Advances in Parkinson's Disease
Vol.2 No.3(2013), Article ID:34966,6 pages DOI:10.4236/apd.2013.23015
Factors affecting early decline of executive function after subthalamic nucleus stimulation in Parkinson’s disease*
![]()
1Department of Research and Therapeutics for Movement Disorders, Juntendo University School of Medicine, Tokyo, Japan; #Corresponding Author: aumemura@juntendo.ac.jp
2Department of Neurosurgery, Nagoya City University Graduate School of Medicine, Nagoya, Japan
3Department of Rehabilitation Medicine, Nagoya City University Graduate School of Medicine, Nagoya, Japan
4Department of Neurology, Nagoya City University Graduate School of Medicine, Nagoya, Japan
Copyright © 2013 Atsushi Umemura et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received 11 April 2013; revised 12 May 2013; accepted 20 May 2013
Keywords: Parkinson’s Disease; Deep Brain Stimulation; Subthalamic Nucleus; Executive Function
ABSTRACT
Subthalamic nucleus deep brain stimulation (STN DBS) is an effective treatment for medically refractory Parkinson’s disease (PD). However, a minority of patients develop cognitive problems, particularly a decline of executive function in the early period after STN DBS. Although this problem is usually transient, it may cause social maladjustment. We investigated factors affecting early decline of executive function after STN-DBS. Fifty-seven patients whose preoperative global cognitive screening was normal (MMSE score; 28 or more) were enrolled in this study. Executive function was evaluated with the Trail-Making Test (TMT) preoperatively and 1- month after surgery. We considered a patient to have decline in executive function if the TMT (B-A) was prolonged more than 30 seconds after STN DBS. Among 57 patients, 25 patients were categorized as having decline of executive function. Univariate analysis revealed that high preoperative UPDRS III motor score in the medication-off period and a depressive state evaluated with BDI-II correlated significantly with decline in executive function. Multiple logistic regression analysis revealed that the only significant independent variable related to early decline of executive function was the preoperative BDI-II score. Postoperative factors such as active contact location or dopaminergic medication reduction had no relation with the decline of executive function. Even in cognitively well-selected patients, STN DBS causes early decline in executive function in a significant number of patients. Preoperative simple cognitive screening alone could not predict early decline in executive function. More detailed neuropsychological evaluation, including mood status, should be undertaken before surgery.
1. INTRODUCTION
Subthalamic nucleus deep brain stimulation (STN DBS) has been widely accepted as an effective treatment for medically refractory Parkinson’s disease (PD). STN DBS improves motor function and also quality of life (QOL) of patients more effectively than medical treatment alone [1,2]. However, it is certain that a minority of patients develop cognitive problem, particularly a decline of executive function in the early period after surgery [3-5]. Among most patients, a dramatic improvement of motor function overcomes cognitive problem after STN DBS and ameliorates QOL.
Executive functions are high-level cognitive functions that are involved in the control and direction of lower-level functions [6]. Executive functions can be conceptualized as having four components: volition, planning, purposive action, and effective performance. All is necessary for appropriate, socially responsible, and effectively self-serving adult conduct [7]. The frontal lobes of the brain seem to play a major role in executive function. Recent studies demonstrate that a decline in executive function after STN DBS is transient [8-10]. However, it may cause problems of social maladjustment in daily life for the patients and their families [11,12]. Therefore, preoperative prediction of decline in executive function would be very desirable.
A decline of executive function after surgery seems to be attributable to surgical intervention, the direct effect of STN stimulation, and postoperative dopaminergic medication changes. However, some patients experience this problem and the other patients do not, even with the same surgical procedure and same method of postoperative adjustment of stimulation and medication. Therefore, there must be some other causative factors at work in postoperative decline of executive function. In this study, we attempted to discover preand postoperative factors affecting early decline of executive function after STNDBS in patients with normal global cognition.
2. MATERIALS AND METHODS
2.1. Patients and Assessments
Fifty-seven patients whose preoperative global cognitive screening was normal (MMSE score; 28 or more) were enrolled in this study. All patients underwent bilateral STN-DBS in one-stage at Nagoya City University Hospital for motor complications resulting from levodopa. Quadripolar DBS electrodes (Activa 3389; Medtronic, Minneapolis, MN) were implanted into the STN stereotactically under MRI guidance with physiological refinement by microelectrode recording. The DBS lead was placed as the most distant contact (contact 0) placed at the bottom of the STN. Electrical stimulation began a few days after surgery. Stimulation parameters were adjusted to produce maximal clinical benefit for cardinal PD symptoms without side effects. A monopolar electrode setting with one or two active contacts was used in all patients. In all cases, stimulation parameters were 90 µsec of pulse width, 130 Hz of pulse rate, and 2 - 3 volts of amplitude. Contact 1 or 2 or both were used as active contact in all cases. After surgery, dopaminergic medication was initially reduced by approximately 50% and then further reduced or increased based on stimulation-induced improvements of PD symptoms. Later, more detailed medication and stimulation adjustment was carried out in the outpatient clinic.
Each patient underwent a clinical assessment including a review of clinical notes, drug history and the Unified Parkinson’s Disease Rating Scale (UPDRS) part III motor score and part IV score, preoperatively (in medication-on and -off conditions) and postoperatively (1- month after surgery). Response to levodopa was estimated as percentage of improvement of preoperative UPDRS III motor score by medication. Axial symptoms were evaluated with the scores of items 29-30, and dyskinesia was evaluated with scores of items 32-35 on UPDRS. The levodopa-equivalent daily dosage (LEDD) was calculated according to the report by Tomlinson et al [13].
Neuropsychological examinations included the MMSE for a screening of global cognition, the Frontal Assessment Battery (FAB) for a screening of frontal lobe function, the Trail Making Test (TMT) for a test of executive function, and the Beck Depression Inventory-second edition (BDI-II) for a test of mood status, and were performed preoperatively and 1-month after surgery. In the preoperative period, evaluations were done when patients were in the medication-on condition. In the postoperative period, patients were assessed under stable conditions after initiation of electrical stimulation. In TMT, the time difference between TMT-B and TMT-A [TMT (B-A)] was evaluated to eliminate the influence of motor speed.
2.2. Statistical Analysis
We assessed a patient as having decline in executive function if the post-DBS score of TMT (B-A) was prolonged more than 30 seconds compared to the preoperative score. Among 57 patients, 25 patients were categorized as having decline of executive function and 32 patients were categorized as having no decline of executive function. Comparing these two groups, we searched for factors affecting early decline of executive function after STN-DBS.
Potential predictive factors affecting early decline of executive function were initially screened using univariate analysis. The Wilcoxon rank sum test was employed to estimate the differences between the two groups in terms of age at surgery, duration of the disease up to surgery, preoperative UPDRS III motor score (medication-on and medication-off period), response to levodopa, axial symptoms score (UPDRS items 29-30), dyskinesia score (UPDRS items 32-35), LEDD (preand post-DBS) and its reduction rate, MMSE, TMT (B-A), FAB, and BDI-II score. Fisher’s exact probability test was employed to estimate the difference in sex and the location of active contact between the two groups.
Then the multiple logistic regression method was employed to identify independent predictive variables significantly associated with early decline of executive function after STN-DBS. Variables for the multivariable models were selected using the stepwise regression procedure. P value for selection criteria was set as 0.2. All statistical analysis was performed using JMP 8 software (SAS Institute Inc).
This study was approved by the medical ethics committee of Nagoya City University Graduate School of Medicine. All patients provided an informed written consent.
3. RESULTS
The results of the differences in all clinical variables between patients with and without early decline of executive function are summarized in Table 1. Overall initial outcomes of motor function were satisfactory in our subjects. STN-DBS significantly improved the UPDRS III motor score and reduced dopaminergic medication in both groups.
Univariate analysis revealed that a high preoperative UPDRS III motor score in the medication-off period (P = 0.048) and a depressive state evaluated with BDI-II (P = 0.023) correlated significantly with decline in executive function. Other factors were not significantly different between the two groups.
The results of multiple logistic regression analysis are summarized in Table 2. Three variables including the preoperative axial symptoms score in the medication-on period, FAB, and BDI-II were selected for multivariable models by the stepwise procedure. Analysis revealed that the only significant independent variable related to early decline of executive function was the preoperative BDI-II score (odds ratio = 1.099 [95% CI: 1.012 - 1.219], P = 0.024). Other factors were not significant (P > 0.05). Consequently, our results demonstrate that patients with a preoperative high BDI-II score have an increased risk of early decline of executive function after STN-DBS. Postoperative factors such as active contact location or dopaminergic medication reduction had no relation to the decline of executive function.

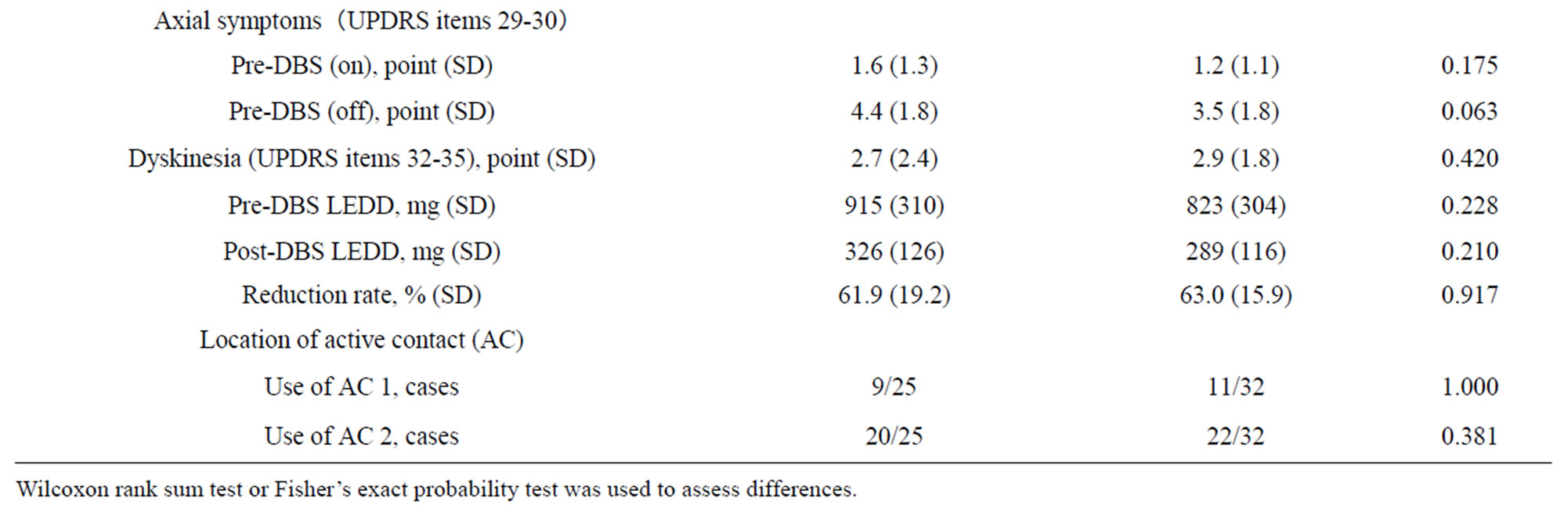
Table 1. The differences in all clinical variables between the patients with and without early decline of executive function.
Wilcoxon rank sum test or Fisher’s exact probability test was used to assess differences.
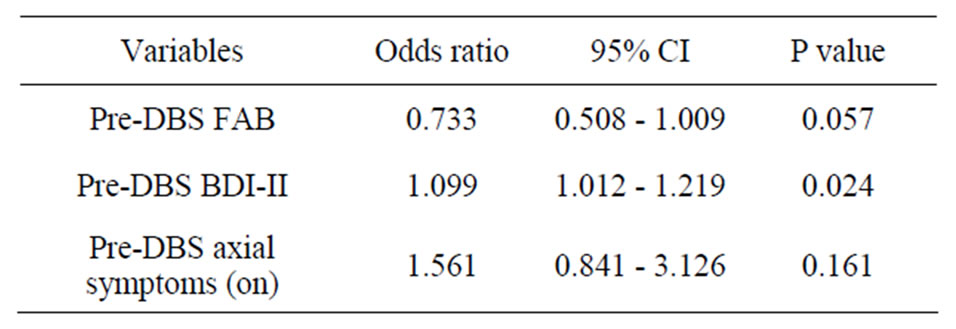
Table 2. Results of multiple logistic regression analysis in relation to early decline of executive function.
4. DISCUSSION
The neuropsychological aspect of STN DBS for PD has recently attracted considerable attention. Numerous studies concerning neuropsychological outcome after STN DBS were performed. Most studies concluded that STN DBS is relatively safe from a cognitive perspective despite mild cognitive morbidity [3-5]. Furthermore, cognitive changes are usually not linked to motor outcome [14]. A meta-analysis of cognitive sequelae by Parsons et al revealed small but significant declines in executive function and verbal learning and memory, and moderate declines in both semantic and phonemic verbal fluency after STN-DBS [3]. A randomized controlled study by Witt et al demonstrated that STN DBS did not reduce overall cognition, but resulted in selective decrease in frontal cognitive function [5]. These changes did not affect improvement of QOL. A decline in verbal fluency after STN DBS is the most consistent finding in the aforementioned studies.
Several causes are considered to contribute to cognitive change after STN-DBS; the impact of surgical intervention, the direct effect of STN stimulation, or drastic postoperative reduction of dopaminergic medication. In our surgical procedure, the trajectory of the lead penetrates the premotor cortex, passes through surrounding anterior limbs of the internal capsule or the anterior portion of the thalamus, and enters the STN. Nerve fibers that connect basal ganglia and the frontal cortex may be damaged in the process. Regarding the direct effect of stimulation, the STN has widespread connections with basal ganglia and the prefrontal cortex [15]. An active role of the STN in processing executive function has also been suggested [16]. Therefore, the stimulation of the STN may directly influence executive function. In addition, levodopa has been shown to ameliorate cognitive deficits in PD patients by inducing blood flow changes in the right dorsolateral prefrontal cortex [17]. Drastic postoperative reduction of dopaminergic medication is associated with apathy after STN DBS [4,18]. Apathy may be attributable to cognitive decline in PD [19,20].
Several factors have been proposed as predictors of cognitive decline after STN DBS. Smeding et al reported that impaired attention, advanced age, and low levodopa response before surgery predicted cognitive decline after STN DBS. Postoperative reduction of dopaminergic medication was not related to cognitive decline [21]. Daniels et al demonstrated a significant decline in executive function after STN DBS in a randomized controlled study comparing DBS with the best medical treatment. They found that higher age, higher baseline LEDD, and a higher axial score in UPDRS were risk factors for deterioration of executive function after STN DBS [22]. These factors seem to reflect progression of PD. Their study did not consider postoperative factors such as active contact location and drastic reduction of dopaminergic medication after surgery.
In this study, we investigated factors affecting early decline of executive function with our simply designed retrospective analysis. Most early studies of STN DBS agree that preoperative cognitive impairment is associated with poorer cognitive and neurobehavioral outcomes [4,23]. Therefore, we recruited only patients who were screened as cognitively normal. The TMT was employed to evaluate executive function. The frontal lobes mediate attentional control in the top-down guidance and direction of other processes. TMT-B, requiring alternating letter-number connecting, is interpreted as a measure of attentional switching and reflects the function of the dorsolateral prefrontal cortex (DLPFC) [6]. From our experience, TMT is easily performed and the most sensitive and reproducible test in PD patients [10]. Our result revealed that 44% (25/57) of patients showed significant decline in TMT in the early period after successful STN DBS even if preoperative global cognition was normal.
In the univariate analysis, we demonstrated that a high motor score in the medication-off period and a depressive state correlated significantly with decline in executive function. A high motor score in the off period seems to reflect an advanced stage of disease. In the multivariate analysis, preoperative BDI-II score was the only significant independent variable related to decline of executive function. Namely, patients with a preoperative depressive state have an increased risk of early decline of executive function after STN-DBS. Depression is one of the most common psychiatric symptoms in PD. Depression is thought to be closely related to cognitive impairment, especially in frontal lobe tasks in PD [24,25]. Hypometabolism and dysfunction of the frontal lobe in PD are considered to relate to depression [26,27]. Even when there are no cognitive problems preoperatively, patients in a depressive state seem to be cognitively vulnerable to STN DBS.
Contrary to initial expectations, postoperative factors such as active contact location or dopaminergic medication reduction had no relation to the decline of executive function. As for active contact location, Smeding et al. reported a case of reversible cognitive decline after STN DBS [28]. Cognitive decline was resolved by changing to dorsal contact in their patient. They suggested that stimulation of the ventral contact caused cognitive decline. There are other reports regarding the relation between the location of active contact and neuropsychological effects [29,30]. In our series, the appropriate adjustment of either active contact location or dopaminergic medication to obtain maximal effect for the improvement of motor function seem not to have affected decline in executive function.
5. CONCLUSION
Even in cognitively well-selected patients, STN DBS causes early decline in executive function in a significant number of patients. We demonstrated that preexisting depressive state was the only significant independent variable related to early decline of executive function after STN-DBS. Simple preoperative cognitive screening alone could not predict early decline in executive function. More detailed neuropsychological evaluation, including mood status, should be undertaken before surgery.
REFERENCES
- Weaver, F.M., Follett, K., Stern, M., Hur, K., Harris, C., Marks Jr., W.J., et al. (2009) Bilateral deep brain stimulation vs best medical therapy for patients with advanced Parkinson disease: A randomized controlled trial. JAMA, 301, 63-73. doi:10.1001/jama.2008.929
- Williams, A., Gill, S., Varma, T., Jenkinson, C., Quinn, N., Mitchell, R., et al. (2010) Deep brain stimulation plus best medical therapy versus best medical therapy alone for advanced Parkinson’s disease (PD SURG trial): A randomised, open-label trial. The Lancet Neurology, 9, 581-591. doi:10.1016/S1474-4422(10)70093-4
- Parsons, T.D., Rogers, S.A., Braaten, A.J., Woods, S.P. and Tröster, A.I. (2006) Cognitive sequelae of subthalamic nucleus deep brain stimulation in Parkinson’s disease: A meta-analysis. The Lancet Neurology, 5, 578-588. doi:10.1016/S1474-4422(06)70475-6
- Voon, V., Kubu, C., Krack, P., Houeto, J.L. and Tröster, A.I. (2006) Deep brain stimulation: Neuropsychological and neuropsychiatric issues. Movement Disorders, 21, S305-S327. doi:10.1002/mds.20963
- Witt, K., Daniels, C., Reiff, J., Krack, P., Volkmann, J., Pinsker, M.O., et al. (2008) Neuropsychological and psychiatric changes after deep brain stimulation for Parkinson’s disease: A randomised, multicentre study. The Lancet Neurology, 7, 605-614. doi:10.1016/S1474-4422(08)70114-5
- Stuss, D.T. and Levine, B. (2002) Adult clinical neuropsychology: Lessons from studies of the frontal lobes. Annual Review of Psychology, 53, 401-433. doi:10.1146/annurev.psych.53.100901.135220
- Lezak, M.D., Howieson, D.B. and Loring, D.W. (2004) Neuropsychological assessment. 4th Edition, Oxford University Press, New York.
- Zangaglia, R., Pacchetti, C., Pasotti, C., Mancini, F., Servello, D., Sinforiani, E., et al. (2009) Deep brain stimulation and cognitive functions in Parkinson’s disease: A three-year controlled study. Movement Disorders, 24, 1621-1628. doi:10.1002/mds.22603
- Auclair-Ouellet, N., Chantal, S., Cantin, L., Prud’homme, M., Langlois, M. and Macoir, J. (2011) Transient executive dysfunction following STN-DBS in Parkinson’s disease. Canadian Journal of Neurological Sciences, 38, 360-363.
- Yamanaka, T., Ishii, F., Umemura, A., Miyata, M., Horiba, M., Oka, Y., et al. (2012) Temporary deterioration of executive function after subthalamic deep brain stimulation in Parkinson’s disease. Clinical Neurology and Neurosurgery, 114, 347-351. doi:10.1016/j.clineuro.2011.11.009
- Schüpbach, M., Gargiulo, M., Welter, M.L., Mallet, L., Béhar, C., Houeto, J.L., et al. (2006) Neurosurgery in Parkinson disease: A distressed mind in a repaired body? Neurology, 66, 1811-1816. doi:10.1212/01.wnl.0000234880.51322.16
- Smeding, H.M., Speelman, J.D., Koning-Haanstra, M., Schuurman, P.R., Nijssen, P., van Laar, T., et al. (2006) Neuropsychological effects of bilateral STN stimulation in Parkinson disease: A controlled study. Neurology, 66, 1830-1836. doi:10.1212/01.wnl.0000234881.77830.66
- Tomlinson, C.L., Stowe, R., Patel, S., Rick, C., Gray, R. and Clarke, C.E. (2010) Systematic review of levodopa dose equivalency reporting in Parkinson’s disease. Movement Disorders, 25, 2649-2653. doi:10.1002/mds.23429
- Perriol, M.P., Krystkowiak, P., Defebvre, L., Blond, S., Destée, A. and Dujardin, K. (2006) Stimulation of the subthalamic nucleus in Parkinson’s disease: Cognitive and affective changes are not linked to the motor outcome. Parkinsonism & Related Disorders, 12, 205-210. doi:10.1016/j.parkreldis.2005.11.009
- Benarroch, E.E. (2008) Subthalamic nucleus and its connections: Anatomic substrate for the network effects of deep brain stimulation. Neurology, 70, 1991-1995. doi:10.1212/01.wnl.0000313022.39329.65
- Baláz, M., Rektor, I. and Pulkrábek, J. (2008) Participation of the subthalamic nucleus in executive functions: An intracerebral recording study. Movement Disorders, 23, 553-557. doi:10.1002/mds.21873
- Cools, R., Stefanova, E., Barker, R.A., Robbins, T.W. and Owen, A.M. (2002) Dopaminergic modulation of highlevel cognition in Parkinson’s disease: The role of the prefrontal cortex revealed by PET. Brain, 125, 584-594. doi:10.1093/brain/awf052
- Funkiewiez, A., Ardouin, C., Krack, P., Fraix, V., Van Blercom, N., Xie, J., et al. (2003) Acute psychotropic effects of bilateral subthalamic nucleus stimulation and levodopa in Parkinson’s disease. Movement Disorders, 18, 524-530. doi:10.1002/mds.10441
- Dujardin, K., Sockeel, P., Delliaux, M., Destée, A. and Defebvre, L. (2009) Apathy may herald cognitive decline and dementia in Parkinson’s disease. 24, 2391-2397. doi:10.1002/mds.22843
- Butterfield, L.C., Cimino, C.R., Oelke, L.E., Hauser, R.A. and Sanchez-Ramos, J. (2010) The independent influence of apathy and depression on cognitive functioning in Parkinson’s disease. Neuropsychology, 24, 721-730. doi:10.1037/a0019650
- Smeding, H.M., Speelman, J.D., Huizenga, H.M., Schuurman, P.R. and Schmand B. (2011) Predictors of cognitive and psychosocial outcome after STN DBS in Parkinson’s disease. Journal of Neurology, Neurosurgery & Psychiatry, 82, 754-760. doi:10.1136/jnnp.2007.140012
- Daniels, C., Krack, P., Volkmann, J., Pinsker, M.O., Krause, M., Tronnier, V., et al. (2010) Risk factors for executive dysfunction after subthalamic nucleus stimulation in Parkinson’s disease. Movement Disorders, 25, 1583-1589. doi:10.1002/mds.23078
- Lang, A.E., Houeto, J.L., Krack, P., Kubu, C., Lyons, K.E., Moro, E., et al. (2006) Deep brain stimulation: Preoperative issues. Movement Disorders, 21, S171-196. doi:10.1002/mds.20955
- Starkstein, S.E., Preziosi, T.J., Berthier, M.L., Bolduc, P.L., Mayberg, H.S., Robinson, R.G. (1989) Depression and cognitive impairment in Parkinson’s disease. Brain, 112, 1141-1153. doi:10.1093/brain/112.5.1141
- Giladi, N., Treves, T.A., Paleacu, D., Shabtai, H., Orlov, Y., Kandinov, B., et al. (2000) Risk factors for dementia, depression and psychosis in long-standing Parkinson’s disease. Journal of Neural Transmission, 107, 59-71. doi:10.1007/s007020050005
- Mayberg, H.S., Starkstein, S.E., Sadzot, B., Preziosi, T., Andrezejewski, P.L., Dannals, R.F., et al. (1990) Selective hypometabolism in the inferior frontal lobe in depressed patients with Parkinson’s disease. Annals of Neurology, 28, 57-64. doi:10.1002/ana.410280111
- Mayberg, H.S. (1994) Frontal lobe dysfunction in secondary depression. The Journal of Neuropsychiatry and Clinical Neurosciences, 6, 428-442.
- Smeding, H.M., van den Munckhof, P., Esselink, R.A., Schmand, B., Schuurman, P.R. and Speelman, J.D. (2007) Reversible cognitive decline after DBS STN in PD and displacement of electrodes. Neurology, 68, 1235-1236. doi:10.1212/01.wnl.0000259067.68691.87
- Tsai, S.T., Lin, S.H., Lin, S.Z., Chen, J.Y., Lee, C.W. and Chen, S.Y. (2007) Neuropsychological effects after chronic subthalamic stimulation and the topography of the nucleus in Parkinson’s disease. Neurosurgery, 61, E1024-E1029. doi:10.1227/01.neu.0000303198.95296.6f
- York, M.K., Wilde, E.A., Simpson, R. and Jankovic, J. (2009) Relationship between neuropsychological outcome and DBS surgical trajectory and electrode location. Journal of the Neurological Sciences, 287, 159-171. doi:10.1016/j.jns.2009.08.003
NOTES
*The authors report no conflict of interest concerning the materials or methods used in this study or the findings specified in this paper.

