Advances in Parkinson's Disease
Vol.2 No.4(2013), Article ID:39069,5 pages DOI:10.4236/apd.2013.24020
Pseudoparkinsonism: A review of a common nonparkinsonian hypokinetic movement disorder
![]()
Atlantic Neuroscience Institute, Overlook Medical Center, Summit, USA; *Corresponding Author: roger.kurlan@atlantichealth.org
Copyright © 2013 Roger Kurlan, Marcie L. Rabin. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received 16 September 2013; revised 16 October 2013; accepted 24 October 2013
Keywords: Parkinsonism; Pseudoparkinsonism; Paratonia; Apraxia; Akinetic-Rigid Syndrome
ABSTRACT
This article reviews the syndrome pseudoparkinsonism, a movement disorder described in the literature that resembles parkinsonism but differs qualitatively. Patients with this disorder have apraxic slowness, paratonic rigidity, frontal gait disorder and elements of akinesia that, taken together, may be mistaken for true parkinsonism. Pseudoparkinsonism appears to be common and is most often due to Alzheimer’s disease or vascular dementia. It seems that patients with even mild cognitive deficits can present with pseudoparkinsonism and that the primary dementing disorder may be overlooked. The authors emphasize the importance of pseudoparkinsonism and implications for clinical diagnosis, treatment and research.
1. INTRODUCTION
History of the Pseudoparkinsonism
Soon after the introduction of neuroleptic antipsychotic medications in the 1950’s, it was recognized that the dopamine receptor antagonist properties of these drugs could induce a clinical syndrome that resembled Parkinson’s disease (PD). Consisting of hypomimia, hypophonia, drooling, resting tremor, rigidity, bradykinesia, and a shuffling and unsteady gait, the condition was referred to as “pseudo-parkinsonism” [1] in order to distinguish it from the neurodegenerative disease. In this case, the term “pseudo” implied a false or non-authentic cause of features of PD. Over the next several decades, “pseudo-parkinsonism” was used in many publications to denote the neuroleptic drug-induced movement disorder [2-4].
Over time, clinicians learned that a variety of entities besides neuroleptic drugs can similarly cause a clinical picture resembling that of PD. The notion that a parkinsonian syndrome could be caused by cerebrovascular disease became popularized in the 1980’s and 1990’s. Some publications referred to the condition as “vascular pseudoparkinsonism” [5,6], similar to the term used for neuroleptic drugs. Other articles, in contrast, used the term “vascular parkinsonism” to describe features with a vascular etiology that are reminiscent of, but distinct from, those found in PD. These include gait disorders such as lower body parkinsonism, frontal-type gait disorders and gait ignition failure [6]. The acute or subacute onset of parkinsonism or pseudoparkinsonism has been described in association with stroke involving specific vascular territories [6]. Eventually, the term “parkinsonism” became used to describe the constellation of signs typically seen in PD, whether caused by PD itself or by another etiology, thus replacing the term “pseudo parkinsonism”. Neuroleptic and other medication-induced forms are now generally referred to as “drug-induced parkinsonism” and the vascular form is now termed “vascular parkinsonism” [7,8]. Non-degenerative causes of parkinsonism, such as drugs, cerebrovascular disease, infections, and others, have often been referred to collectively as “secondary parkinsonism”. Other neurodegenerative diseases that can resemble PD, such as progressive supranuclear palsy and multiple system atrophy, are often referred to as “parkinson plus syndromes” or “atypical parkinsonism” to denote the presence of true parkinsonian features while at the same time highlight the presence of features considered atypical of idiopathic PD. Some of these atypical features include symmetric motor dysfunction, absence of resting tremor, poor response to levodopa, and presence of certain characteristics such as prominent autonomic dysfunction, cerebellar signs and early dementia.
In 2000, we published an article [9] that discussed the confusing literature on extrapyramidal parkinsonian signs (slowness, rigidity, shuffling gait) being reported in patients with Alzheimer’s disease [10-15] and how the proper classification of hypokinetic movement disturbances required a careful consideration of not only their presence or absence but also their quality. Thus, we suggested that the term “parkinsonism” should be reserved for conditions that showed the qualitative motor features of PD and were due to dysfunction of the nigrostriatal dopaminergic pathway. Parkinsonism includes bradykinesia (slowness and paucity of movement), lead pipe (non-varying resistance to passive movement of a limb; i.e., like bending a lead pipe) rigidity, resting tremor, postural imbalance, and a slow, shuffling gait. We further suggested that the term “pseudoparkinsonism” be used for the hypokinetic syndrome that superficially resembled PD but was qualitatively different (Table 1). The “pseudo” component implies that the motor dysfunction resembles but is qualitatively not what occurs in PD or parkinsonism and is not related to nigrostriatal dysfunction, but rather to diffuse or multifocal cortical or subcortical dysfunction.
2. DISTINGUISHING PSEUDOPARKINSONISM FROM TRUE PARKINSONISM
In both parkinsonism and pseudoparkinsonism patients can appear slow, rigid and have a shuffling gait. In true parkinsonism, these clinical features are due to bradykinesia, lead pipe rigidity and parkinsonian gait. In pseudoparkinsonism, the similar clinical appearance is due to apraxic slowness, paratonic rigidity and frontal gait disorder. Apraxia in pseudoparkinsonism is an inability to perform or slowness (due to slowed cognitive processing) in performing skilled motor acts, such as dressing, eating, or walking, despite intact primary neurological functions (comprehension of the task, motor strength, sensation and coordination). Apraxia results from a disturbance of cortical association function and can lead to the appearance of akinesia (the failure of willed movement to occur), hypokinesia (reduced amplitude of movement) and
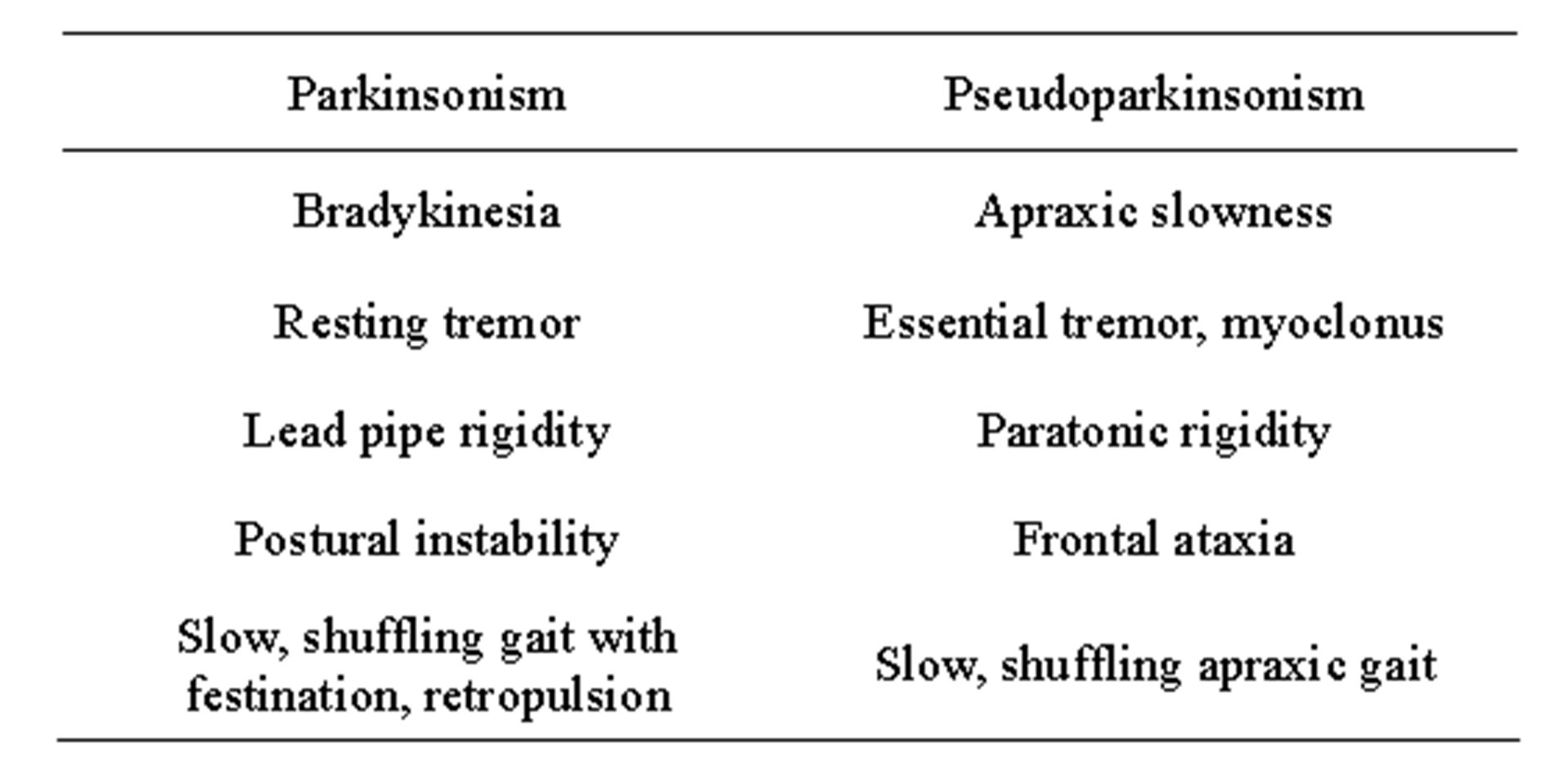
Table 1. Parkinsonism vs. pseudoparkinsonism.
bradykinesia (slowness or poverty of movement), and thus closely resembles parkinsonism [16]. The physiological sequence effect of parkinsonian bradykinesia [22], namely reduced starting force and progressive reduction of speed and amplitude which can be seen with repetitive finger movements, would not be expected to be observed in pseudoparkinsonism. Bedside or neurocognitive testing of praxis can also help distinguish apraxic slowness from bradykinesia [9]. Rigidity in pseudoparkinsonism is paratonia (gegenhalten; varying resistance to passive movement of a limb) [17]. The degree of resistance in paratonia typically depends on the speed of movement, usually being greater with faster movements and less or absent with slower movements. In contrast, in parkinsonism the degree of resistance is not speed-dependent.
The gait of patients with pseudoparkinsonism has been described as a frontal (apraxic, cortical) gait disorder, consisting of short steps, shuffling, “magnetic” (“glued to the floor”) qualities, start and turn hesitation and transient freezing, reduced arm swing, stooped posture and imbalance [6,18]. However, these are all also qualities that can occur PD so they are not helpful in distinguishing a frontal gait disorder from a parkinsonian gait [18]. The presence of festination and retropulsion, in contrast, are more characteristic of a parkinsonian gait while a wide-based stance is more common in frontal gait disorders [17]. The frontal ataxia due to pseudoparkinsonism can also be mistaken for the postural imbalance due to the extrapyramidal etiology seen in true parkinsonism. Another important way of distinguishing true parkinsonism from pseudoparkinsonism is that the motor features of the former generally respond to dopaminergic medications while those of the latter do not.
Beyond apraxic slowness, paratonic rigidity and frontal gait disorder, there are some additional phenomenological considerations relevant to the identification of pseudoparkinsonism. Paratonic rigidity is often accompanied by mitgehen (“go with”), a phenomenon in which the patient anticipates the movements and initiates them before the examiner. Mitgehen is not part of parkinsonism. Although cogwheeling has been viewed as a specific sign of parkinsonism, this phenomenon can also occur when there is a combination of paratonic rigidity and essential tremor or myoclonus; thus, it can be seen in pseudoparkinsonism. Because myoclonus is common in patients with Alzheimer’s disease and vascular dementia, it is often seen in patients with pseudoparkinsonism. In our experience, myoclonus that is very frequent can be mistaken for a resting tremor by referring physicians. Resting tremor has a predictable to-and-fro rhythmic pattern, while myoclonus, when closely examined, is generally not truly rhythmic and has multiple directions of movement. Clinical features that distinguish pseudoparkinsonism and true parkinsonism are summarized in Table 1.
Pseudoparkinsonism appears to be most common in patients with Alzheimer’s disease or vascular dementia [8]. Thus, most patients with pseudoparkinsonism will have coexisting dementia. Cognitive testing may be useful in identifying dementia. When encountering patients with vascular risk factors or a history of transient ischemic attacks or stroke who present with a hypokinetic movement disorder, a high index of suspicion should be present for a vascular etiology, including vascular pseudoparkinsonism. Pseudoparkinsonism can also be seen as a result of other causes of diffuse or multifocal cortical dysfunction, such as infection, trauma and anoxia, and has sometimes been referred to as an “akinetic-rigid syndrome” [19-21]. The presence of features like pyramidal tract signs, unilateral central facial weakness or seizures suggests involvement of brain regions outside of the basal ganglia and should also raise suspicion for pseudoparkinsonism. In certain conditions, there may be a combination of both parkinsonism and pseudoparkinsonism. This occurs when pathology involves both basal ganglia and cortical regions. Examples include cerebrovascular disease, dementia with Lewy bodies, frontotemporal dementia with parkinsonism, and PD with dementia.
3. CLINICAL IMPORTANCE OF PSEUDOPARKISONISM
The term “pseudoparkinsonism” has been used very little in the recent literature. This may reflect some discomfort or confusion with the term. Indeed, the “parkinsonism” component of the term is confusing since the condition does not include true parkinsonism. Furthermore, the “pseudo” aspect has been used in the neurological literature for different purposes, often related to conversion disorders, which is not the case here. However, the concept of pseudoparkinsonism appears to be very important in clinical diagnosis.
Recognition of the entity pseudoparkinsonism has a number of important implications. First, the existence of pseudoparkinsonism suggests that the initial clinical approach to assessing a patient with a hypokinetic movement disorder should first involve distinguishing parkinsonism from the nonparkinsonian mimic pseudoparkinsonism. This is accomplished mainly by carefully evaluating the quality of the motor signs as discussed above.
An obvious implication of pseudoparkinsonism is that failure to recognize it can lead to a mistaken diagnosis of PD or another parkinsonian condition which usually has a very different course, prognosis and treatment approach. Thus, patients and their families may obtain inaccurate information and form mistaken expectations about the future. Although there is little published information, in our experience antiparkinsonian medications do not lead to substantial improvement in patients with pseudoparkinsonism and often cause significant side effects, such as confusion, psychosis and orthostatic hypotension. These, in turn, could worsen the patient’s overall condition and increase morbidity, from such things as falling. Furthermore, if the clinician focuses only on the motor disturbance, these patients may miss out on more appropriate evaluations (e.g., tests for potentially reversible causes of dementia) and treatments (e.g., cholinesterase inhibitors). Published clinicopathological series have shown that about one-quarter of patients diagnosed with PD by neurologists did not demonstrate this pathology at autopsy [23,24]. Alzheimer’s disease and cerebrovascular disease, the two most common causes of pseudoparkinsonism, were the two most common actual diagnoses after essential tremor.
4. PSEUDOPARKINSONISM AND MILD COGNITIVE IMPAIRMENT
For the vascular dementia, there is growing evidence that even mild signs of microangiopathic pathology on MRI correlate with cognitive decline [25]. Likewise, minor degrees of small vessel cerebrovascular disease appear to be associated with motor dysfunction. Slow gait in older individuals is strongly associated with cerebrovascular disease [26] and the extent of cerebral white matter hyperintensities on MRI closely correlate with slow and unsteady gait [27,28]. A recent study found that neurovascular coupling (the link between regional cerebral synaptic activity and blood flow) is impaired in slow walkers [29]. It has yet to be determined whether these motor changes are linked to the degree of resultant cognitive impairment or are independent of them. For Alzheimer’s disease, it is unclear what degree of cognitive impairment is needed to cause pseudoparkinsonism. However, a recent study found that measured in-home walking speeds are slower in patients with mild cognitive impairment compared to matched individuals with normal cognition [30]. It is possible that the cognitive deficits may be mild enough early on that the clinician fails to consider a primary dementing illness in patients presenting with pseudoparkinsonism. Formal mental status testing may be advisable to detect the more subtle cognitive dysfunction.
5. RESEARCH IMPORTANCE OF PSEUDOPARKINSONISM
If patients with pseudoparkinsonism are being enrolled in research studies aimed at clarifying the etiology, pathogenesis, epidemiology or therapy of PD, inaccurate results may be obtained. It thus appears to be advisable for inclusion criteria in such studies to focus not only on the presence or absence of features like rigidity or slowness of movement, but also on the quality. Many studies of PD rely on the Unified PD Rating Scale to establish the presence of parkinsonism, but it is important to recognize that this scale was developed to assess the severity of illness in patients already diagnosed with PD. It fails to distinguish, for example, paratonic from parkinsonian rigidity or apraxic slowness from parkinsonian bradykinesia.
6. CONCLUSION
The condition pseudoparkinsonism consists of motor features that resemble PD or parkinsonism but are qualitatively different. It appears to be a common and important yet under-appreciated entity. Patients with pseudoparkinsonism may be misdiagnosed as having PD or another parkinsonian condition with inappropriate therapies prescribed and more appropriate assessments and treatments are delayed or not provided at all. Alzheimer’s disease and vascular dementia appear to be common causes of pseudoparkinsonism, suggesting that cognitive disturbances are associated with or may cause motor dysfunction. It appears that patients with even mild cognitive impairments can present with the motor disturbance of pseudoparkinsonism and that the underlying primary cognitive disturbance may be overlooked.
REFERENCES
- Kabat, H. (1959) Drug therapy of cerebellar ataxia and disorders of the basal ganglia, based on cerebellar-striatal antagonism. Annals of Internal Medicine, 50, 1438-1448. http://dx.doi.org/10.7326/0003-4819-50-6-1438
- Crane, G.E. (1972) Pseudoparkinsonism and tardive dyskinesia. Archives of Neurology, 27, 426-430. http://dx.doi.org/10.1001/archneur.1972.00490170058008
- Janowsky, D.S., el-Yousef, M.K., Davis, J.M., et al. (1972) Effects of amantadine on tardive dyskinesia and pseudo-parkinsonism. The New England Journal of Medicine, 286, 785. http://dx.doi.org/10.1056/NEJM197204062861424
- Yassa, R. and Nair, V. (1988) The association of tardive dyskinesia and pseudoparkinsonism. Neuro-Psychopharmacology and Biological Psychiatry, 12, 909-914.
- Chang, C.M., Yu, Y.L., Ng, H.K., et al. (1992) Vascular pseudoparkinsonism. Acta Neurologica Scandinavica, 86, 588-592. http://dx.doi.org/10.1111/j.1600-0404.1992.tb05492.x
- Sibon, I., Fenelon, G., Quinn, N.P. and Tison, F. (2004) Vascular parkinsonism. Journal of Neurology, 251, 513- 524. http://dx.doi.org/10.1007/s00415-004-0421-4
- Demirkirn, M., Bozdemir, H. and Sarica, Y. (2001) Vascular parkinsonism: A distinct, heterogeneous clinical entity. Acta Neurologica Scandinavica, 104, 63-67. http://dx.doi.org/10.1034/j.1600-0404.2001.104002063.x
- Zijlmans, J.C., Daniel, S.E., Hughes, A.J., et al. (2004) Clinicopathological investigation of vascular Parkinsonism. Movement Disorders, 19, 630-640. http://dx.doi.org/10.1002/mds.20083
- Kurlan, R., Richard, I.H., Papka, M. and Marshall, F. (2000) Movement disorders in Alzheimer’s disease: More rigidity of definitions is needed. Movement Disorders, 15, 24-29. http://dx.doi.org/10.1002/1531-8257(200001)15:1<24::AID-MDS1006>3.0.CO;2-X
- Pearce, J. (1974) The extrapyramidal disorder of Alzheimer’s disease. European Neurology, 12, 94-116. http://dx.doi.org/10.1159/000114608
- Molsa, P.K., Marttila, R.J. and Rinne, U.K. (1984) Extrapyramidal signs in Alzheimer’s disease. Neurology, 34, 1114-1116. http://dx.doi.org/10.1212/WNL.34.8.1114
- Chui, H.C., Lyness, S.A., Sobel, E. and Schneider, L.S. (1994) Extrapyramidal signs and psychiatric symptoms predict faster cognitive decline in Alzheimer’s disease. Archives of Neurology, 51, 676-681. http://dx.doi.org/10.1001/archneur.1994.00540190056015
- Chui, H.C., Teng, E.L., Henderson, V.W. and Moy, A.C. (1988) Clinical sub-types of dementia of the Alzheimer’s type. Neurology, 35, 1544-1550. http://dx.doi.org/10.1212/WNL.35.11.1544
- Stern, Y., Albert, M., Brandt, J., et al. (1994) Utility of extrapyramidal signs and psychosis as predictors of cognitive and functional decline, nursing home admission and death in Alzheimer’s disease: Prospective analyses from the Predictors Study. Neurology, 44, 2300-2307. http://dx.doi.org/10.1212/WNL.44.12.2300
- Schmidt, C., Wolff, M., Weitz, M., et al. (2001) Rapidly progressive Alzheimer’s disease. Archives of Neurology, 68, 1124-1130. http://dx.doi.org/10.1001/archneurol.2011.189
- Berardelli, A., Rothwell, J.C., Thompson, P.D., et al. (2001) Pathophysiology of bradykinesia in Parkinson’s disease. Brain, 124, 2131-2146. http://dx.doi.org/10.1093/brain/124.11.2131
- Hobbelen, J.H.S.M., Koopmans, R.T.C.M., Verhey, F.R.J., et al. (2006) Paratonia: A Delphi procedure for consensus definition. Journal of Geriatric Physical Therapy, 29, 6- 12. http://dx.doi.org/10.1519/00139143-200608000-00002
- Nutt, J.G., Marsden, C.D. and Thompson, P.D. (1993) Human walking and higher level gait disorders, particularly in the elderly. Neurology, 43, 268-279. http://dx.doi.org/10.1212/WNL.43.2.268
- Bhatt, M.H., Obeso, J.A. and Marsden, C.D. (1993) Time course of postanoxic akinetic-rigid and dystonic syndromes. Movement Disorders, 43, 314-322.
- Singer, C., Berger, J.R., Bowen, B.C., et al. (1993) Akineticrigid syndrome in a 13-year-old girl with HIV-related progressive multifocal leukoencephalopathy. Movement Disorders, 8, 113-116. http://dx.doi.org/10.1002/mds.870080120
- Bhatt, M., Desai, J., Mankodi, A., et al. (2000) Posttraumatic akinetic-rigid syndrome resembling Parkinson’s disease: A report on three patients. Movement Disorders, 15, 313-317. http://dx.doi.org/10.1002/1531-8257(200003)15:2<313::AID-MDS1017>3.0.CO;2-P
- Iansek, R., Huxham, F. and McGinley, J. (2006) The sequence effect and gait festination in Parkinson’s disease: Contributors to freezing of gait? Movement Disorders, 21, 1419-1424. http://dx.doi.org/10.1002/mds.20998
- Hughes, T.A., Daniel, S.E., Blankson, S. and Lees, A.J. (1993) A clinicopathologic study of 100 cases of Parkinson’s disease. Archives of Neurology, 50, 140-148. http://dx.doi.org/10.1001/archneur.1993.00540020018011
- Rajput, A.H., Rozdilsky, B. and Rajput, A. (1991) Accuracy of clinical diagnosis in parkinsonism: A prospective study. Canadian Journal of Neurological Sciences, 18, 275-278.
- Gorelick, P.B., Scuteri, A., Black, S.E., et al. (2011) AHA/ ASA Scientific Statement: Vascular contributions to cognitive impairment and dementia. Stroke, 42, 2672-2713. http://dx.doi.org/10.1161/STR.0b013e3182299496
- McGinn, A.P., Kaplan, R.C., Verghese, J., et al. (2008) Walking speed and risk of incident ischemic stroke among postmenopausal women. Stroke, 39, 1233-1239. http://dx.doi.org/10.1161/STROKEAHA.107.500850
- Briley, D.P., Wasay, M., Sergent, S. and Thomas, S. (1997) Cerebral white matter changes (leukoariosis), stroke, and gait disturbance. Journal of the American Geriatrics Society, 45, 1434-1438.
- Baloh, R.W., Yue, Q., Socotch, T.M. and Jacobsen, K.M. (1995) White matter lesions and disequilibrium in older people. Archives of Neurology, 52, 970-974. http://dx.doi.org/10.1001/archneur.1995.00540340062013
- Sorond, F.A., Kiely, D.K., Galica, A., et al. (2011) Neurovascular coupling is impaired in slow walkers: The MOBILIZE Boston study. Annals of Neurology, 70, 213-220. http://dx.doi.org/10.1002/ana.22433
- Dodge, H.H., Mattek, N.C., Austin, D., et al. (2012) Inhome walking speed and variability trajectories associated with mild cognitive impairment. Neurology, 78, 1946- 1952. http://dx.doi.org/10.1212/WNL.0b013e318259e1de

