks and are formed when natural gas or oil containing molecules larger than ethane but smaller than pentane. The structure II hydrates are usually found in oil and gas production and processing systems. They are also formed from two different shapes and sizes of cages. These are:
I. Small Cages
The small cages comprise 16 dodecahedrons which are formed from 12 pentagons (512).
II. Large Cages
The large cages consist of 8 hexadecahedrons which are formed from 12 pentagons and 4 hexagons (51264).
3.1.3. Structure H Gas Hydrates (sH)
This is the newest structure of gas hydrates discovered and was also found to occur in the Gulf of Mexico. The structure H hydrates have hexagonal framework and also possess cavities large enough to hold molecules as big as naphtha and gasoline.
Table 1 shows the properties of the different structures of gas hydrates. The variation in distance of oxygen atoms from center of cage is the cavity radius and the number of oxygen molecules at the periphery of each cavity signifies the variation in radius. The estimation of structure H cavities was based on geometric models. The three different crystal structures of gas hydrates are cubic structure type I (sI), cubic structure type II (sII) and hexagonal structure (sH) as shown in Figure 5. The unit cell of the cubic structure type I requires 46 water molecules as host to form small and large cages. There are two small cages and
Figure 5. The structures of the classes of gas hydrate.
six large cages in the unit cell. The small cage has the shape of a pentagonal dodecahedron and the large cage has hexagonal truncated trapezohedron shape. Typical guests molecules that form the type I hydrates are carbon dioxide and methane gas. The cubic structure type II requires 136 molecules of water to form small and large cages. There are sixteen small cages and eight large cages in a unit cell. The small cage has the shape of pentagonal dodecahedron while the large one has hexadecahedron shape. The structure type II hydrates are formed by oxygen and nitrogen gases. The hexagonal structure (sH) requires 34 molecules of water to form two small cages of different types and a huge cage. The formation of structure type H hydrate requires the guest molecules to be stable. The size of the large cavity allows large molecules (i.e. butane) to fit into the structure type H hydrates in the presence of other small gases to support the remaining cavities.
3.2. Properties of Gas Hydrates
Gas hydrates structures are in three different crystal structures: cubic structure type I (sI), cubic structure type II (sII), and hexagonal structure (sH) as shown in Figure 5. The type I (sI) gas hydrate is formed with those gas molecules having diameters between 4.2 and 6 Å, i.e. methane, ethane, carbon dioxide, and hydrogen sulfide. Other small molecules like nitrogen (d < 4.2 Å) form the type II (sII) gas hydrates. Molecules with diameters: 6 Å < d < 7 Å, i.e. propane and iso-butane also form sII gas hydrates. Larger molecules with diameters: 7 Å < d < 9 Å, i.e. iso-pentane and 2, 2-dimethylbutane form sH gas hydrates in the presence of smaller molecules like methane or nitrogen [4] as shown in Table 2.
Flow assurance problems caused by gas hydrates occur as a result of slow cooling of oil and gas in pipeline or rapid cooling due to depressurizing across the valves installed with the pipeline or distribution systems. Recent studies on gas hydrate showed that there are three primary conditions that influence hydrate
Table 2. Properties of Ice, structures I and II gas hydrates.
formation in oil and gas pipelines and in petrochemical processes and they are:
1) The presence of water and gas components,
2) Low temperatures and,
3) High pressures.
There are also secondary influencing factors which favor hydrate formation such as high fluid velocities, agitation, pressure, pulsations or any source of fluid turbulence, the presence of CO2 and H2S [4] . Understanding the properties of gas hydrates is crucial to the exploration and production of these enormous deposits of methane gas. The presence of hydrate in the marine sediments significantly influences some of the properties of the sediments. This can be capitalized on and detected by field measurements and down hole logs.
3.3. Rate of Hydrate Formation
The formation of hydrate has two major steps-nucleation and growth and is a time-dependent process. The knowledge of the dynamics of hydrate formation and accumulation of hydrate crystals is important in determining the parameters for mass production of gas hydrates, and in understanding plug conditions in the gas pipeline and other equipment [17] . The former has an advantage for natural gas storage as hydrate and the later has an advantage for preventing of hydrate blockage in offshore processing, production and transportation of oil and gas. The rate of hydrate formation can be calculated using the Equation (2.0) [17] :
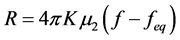 (2.0)
(2.0)
where R is the rate of hydrate formation, K is the empirical kinetic parameter obtained by Englezos 1987, 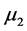 is the second moment of the particle size distribution for the hydrate crystals, f is the fugacity of the gas at the given conditions, and
is the second moment of the particle size distribution for the hydrate crystals, f is the fugacity of the gas at the given conditions, and 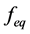 is the fugacity of the gas at equilibrium conditions. The fugacities required can be calculated using the CNGA equation of state for natural gas mixtures where the compressibility factor (Z) is:
is the fugacity of the gas at equilibrium conditions. The fugacities required can be calculated using the CNGA equation of state for natural gas mixtures where the compressibility factor (Z) is:
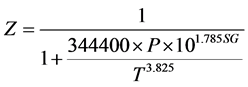 (3.0)
(3.0)
P is the pressure (psig), SG the specific gravity of the gas relative to air, and T the temperature (˚R). The second moment of the particle size distribution (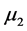 ) can be calculated by the following equations (Englezos et al. 1987):
) can be calculated by the following equations (Englezos et al. 1987):
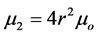 (4.0)
(4.0)
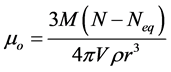 (5.0)
(5.0)
M is the molecular mass of the hydrate, N is the number of moles of gas in solution at the given conditions, 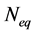 is the number of moles of gas in solution at the equilibrium conditions, V is the volume of water for the above number of moles of gas, ρ is the density of the hydrate, and r is the mean particle radius for the hydrate crystals.
is the number of moles of gas in solution at the equilibrium conditions, V is the volume of water for the above number of moles of gas, ρ is the density of the hydrate, and r is the mean particle radius for the hydrate crystals.
4. Benefits of Gas Hydrate
Methane hydrate, commonly known as gas hydrate holds enormous amount of energy which cancan be used for different purposes. Natural gas contains mainly methane gas which is colorless, odorless and combusts completely to generate carbon dioxide, water and significant amount of energy. There are many gas hydrate deposits in permafrost and deepwater marines. Gas hydrate deposits can be explored to produce methane gas which can serve as a sustainable energy resource. Gas hydrate can also serves as a means of storing and transporting natural gas. This will serve as alternative solution to gas flaring of useful natural resource.
4.1. Gas Hydrate for Natural Gas Storage
Natural gas can be effectively and efficiently stored as gas hydrate. This can be achieved through proper study and understanding of the processes involved to convert the gas to hydrate and the processes required to prevent the hydrate from dissociating. The storage of natural gas as hydrates will require the synthesis of the hydrate and its regasification. This process is beneficial because the density of natural gas hydrates reduces the space requirements for the storage of natural gas. The stored gas can be used in the future and for peak-shaving applications to obtain a higher price for the natural gas as well as to ensure adequate natural gas supplies during periods of peak usage. Peak-shaving application is storing natural gas when natural gas demand is low, then selling the natural gas during periods of high demand.
Gas hydrate can be stored at equilibrium conditions with either its saturation temperature or pressure. The main factors that determine the optimum/limiting pressure and temperature are cost and weight of material, i.e. steel needed for hydrate storage vessel. There are basically three approaches to this operation to make it an economical process:
1) The first is the initial formation of large quantity of gas hydrates to avoid high pressure recompression on recycle,
2) In addition, reproducible, rapid conversion of the gas and water to hydrate to reduce the amount of water transported, and
3) Lastly, the transportation of gas hydrates to locations with small amount of refrigeration and dissociation units needed for the whole operation.
4.2. Sustainable Energy Resource
The estimate of the gas hydrate reserves in the world vary widely and a range between 1015 - 1018 m3 has been widely reported [19] . These estimates are based on research investigation and data obtained by different researchers on conventional hydrocarbon resources. The total quantity of gas in hydrate deposits exceeds, by a factor of two, the total energy content of fuel fossil recoverable reserves by conventional methods [19] . The magnitude of this resource can make gas hydrate reserves a sustainable energy resource. The potential benefit of gas hydrates is also supported by the environmentally friendly nature of natural gas compared to other fossil fuels [14] . Gas can be produced from hydrate deposits through dissociation of hydrate. The three methods of dissociation of gas hydrate are:
1) Depressurization: the pressure of the system containing the hydrate is reduced to a value lower than the hydration pressure at the corresponding temperature,
2) Thermal stimulation: the temperature of the system is allowed to increase above the hydration temperature at the corresponding pressure, and
3) The use of hydrate inhibitors: inhibitors such as salts and alcohols can be used to shift the equilibrium temperature and pressure of hydrate to the region of instability of the hydrate for effective dissociation of the hydrate to its gas and water components.
The most producible hydrate reserves are those directly adjacent gas reserves, such that gas production causes hydrate dissociation by reducing the reservoir pressure below hydrate stability pressure. A similar technique was used in a Siberian permafrost reservoir (Messoyakha) to produce for almost a decade in the 1970s. Gas production from hydrate deposits in the permafrost and ocean sediments containing hydrates is costly but technically feasible. In 2002, Mackenzie delta of Canada used depressurization and thermal stimulation to produce gas from hydrate reserves. The objective of the well was extended to other findings to produce gas from leaner hydrate reserves.
4.3. Gas Hydrate as a Transportation Means
Natural gas can be shipped or transported to different locations where there is complex terrain for pipeline and the quantity of the gas is insufficient to justify liquefaction. It may be economical and viable to transport by converting the gas to hydrate. Hydrate concentrates the gas by a factor that ranges from 150 - 180 and without the cost of liquefaction/compression or transporting the gas at high pressure. In hydrate transportation or shipment, as shown in Figure 6, preservation of the hydrate is vital for preventing losses due to dissociation of the hydrate [17] . Recent research works on hydrates have shown that only a minute amount of refrigeration, i.e. 20 ˚F is required to prevent dissociation of hydrate with rates that are several magnitudes less than those stipulated for lower or higher temperatures. The reason for the anomalous behavior of the hydrate is due to the presence of outer ice barriers that prevent inner-particle dissociation. The ice protective shell is as a result of re-freezing of water from the melted hydrate surface caused by endothermic process.
Research experiments and calculations on gas hydrate have shown that hydrate-gas transportation is economical for distances greater than 400 m. British Gas Ltd., an energy company in the United Kingdom has a pilot facility that converts natural gas to hydrate. The Japanese National Marine Research Institute also started a similar project to transport natural gas as hydrate.
Hydrate can be transported either as slurry or solid blocks depending on the location of target or destination [17] . Hydrate transportation in slurry form has a faster loading, unloading and also ease of handling. This form of transporting hydrate loses storage capacity to anti-freezing agents and extra equipment required to remove anti-freezing agents. The solid form of transporting hydrate uses simplified regasification facility and offers slightly more capacity per storage than that of the slurry form. In this form of transporting hydrate, there is slower loading, unloading and additional solid handling equipment is needed. The slurry form of transporting hydrates is usually a better option for distances of approximately 2500 miles or less while the solid form is suitable for distances of roughly 3500 miles or more [17] . The economics of the two forms of hydrate transportation is shown in Table 3.
The transportation of hydrate at high pressure can be highly expensive because the storage vessel must have thicker walls increasing the cost of transportation
Figure 6. Schematic of a gas hydrate formation, transportation and dissociation system.
Table 3. Economics of hydrate transportation in slurry and solid forms.
and decreasing the amount of hydrate transported. Unlike CNG, shipping gas hydrates at elevated pressures provides no increase in capacity due to the incompressibility of the gas hydrates. Also, polyurethane insulation is relatively more efficient at reducing hydrate losses due to dissociation than increasing the pressure rating of the vessel in order to transport hydrates at elevated temperature. However, at atmospheric pressure, the stable temperature for hydrates is below the freezing point of water which can make the hydrate to freeze any solid in the vessel. Therefore, to effectively handle the hydrate as fluid, ethanol can be added to depress the freezing point of the water. Alternatively, the hydrate slurry mixture can be frozen into manageable blocks and loaded onto the vessel for transportation. The ethanol-water-hydrate system will be more easily loaded and unloaded by the use of pumps, which will reduce the amount of time spent while loading and unloading. But, additional equipment will be required to separate the ethanol vapor from the natural gas. Also, the ethanol displaces natural gas hydrate, thereby reducing the effective capacity of the vessel.
Natural gas transportation as LNG is better economically because the shipping costs for gas hydrates are much higher than the shipping costs for LNG for high transportation distances. The costs for shipping gas hydrates are high because for every ton of natural gas shipped, 6.5 tons of water, which generates no revenue, is also shipped [17] . But for low transportation distances and low production capacities, the costs for shipping gas hydrates are lower than the costs for the entire LNG process. However, as transportation distance increases and capacity increases, LNG becomes a better economic option than gas hydrates in transporting natural gas.
Peak-shaving application is storing natural gas when natural gas demand is low, then selling the natural gas during periods of high demand. For peak-shaving application, shipping of the hydrate is not applicable because they are designed for the temporary storage of natural gas. Production and regasification of these hydrates is cheaper than that of LNG. The production of LNG requires very low temperatures with the use of expensive refrigeration systems while gas hydrates are produced at moderate temperatures with the use of less expensive refrigeration systems. This production condition of gas hydrates makes it economically more favorable than LNG for the temporary storage of natural gas.
5. Conclusions
The abundance of gas hydrate reserves can ultimately make gas hydrate to be a sustainable energy resource all over the world. These hydrate reserves hold significant amount of energy that is estimated to be more than twice the combined carbon of coal, conventional gas and petroleum reserves. The properties and formation of hydrates were studied and examined because they are paramount to efficient and effective exploration and development of hydrate reserves.
Gas hydrate can be stored at equilibrium conditions with either its saturation temperature or pressure. The factors that determine the optimum/limiting pressure and temperature are cost and weight of hydrate storage vessel. The stored gas can be used in the future and for peak-shaving applications to obtain a higher price for the natural gas as well as to ensure adequate natural gas supplies during periods of peak usage. Peak-shaving application is storing natural gas when natural gas demand is low, then selling the natural gas during periods of high demand. The possibility of storing and transporting natural gas as either gas hydrate solid or slurry was also considered. The advantages and disadvantages of solid and slurry forms of transporting gas hydrate were also examined. The slurry form of transport is usually a better option for distances of approximately 2500 miles or less while the solid form is suitable for distances of roughly 3500 miles or more.
Hydrate can serve as a sustainable energy resource and a means of storing and transporting natural gas from one end to the other. This will create an effective and efficient alternative for bulk gas transportation and storage for future use of the gas. Natural gas transportation as LNG is more economical because the shipping costs for gas hydrates are much higher than the shipping costs for LNG and for high transportation distances. The costs for shipping gas hydrates are high because for every ton of natural gas shipped, 6.5 tons of water, which generates no revenue, is also shipped. But for low transportation distances and low production capacities the costs for shipping gas hydrates are lower than the costs for the entire LNG process.
Cite this paper
Aregba, A.G. (2017) Gas Hydrate―Properties, Formation and Benefits. Open Journal of Yangtze Gas and Oil, 2, 27-44. https://doi.org/10.4236/ojogas.2017.21003
References
- 1. Rempel, A.W. and Buffet, B.A. (1997) Formation and Accumulation of Gas Hydrate in Porous Media. Journal of Geophysical Research, 102, 151-164.
https://doi.org/10.1029/97JB00392 - 2. Tohidi, B. (2014) Advances in Avoiding Gas Hydrate Problems. Centre for Gas Hydrate Research & Hydrafact Ltd., Institute of Petroleum Engineering, Heriot-Watt University, 1-47.
- 3. Ruffine, L., Donvala, J.P., Charloua, J.L., Cremièrea, A. and Zehnderb, B.H. (2010) Experimental Study of Gas Hydrate Formation and Destabilisation Using a Novel High-Pressure Apparatus. Marine and Petroleum Geology, 27, 1157-1165.
- 4. Gabitto, J.F. and Tsouris, C. (2010) Physical Properties of Gas Hydrates: A Review. Journal of Thermodynamics, 2010, 1-12.
https://doi.org/10.1155/2010/271291 - 5. Atilhan, M., Aparicio, S., Benyahia, F. and Deniz, E. (2012) Natural Gas Hydrate. Advances in Natural Gas Technology, 10, 194-212.
https://doi.org/10.5772/38301 - 6. Raz, S.B. (2012) A Study of Formation and Dissociation of Gas Hydrate. Master’s Thesis, Office of Graduate Studies of Texas A&M University, College Station, 1-129.
- 7. Jin, S., Nagao, J. and Takeya, S. (2006) Structural Investigation of Methane Hydrates Sediments by Microfocus X-Ray Computed Tomography Technique under High Pressure Conditions. Japanese Journal of Applied Physics, 45, 714-716.
https://doi.org/10.1143/JJAP.45.L714 - 8. Minagawa, H., Nishikawa, Y. and Ikeda, I. (2008) Relation between Permeability and Pore-Size Distribution of Methane-Bearing Hydrates Sediments. Proceeding of the Offshore Technology Conference, Houston, 1-6.
https://doi.org/10.4043/19305-ms - 9. Santamarina, J.C. and Ruppel, C. (2008) The Impacts of Hydrate Saturation on the Mechanical, Electrical and Thermal Properties of Hydrate-Bearing Sand, Silts and Clay. Proceedings of the 6th International Conference on Gas Hydrates, Vancouver, 1-12
- 10. Stoll, R.D. and Bryan, G.M. (1979) Physical Properties of Sediments Containing Gas Hydrate. Journal of Geophysical Research, 84, 1629-1634.
https://doi.org/10.1029/JB084iB04p01629 - 11. Pearson, C., Halleck, P.M., McGuire, P.L., Hermes, R. and Mathews, M. (1983) Natural Gas Hydrate Deposit: A Review of in Situ Properties. The Journal of Physical Chemistry, 87, 4180-4185.
https://doi.org/10.1021/j100244a041 - 12. Winters, W.J., Henry, P., Booth, J.S., Hovland, M. and Clennell, M.B. (1999) Formation of Natural Gas Hydrates in Marine Sediments. Conceptual Model of Gas Hydrate Growth conditioned by Host Sediments Properties, 104, 22985-23003.
- 13. Tinivella, U., Accaino, F., Guistiniani, M. and Loreto, M.F. (2009) Gas Hydrates and Mud Volcanoes Offshore Antarctic Peninsula: A Geophysical Study. Proceedings of the Goldschmidt Conference, Davos, Switzerland, 1-8.
- 14. Tohidi, B., Anderson, R., Clennell, M.B., Burgass, R.W. and Biderkab, A.B. (2001) Visualobservation of Gas Hydrate Formation and Dissociation in Synthetic Porous Media by Means of Glass Micro-Models. Journal of Geology, 29, 869-870.
- 15. Kingston, E., Clayton, C. and Priest, J. (2008) Gas Hydrate Growth Morphologies and Their Effects on the Stiffness and Damping of a Hydrate-Bearing Sand. Proceedings of the 6th International Conference on Gas Hydrates, Vancouver, Vancouver, Canada, 1-7.
- 16. Moridis, G.J. and Kowalsky, M. (2006) Gas Production from Unconfined Class 2 Hydrate Accumulations in the Oceanic Subsurface. Lawrence Berkeley National Laboratory, Earth Sciences Division, 1-8.
- 17. Mannel, D. and Puckett, D. (2008) Natural Gas Hydrate Transportation. University of Oklahoma, Norman, 1-54.
- 18. USGS—Science for a Changing World (2014) Review on International Gas Hydrate R&D Research Programs. USGS-Science for a Changing World, 1-79.
- 19. Birchwood, R., Dai. J., Shelander, D., Boswell, R., Collett, T., Cook, A., Dallimore, S., Fujii, K., Imasato, Y., Fukuhara, M., Kusaka K., Murray, D. and Saeki, T. (2010) Developments in Gas Hydrates. Oilfield Review, 22, 18-33.








