International Journal of Medical Physics, Clinical Engineering and Radiation Oncology
Vol.3 No.1(2014), Article ID:42561,6 pages DOI:10.4236/ijmpcero.2014.31003
Dosimetric Comparison of Different Prescription Modes in Lung Stereotactic Body Radiation Therapy
1Miyakojima IGRT Clinic, Osaka, Japan
2Department of Medical Physics & Engineering, Osaka University Graduate School of Medicine, Osaka, Japan
Email: *hide-miura@osaka-igrt.or.jp
Copyright © 2014 Hideharu Miura et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. In accordance of the Creative Commons Attribution License all Copyrights © 2014 are reserved for SCIRP and the owner of the intellectual property Hideharu Miura et al. All Copyright © 2014 are guarded by law and by SCIRP as a guardian.
Received November 26, 2013; revised December 23, 2013; accepted January 20, 2014
KEYWORDS
Dose Prescription; Monte Carlo Calculation; Stereotactic Body Radiation Therapy; Lung Cancer
ABSTRACT
The purpose of this study was to compare the dose-volume statistics of stereotactic body radiotherapy (SBRT) for lung cancer between planning target volume (PTV): D95 and gross tumor volume (GTV): D99 dose prescriptions using Monte Carlo (MC) calculation. Plans for 183 patients treated between October 2010 and April 2013 were generated based on four-dimensional (4D) computed tomography (CT) under free breathing. A uniform margin of 8 mm was added to the internal target volume (ITV) to generate PTV. A leaf margin of 2 mm was added to the PTV. The plans were calculated with two different dose prescription methods: 40 Gy to cover 95% of the PTV (PTV prescription) and 44 Gy to cover 99% of the GTV (GTV prescription). A 6-MV photon beam was used. A dose-volume histogram (DVH) analysis was performed for dose to the GTV using PTV and GTV dose prescriptions. For each treatment plan, we evaluated the minimum dose to 99% of the GTV (D99). The D99 of GTV was 44.5 ± 1.9 Gy and 44.0 ± 0.0 Gy for PTV and GTV prescriptions, respectively. The dose to the GTV had wide variations with PTV prescription. We recommend that GTV based dose prescription should be used to standardize dose to the tumor and to achieve highly conformal dose distributions in SBRT for lung cancer.
1. Introduction
Stereotactic body radiation therapy (SBRT) plays an increasingly important role in non-surgical treatment of early-stage primary and secondary lung cancers. Multiple institutions published local control rates between 80% and 90% for a large range of treatment doses [1-4]. Because these reports do not cover the results of inhomogeneity correction, the actual dose delivered to the tumor cannot be accurately determined. The influences of heterogeneity correction on dose distribution are reported to result in the larger dose differences for lung SBRT [5-7]. Dose prescription regarding lung SBRT treatment planning is a significant issue. The International Commission on Radiation Units and Measurements (ICRU) recommended methods of dose prescription have been changed from prescription at the isocenter point of the treatment plan (IC prescription) to prescription at the periphery of the planning target volume (PTV) (PTV prescription) [8]. As SBRT has been introduced by the national health insurance system in Japan since 2004, the number of institutions performing SBRT is increasing rapidly. According to Japan Clinical Oncology Group (JCOG) 0403 protocol, the dose prescription is defined as the point dose at the isocenter of the PTV with inhomogeneity correction, such as the Pencil Beam convolution with Batho power law and Clarkson with effective path length correction, but this prescription is not accurate for dose calculations of lung cancer [9]. Total dose of 48 Gy at isocenter is delivered with a daily dose of 12 Gy in 4 fractions within 2 weeks. PTV prescription was adopted in the JCOG 0702 phase I trial instead of the IC prescription adopted in the previous JCOG 0403 phase II trial. In JCOG 0702 protocol, the heterogeneity correction algorithm equivalent to superposition algorithms is required for dose calculation.
In our previous report, we used the iPlan RT Dose to evaluate the dosimetric impact of different dose calculation algorithms (n = 53). Prescribed dose was defined as 95% of the PTV, which should receive 100% of the dose (48 Gy/4 fractions) using Pencil Beam (PB) calculation. We recalculated dose distribution using MC calculation with same parameters (beam arrangement, leaf positions, isocenter position and monitor unit). Average doses to the D95 of the PTV and D99 of the GTV using the MC calculation plan were approximately 20% and 10% lower than those by the PB calculation plan, respectively [10]. The purpose of this study was to compare the dose to the target between PTV: D95 and GTV: D99 dose prescriptions using MC calculation.
2. Methods and Materials
2.1. Patient Selection
183 patients treated with SBRT between October 2010 and April 2013 were included in this analysis. Patient characteristics (Age, GTV, ITV, PTV and Tumor location) are shown in Table 1.
2.2. Treatment Planning
As a routine procedure for the planning of stereotactic radiotherapy, 3D-CT scans were performed on a 4-slice Brightspeed QX/i scanner (GE Medical Systems, Waukesha, WI,USA) to acquire a whole chest image series under free breathing using a motion suppression system, the “Air-bag System” (Niigata Mechatronics Co., Ltd., Niigata, Japan) [11]. The Air-bag System consists of a non-elastic air bag connected to a second smaller elastic air bag. The first air bag is placed between the patient’s body surface and a HipFix device (CIVCO, USA) and secured by pressure adjustment via the elastic air bag.

Table 1. Patient characteristics.
4D-CT was performed to more accurately determine tumor shape, volume, and position at different phases of the breathing cycle. The CT images had a slice thickness of 2.5 mm with a gantry rotation time of 0.5 second. Each image was tagged with the corresponding phase of the respiratory cycle and then sent to the Advantage Workstation (General Electric Company, Waukesha, WI, USA) using the Advantage 4D-CT software. The 4D datasets were categorized into four phases of the respiratory cycle: 0%, 25%, 50%, and 75%, with 0% representing maximum inspiration. Image quality of the 4DCT was sufficient for tumor evaluation in all patients. The visible tumor was delineated as the GTV in the CT pulmonary window of the 4D-CT images. No additional margin was added to GTV for generation of the clinical target volume (CTV). The internal target volume (ITV) was defined as the sum of the CTV positions in all respiratory phases. The PTV was generated by adding a uniform margin of 8 mm to the ITV to account for setup uncertainties and mechanical accuracy. The treatment fields were conformed around the PTV. A leaf margin of 2 mm was added to the PTV, and the isocenter was positioned in the center of the PTV. Beam arrangement used non-coplanar and non-opposing beams. The irradiated lung volume was made as small as possible. The plans were calculated with an iPlan RT Dose, ver 4.1.2 (Brainlab, Munich, Germany) using MC calculation. The iPlan RT dose treatment planning system has an X-ray Voxel Monte Carlo (XVMC) dose calculation engine. XVMC has three main stages for the calculation. The first component of the algorithm is a virtual energy fluence model which is used for the modeling of the upper part of the linac treatment head. The second component of the algorithm models the beam collimating system. The third component of the algorithm computes the dose distribution inside the model of the patient. For the MC photon transport simulations, Compton interactions, pair production events and photoelectric absorptions are considered [12-15]. The plans were calculated with two different dose prescription methods: 40 Gy (10 Gy × 4 fractions) to cover 95% of the PTV (PTV prescription) and 44 Gy (11 Gy × 4 fractions) to cover 99% of the GTV (GTV prescription). A 6-MV photon beam was used.
2.3. Plan Analyses
A dose-volume histogram (DVH) analysis was performed for dose to the GTV using PTV and GTV dose prescriptions. For each treatment plan, we evaluated the minimum dose to 99% of the GTV (D99). The analyzed data were displayed as mean ± standard deviation among 183 clinical plans.
3. Results
The dose distributions and DVH for PTV and GTV prescriptions are shown for three representative cases. Dose to the GTV was almost same using the two different dose prescriptions for the same patient (Figure 1). Dose to the GTV with PTV prescription was about 9.4% lower than the prescribed dose (44 Gy) using the two different dose prescriptions for the same patient (Figure 2). Dose to the GTV with PTV prescription was about 12.5% higher than the prescribed dose (44 Gy) using the two different dose prescriptions for the same patient (Figure 3). Figure 4 shows the histogram of the dose to the GTV using PTV and GTV prescriptions. The D99 of GTV was 44.5 ± 1.9 Gy and 44.0 ± 0.0 Gy for PTV and GTV prescriptions, respectively. The dose to the GTV had wide variations with PTV prescription.
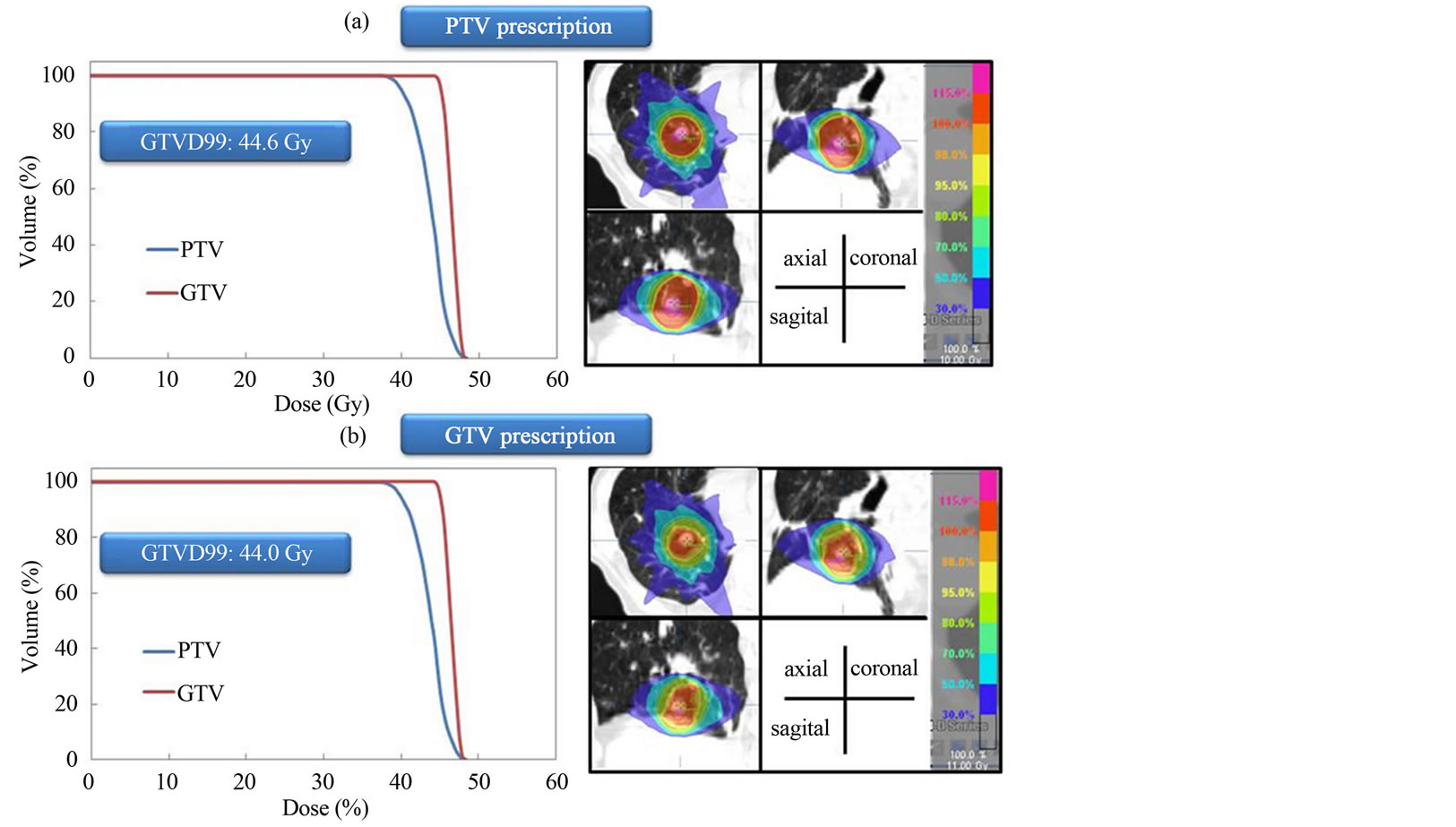
Figure 1. Dose distributions and dose volume histograms calculated at (a) PTV and (b) GTV prescriptions for lung SBRT. Dose to the GTV is almost same using the two different dose prescriptions.
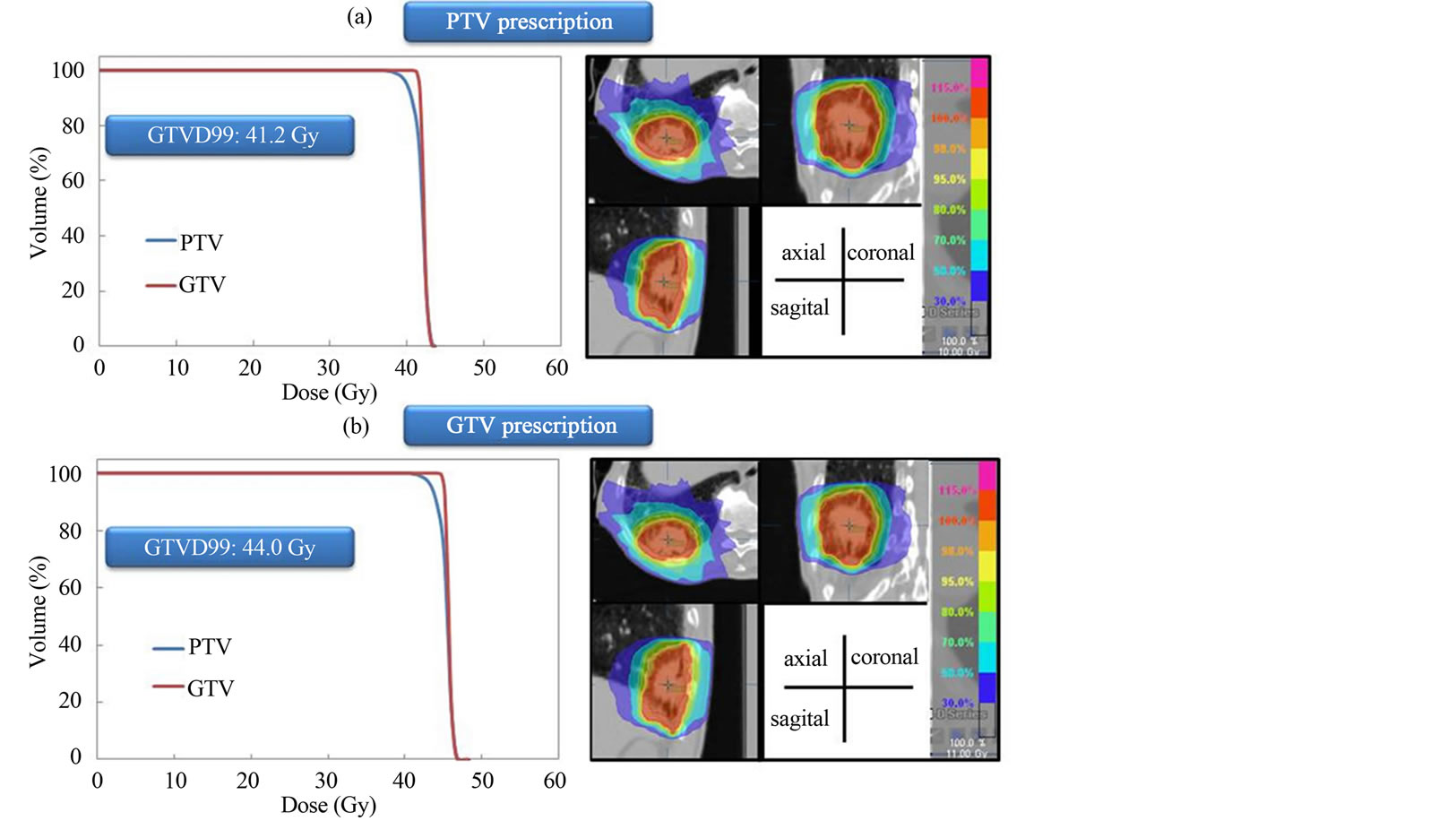
Figure 2. Dose distributions and dose volume histograms calculated at PTV and GTV prescriptions for lung SBRT. Dose to the GTV with PTV prescription is 9.4% lower than prescribed dose (44 Gy).
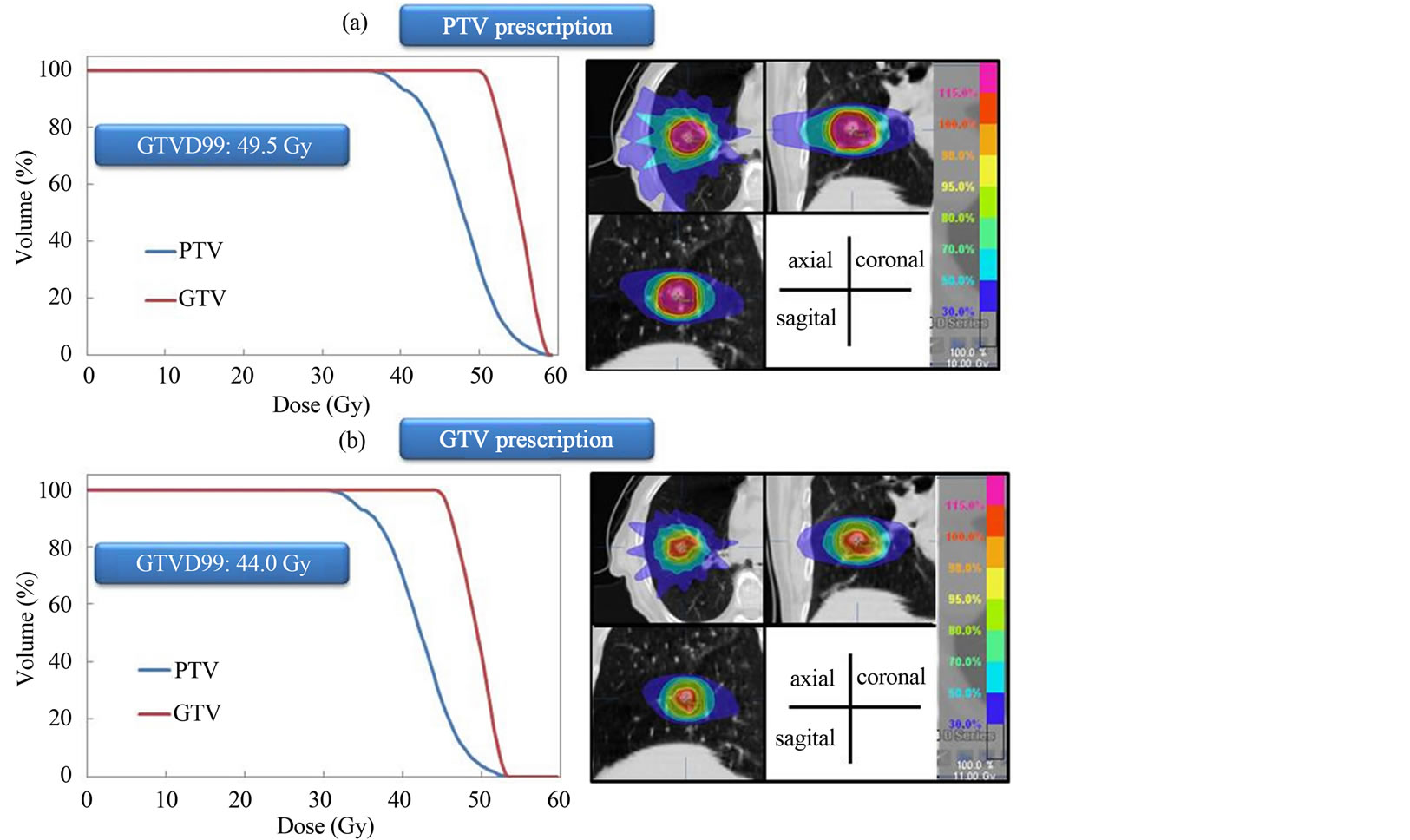
Figure 3. (a) Dose distributions and dose volume histograms calculated at PTV and GTV prescriptions for lung SBRT. Dose to the GTV with PTV prescription is 12.5% higher than prescribed dose (44 Gy).
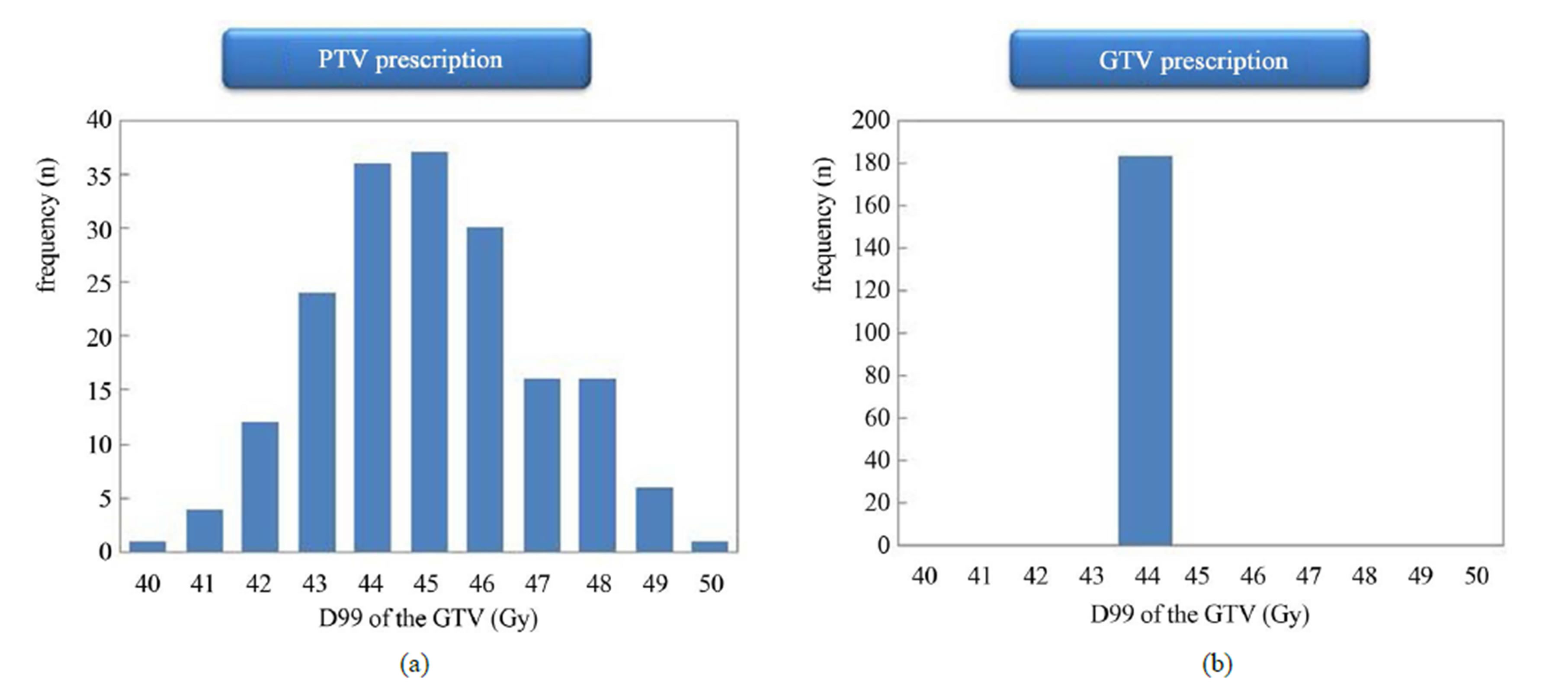
Figure 4. Histograms of the dose to the GTV for (a) PTV and (b) GTV prescriptions (n = 183). With PTV prescription, large variations can be observed in dose to GTV.
4. Discussions
The results of the present study demonstrate the variations in the target dose distributions caused by different prescription modes. Large variations were observed between individual patients. Factors of potential influence on the dose coverage of the GTV are strongly dependent on the lung density [10]. Careful attention should be paid when applying MC calculation using PTV prescription, because dose to the GTV may vary by approximately 10%.
For GTV prescription, respiratory-induced tumor motion and tumor position error raise concern over a decrease between the planned and the delivered dose. Our previous study supported the clinical acceptability of treatment planning for breathing-induced tumor motion based on the dose prescription defined for the GTV. In addition, for most patients tumor motion can be suppressed to less than 5 mm using the Air-bag System [16]. Some patients have a larger tumor motion. Larger tumor motion results in an increased dose to the normal tissue, which increases the risk of normal tissue toxicity (V20), and in turn leads to a higher probability of radiation pneumonitis [17,18]. Most lung cancer patients are old, and gate and synchronize techniques require a longer time for beam delivery, making it more difficult for patients as they must maintain normal breathing over the delivery time. We therefore selected the free-breathing technique using abdominal compression.
Dose prescriptions defined to enclose the PTV often vary widely among institutions, ranging from 65% to 90% relative to the dose at the isocenter [19-21]. The dose to the GTV showed large variations when dose prescription was defined at PTV. In one study, the dose definition was reported as the PTV covering the 100% isodose line, with normalization to 150% at the isocenter by use of an inhomogeneity correction [22]. Target is surrounded by lung normal tissues and some of them are always included in PTV. As PTV is irradiated by higher dose, the lung normal tissues also receive higher dose which may lead to radiation pneumonitis [17,18] and chest wall injuries such as radiation-induced rib fracture [23,24]. Lower dose to the GTV may lead to a negative effect on tumor local control probability. We suggest that the target dose is more appropriately defined at the GTV than at the PTV, because dose to the GTV remains invariant under GTV dose prescription. For local tumor control, dose prescription defined at the GTV may offer advantages over prescriptions defined at the PTV and it may also be used for comparison and standardization of dose prescriptions among institutions. It is stated in ICRU-83 that concept of a PTV might be utilized in unconventional ways to ensure that the prescribed absorbed dose is delivered to the CTV [25]. In our clinic, dose prescription was defined as 99% of the GTV should be covered by 100% of the prescribed dose (D99 = 100%).
5. Conclusion
In conclusion, dose prescription can have significant impacts on dose distributions of SBRT for lung tumor. In particular, the application of MC calculation using PTV prescription can cause a variation in potential dose by approximately 10%. Based on the dose-volume statistics, we recommend that GTV based dose prescription should be used to standardize the dose to the tumor and to achieve highly conformal dose distributions in SBRT for lung cancer.
Conflict of Interest
None.
REFERENCES
- Y. Nagata, J. Wulf, I. Lax, et al., “Stereotactic Radiotherapy of Primary Lung Cancer and Other Targets: Results of Consultant Meeting of the International Atomic Energy Agency,” International Journal of Radiation Oncology * Biology * Physics, Vol. 79, No. 3, 2011, pp. 660-669.
- H. Onishi, T. Araki, H. Shirato, et al., “Stereotactic Hypofractionated High-Dose Irradiation for Stage I NonSmall Cell Lung Carcinoma,” Cancer, Vol. 101, No. 7, 2004, pp. 1623-1631. http://dx.doi.org/10.1002/cncr.20539
- R. Timmerman, L. Papiez, R. McGarry, et al., “Extracranial Stereotactic Radioablation: Results of a Phase I Study in Medically Inoperable Stage I Non-Small Cell Lung Cancer,” Chest, Vol. 124, No. 5, 2003, pp. 1946- 1955. http://dx.doi.org/10.1378/chest.124.5.1946
- Y. Nagata, K. Takayama, Y. Matsuo, et al., “Clinical Outcomes of a Phase I/II Study of 48 Gy of Stereotactic Body Radiotherapy in 4 Fractions for Primary Lung Cancer Using a Stereotactic Body Frame,” International Journal of Radiation Oncology * Biology * Physics, Vol. 63, No. 5, 2005, pp. 1427-1431.
- L. R. Aarup, A. E. Nahum, C. Zacharatou, et al., “The Effect of Different Lung Densities on the Accuracy of Various Radiotherapy Dose Calculation Methods: Implications for Tumour Coverage,” Radiotherapy & Oncology, Vol. 91, No. 3, 2009, pp. 405-414. http://dx.doi.org/10.1016/j.radonc.2009.01.008
- V. Panettieri, B. Wennberg, G. Gagliardi M. Ginjaume and I. Lax, “SBRT of Lung Tumours: Monte Carlo Simulation with PENELOPE of Dose Distributions Including Respiratory Motion and Comparison with Different Treatment Planning Systems,” Physics in Medicine and Biology, Vol. 52, No. 14, 2007, pp. 4265-4281. http://dx.doi.org/10.1088/0031-9155/52/14/016
- U. Haedinger, T. Krieger, M. Flentje and J. Wulf, “Influence of Calculation Model on Dose Distribution in Stereotactic Radiotherapy for Pulmonary Targets,” International Journal of Radiation Oncology * Biology * Physics, Vol. 61, No. 1, 2005, pp. 239-249.
- ICRU, “Prescribing, Recording, and Reporting Photon Beam Therapy,” ICRU Report Volume 50, International Commission on Radiation Units and Measurements, Bethesda, 1993.
- C. Kappas and J. C. Rosenwald, “Quality Control of Inhomogeneity Correction Algorithms Used in Treatment Planning Systems,” International Journal of Radiation Oncology * Biology * Physics, Vol. 32, No. 3, 1995, pp. 847-858.
- H. Miura, N. Masai, R. Oh, et al., “Clinical Introduction of Monte Carlo Treatment Planning for Lung Stereotactic Body Radiotherapy,” Journal of Applied Clinical Medical Physics. (In Press)
- R. Oh, N. Masai, H. Shiomi and T. Inoue. “The ‘Air-Bag System’: A Novel Respiratory Monitoring Device Collaborated with RPM System,” International Journal of Radiation Oncology * Biology * Physics, Vol. 78, No. 3, 2010, pp. 824-825 (Abstract).
- M. Fippel, “Fast Monte Carlo Dose Calculation for Photon Beams Based on the VMC Electron Algorithm,” Medical Physics, Vol. 26, No. 8, 1999, pp. 1466-1475. http://dx.doi.org/10.1118/1.598676
- M. Fippel, F. Haryanto, O. Dohm, F. Nüsslin and S. Kriesen, “A Virtual Photon Energy Fluence Model for Monte Carlo Dose Calculation,” Medical Physics, Vol. 30, No. 3, 2003, pp. 301-311. http://dx.doi.org/10.1118/1.1543152
- M. Fippel, “Efficient Particle Transport Simulation through Beam Modulating Devices for Monte Carlo Treatment Planning,” Medical Physics, Vol. 31, No. 5, 2004, pp. 1235-1242. http://dx.doi.org/10.1118/1.1710734
- I. Kawrakow, M. Fippel and K. Friedrich, “3D Electron Dose Calculation Using a Voxel Based Monte Carlo Algorithm (VMC),” Medical Physics, Vol. 23, No. 4, 1996, pp. 445-457. http://dx.doi.org/10.1118/1.597673
- H. Miura, N. Masai, R. J. Oh, H. Shiomi, J. Sasaki and T. Inoue, “Approach to Dose Prescription of the Gross Tumor Volume for Lung Cancer with Respiratory Tumor Motion,” Journal of Radiation Research, Vol. 54, No. 1, 2013, pp. 140-145. http://dx.doi.org/10.1093/jrr/rrs054
- H. Hof, J. Zgoda, S. Nill, et al., “Timeand Dose-Dependency of Radiographic Normal Tissue Changes of the Lung after Stereotactic Radiotherapy,” International Journal of Radiation Oncology * Biology * Physics, Vol. 77, No. 5, 2010, pp. 1369-1374.
- A. Takeda, T. Ohashi, E. Kunieda, et al., “Early Graphical Appearance of Radiation Pneumonitis Correlates with the Severity of Radiation Pneumonitis after Stereotactic Body Radiotherapy (SBRT) in Patients with Lung Tumors,” International Journal of Radiation Oncology * Biology * Physics, Vol. 77, No. 3, 2010, pp. 685-690.
- M. Guckenberger, J. Wilbert, T. Krieger, et al., “FourDimensional Treatment Planning for Stereotactic Body Radiotherapy,” International Journal of Radiation Oncology * Biology * Physics, Vol. 69, No. 1, 2007, pp. 276-285.
- P. Baumann, J. Nyman, M. Hoyer, et al., “Outcome in a Prospective Phase II Trial of Medically Inoperable Stage I Non-Small Cell Lung Cancer Patients Treated with Stereotactic Body Radiotherapy,” Journal of Clinical Oncology, Vol. 27, No. 20, 2009, pp. 3290-3296. http://dx.doi.org/10.1200/JCO.2008.21.5681
- S. W. Lee, E. K. Choi, H. J. Park, et al., “Stereotactic Body Frame Based Fractionated Radiosurgery on Consecutive Days for Primary or Metastatic Tumors in the Lung,” Lung Cancer, Vol. 40, No. 3, 2003, pp. 309-315. http://dx.doi.org/10.1016/S0169-5002(03)00040-0
- I. Lax, “Target Dose versus Extra-Target Dose in Stereotactic Radiosurgery,” Acta Oncologica, Vol. 32, No. 4, 1993, pp. 453-457. http://dx.doi.org/10.3109/02841869309093624
- N. Pettersson, J. Nyman and K. A. Johansson, “RadiationInduced Rib Fracture after Hypofractionated Stereotactic Body Radiation Therapy of Non-Small Cell Lung Cancer: A Doseand Volume-Response Analysis,” Radiotherapy & Oncology, Vol. 91, No. 3, 2009, pp. 360-368. http://dx.doi.org/10.1016/j.radonc.2009.03.022
- S. S. Kim, S. Y. Song, J. Kwak, et al., “Clinical Prognostic Factors and Grading System for Rib Fracture Following Stereotactic Body Radiation Therapy (SBRT) in Patients with Peripheral Lung Tumors,” Lung Cancer, Vol. 79, No. 2, 2013, pp. 161-166. http://dx.doi.org/10.1016/j.lungcan.2012.10.011
- V. Gregoire, T. R. Mackie, W. D. Neve, et al., “Prescribing Recording and Reporting Photon-Beam IntensityModulated Radiation Therapy (IMRT),” Journal of the ICRU, Vol. 10, No. 1, 2010, pp. 1-112.
NOTES
*Corresponding author.

