Advances in Nanoparticles
Vol.3 No.3(2014), Article
ID:48839,6
pages
DOI:10.4236/anp.2014.33011
Spectral Investigations of Hybrid Nanostructures Including Modified Fullerene C60 and Carbazole-Derivative Fluorophore
Evgeny P. Grebennikov1, Grigory E. Adamov1*, Valery N. Charushin2, Konstantin S. Levchenko1, Gennady L. Rusinov2, Evgeny V. Zinoviev1, Roman A. Irgashev2, Pavel S. Shmelin1
1OJSC “CSRIT Technomash”, Moscow, Russia
2Institute of Organic Synthesis, Urals Branch of Russian Academy of Sciences, Ekaterinburg, Russia
Email: *adamov@cnititm.ru
Copyright © 2014 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/



Received 6 May 2014; revised 21 June 2014; accepted 12 July 2014
ABSTRACT
Hybrid nanostructures including modified fullerene C60 and fluorophoric (E)-2-Cyano-3-(9-ethyl- 9Н-carbazole-3-yl)-acrylic acid (cyanoacrylic acid I) were created. Spectral properties (absorption and luminescence spectra) of the hybrid nanostructures were investigated. It was established what molecules C60 strongly affected spectral properties of cyanoacrylic acid I in the hybrid nanostructures. Luminescence quenching of fluorophore and a change of luminescence features were observed in the hybrid nanostructures.
Keywords:Hybrid Nanostructures, Water-Soluble Fullerene C60, Luminescence Quenching

1. Introduction
An investigation of influence of nanoobjects onto properties of optically active molecules is the one of the interesting fundamental problems of modern science. This phenomenon is strongly depended on a distance “molecule-nanoobjects” and exhibited in changing lifetime of excited states of atoms or molecules arranged at distances less than the wavelength of the irradiation [1] . Strong local fields affect the rate of electronic transitions, i.e. on the lifetime of excited states. They can affect light absorption and radiation parameters, energy (photo or electrical) transfer, as well as much enhance various nonlinear optical effects [2] . If optically active molecules are linked to a surface of nanoobjects by chemical bonds, it would be mean a formation of hybrid nanostructures [3] . In a general case, a hybrid nanostructure is a cross-linked system including nanoparticles of different natures (metal colloid nanoparticles, semiconductor quantum dots, oxide or polymer nanoparticles, carbon nanotubes, fullerenes, etc.) and functional molecules (photochromic, photoluminescent, electroluminescent, magnetically active, etc.).
Carbon nanomaterials (carbon nanotubes, graphene, fullerenes) have unique electronic and mechanical properties and are widely applied for a creation of novel composite materials [4] -[6] , electronic functional components [7] [8] , etc. Fullerenes having very interesting properties due to unique electronic structure can be widely used for application in a construction of the hybrid nanostructures. Focus of this investigation is on an influence of fullerene C60 onto luminescence of fluorophore compound linked to fullerene surface.
2. Material and Methods
Carbazole derivative, (E)-2-Cyano-3-(9-ethyl-9Н-carbazole-3-yl)-acrylic acid (cyanoacrylic acid I), was used as fluorophore compound. The choose of the compound is caused of presence in the molecule structure two binding centers capable to link to nanoobject surfaces—1) carboxylic group and 2) aromatic π-conjugated electron system of carbazole cycle. A synthesis of the compound was made by a modified method [9] (Figure 1). Characterization and confirmation of the structure were made by NMR1H and element analysis. A water solution of cyanoacrylic acid I (c = 0.2 mg/ml) and NaOH in relation of cyanoacrylic acid I:NaOH = 1:1.5 (mol) was used.
Fullerene С60 was purchased from NeoTechProduct Research & Production Company, Ltd. (Russia) [10] . Fullerene is needed in a surface functionalization for an application to create hybrid nanostructures. The functionalization leads to involve structure substitutions for bounding fullerene and cyanoacrylic acid I. Method [11] was used for the functionalization of C60. Method is based on functionaling of C60 by N,N’-Bis-(3-diethylamino-propyl)malonamide (Figure 2).
Reaction was made in dried toluene in presence of CBr4 and under 4-fold excess of malonate. Mixture of mono-, diand tri-[N,N’-Bis-(3-diethylamino-propyl)-malonamide-2,2-yl]fullerene was obtained. Solid was dissolved in HCl (15%) and result solution was filtered, evaporated until 10 ml of solvent. A water solution of functionalized C60 (c = 0.02 mg/ml) was used for creation of hybrid nanostructures.
A principle of formation of hybrid nanostructures including modified C60 and cyanoacrylic acid I is based on

Figure 1. Structure formula of cyanoacrylic acid I.
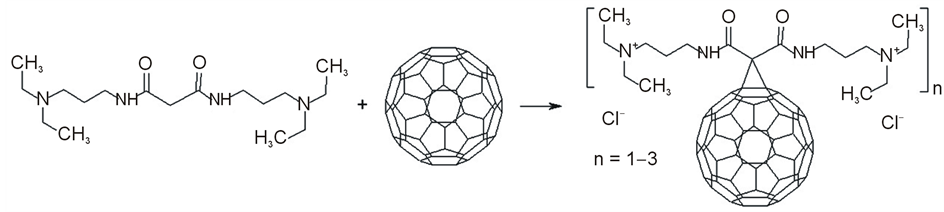
Figure 2. Schematic reaction of a functionalization of fullerene C60.
self-assembling process. It is an interaction of positive-charged ammonium substitutions of fullerene and negative-charged carboxylic groups of cyanoacrylic acid I. Such interaction is strongly depended on pH. A manage of pH is very important to synthesize of the hybrid nanostructures.
In these work spectral characteristics (absorption and luminescence) of created hybrid nanostructures was investigated under different pH conditions. Absorption spectra were given by a spectrophotometer Lambda 35 (Perkin Elmer). Parameters of coverage were: optical length 2 mm; optical scan diapason 250 - 700 nm; scan speed 480 nm/min; slit width 2 nm; data interval 1 nm. Luminescence spectra were given by a spectrofluorometer Shimadzu RF-5301 PC. Parameters of coverage were: excitation wavelength 380 nm, excitation slit width 5 nm; emission slit width 5 nm; emission scan diapason 400 - 700 nm; sampling interval 1 nm.
3. Results and Discussion
In first time spectral characteristics of solution of separated components (modified C60 and cyanoacrylic acid I) were investigated. It was shown that absorption and luminescence spectra of cyanoacrylic acid I were strongly changed in dependence on pH (Figure 3 and Figure 4). The spectral characteristics of modified C60 didn’t depend on pH.
It was shown that magnitude of absorption on 289 nm was decreased, a pike on 316 nm was split in two with low intensity and a pike on 376 nm was shifted on 396 nm and significantly broadened (Figure 3). Also substantial shift of luminescence band maximum was appeared from 500 nm (pH 9.76) to 542 nm (pH 3.23) (Figure 4). It is probably explained that a protonated state of fluorophore molecule is formed. It is led to a redistribution of electronic density, absorption ability and changing magnitude of Stokes shift. Herewith luminescence intensity was risen more than 1.5 fold.
Other behavior was observed for the hybrid nanostructures including modified fullerene C60 and cyanoacrylic acid I (see Figure 5 and Figure 6). A shift of maximum luminescence band was not appeared and luminescence was quenched.
Dissociation equation of cyanoacrylic acid I in water solution can be shown by a schematic equation:
С17Н13N2-COOH + H2O ⇄ С17Н13N2-COO− + H3O+.
In alkaline solution equilibrium is shifted to form of true solution of salt (deprotonated) state of cyanoacrylic acid I. In acid media molecules of cyanoacrylic acid I form a suspension due to a transition in undissociated and insoluble state (Figure 7). In presence of modified C60 a solution of cyanoacrylic acid I states true at all magnitude of pH from 2.23 to 9.79. This fact proves binding of fluorophore molecules to C60 surface and a formation
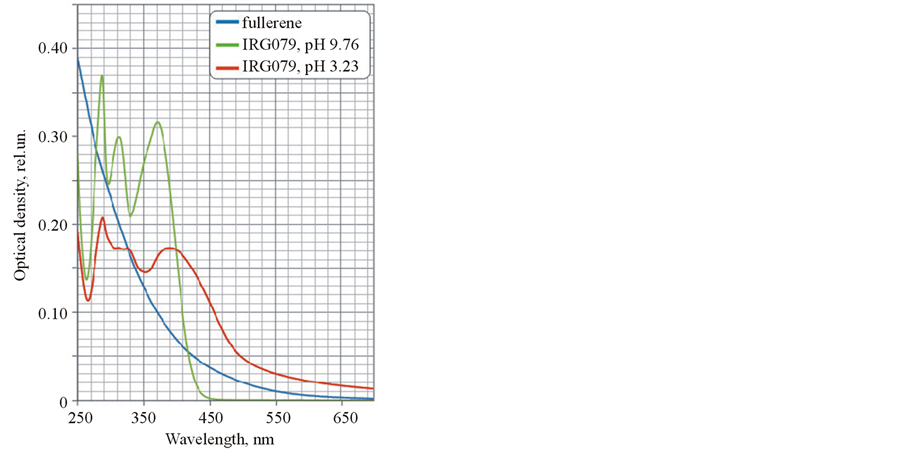
Figure 3. Absorption spectra of separated components of the hybrid nanostructures.
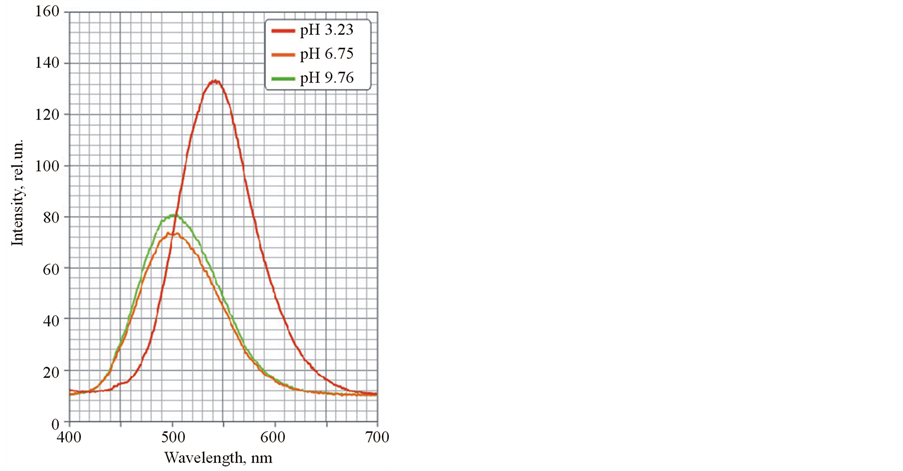
Figure 4. Luminescence spectra of cyanoacrylic acid I at different pH.

Figure 5. Absorption spectra of the hybrid nanostructures at different pH.
of the hybrid nanostructures. Test solutions of cyanoacrylic acid I in presence of N,N’-Bis-(3-diethylaminopropyl)malonamide were investigated. No significant influence of separated malonate onto spectral properties of fluorophore was given. Therefore, just molecules C60 affect spectral properties of cyanoacrylic acid I in the hybrid nanostructures.
Fullerene C60 is inclined to form of charge-transfer complexes with organic molecules [12] -[14] . The complexes are characterized a Ferster energy transfer from donor molecule (in our case, fluorophore molecule) to acceptor molecule (fullerene) with following fast oscillating (notably, nonradiative) relaxation [15] . It was appeared in luminescence quenching and it was proved by our experimental data (Figure 6). A stabilization of deprotonated state of cyanoacrylic acid I and changing character of luminescence in the hybrid nanostructures are
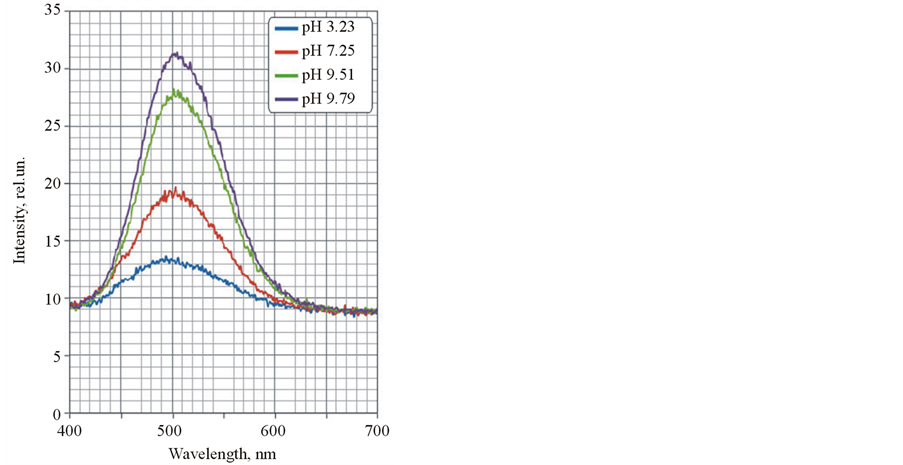
Figure 6. Luminescence spectra of the hybrid nanostructures at different pH.

Figure 7. 1—Water solution of cyanoacrylic acid I (рН 9.76); 2— Water suspension of cyanoacrylic acid I (рН 3.23); 3—Water solution of the hybrid nanostructures including modified C60 and cyanoacrylic acid I (рН 3.23).
explained by a formation of charge-transfer complexes between С60 and cyanoacrylic acid I. It was proved by fluorescence spectroscopy observing no luminescence maximum shift in the hybrid nanostructures whereas the shift was observed for separated cyanoacrylic acid I without modified C60 (Figure 4).
4. Conclusions
Hybrid nanostructures including modified fullerene C60 and carbazole derivative fluorophore were investigated in this work. A luminescence quenching of fluorophore was observed in the hybrid nanostructures due to Ferster energy transfer. A change of luminescence features (wavelength and magnitude) was observed due to a formation of charge-transfer complexes between fluorophore and C60 molecules.
This work is supported by Russian Foundation for Basic Research, grant No.14-03-00479 А.
References
- Klimov, V. (2012) Nanoplasmonics: Fundamentals and Applications. Pan Stanford Publishing, Singapore.
- Klimov, V.V., Ducloy, M. and Letokhov, V.S. (2001) REVIEW: Spontaneous Emission of an Atom in the Presence of Nanobodies. Quantum Electronics, 31, 569-586. http://dx.doi.org/10.1070/QE2001v031n07ABEH002007
- Adamov, G.E., Levchenko, K.S., Kurbangaleev, V.R., Shmelin, P.S. and Grebennikov, E.P. (2013) Functional Hybrid Nanostructures for Nanophotonics: Synthesis, Properties, and Application. Russian Journal of General Chemistry, 83, 2195-2202. http://dx.doi.org/10.1134/S107036321311039X
- Lionetto, F., Calò, E., Di Benedetto, F., Pisignano, D. and Maffezzoli, A. (2014) A Methodology to Orient Carbon Nanotubes in a Thermosetting Matrix. Composite Science and Technology, 96, 47-55. http://dx.doi.org/10.1016/j.compscitech.2014.02.016
- Corcione, C.E. and Maffezzoli, A. (2013) Transport Properties of Graphite/Epoxy Composites: Thermal, Permeability and Dielectric Characterization. Polymer Testing, 32, 880-888. http://dx.doi.org/10.1016/j.polymertesting.2013.03.023
- Corcione, C.E., Freuli, F. and Maffezzoli, A. (2013) The Aspect Ratio of Epoxy Matrix Nanocomposites Reinforced with Graphene Stacks. Polymer Engineering and Science, 53, 531-539. http://dx.doi.org/10.1002/pen.23292
- Susarova, D.K., Goryachev, A.E., Novikov, D.V., Dremova, N.N., Peregudova, S.M., Razumov, V.F. and Troshin, P.A. (2014) Material Solubility Effects in Bulk Heterojunction Solar Cells Based on the Bis-Cyclopropane Fullerene Adducts and P3HT. Solar Energy Materials and Solar Cells, 120, 30-36. http://dx.doi.org/10.1016/j.solmat.2013.08.005
- Takeda, A., Oku, T., Suzuki, A., Akiyama, T. and Yamasaki, Y. (2012) Fabrication and Characterization of FullereneBased Solar Cells Containing Phthalocyanine and Naphthalocyanine Dimers. Synthetic Metals, 177, 48-51. http://dx.doi.org/10.1016/j.synthmet.2013.06.011
- Wu, T.-Y., Tsao, M.-H., Chen, F.-L., Su, S.-G., Chang, C.-W., Wang, H.-P., Lin, Y.-C., Ou-Yang, W.-C. and Sun, I-W. (2010) Synthesis and Characterization of Organic Dyes Containing Various Donors and Acceptors. International Journal of Molecular Sciences, 11, 329-353. http://dx.doi.org/10.3390/ijms11010329
- http://www.neotechproduct.ru
- Richardson, C.F., Schuster, D.I. and Wilson, S.R. (2000) Synthesis and Characterization of Water-Soluble Amino Fullerene Derivatives. Organic Letters, 2, 1011-1014. http://dx.doi.org/10.1021/ol990312a
- Zhanga, C., Shena, W., Fana, R., Zhanga, G., Shangguana, L., Chaoa, J., Shuanga, S., Donga, Ch. and Choib, M.M.F. (2009) Study of the Contact Charge Transfer Behavior between Cryptophanes (A and E) and Fullerene by Absorption, Fluorescence and 1H NMR Spectroscopy. Analytica Chimica Acta, 650, 118-123. http://dx.doi.org/10.1016/j.aca.2009.02.046
- Wrobel, D. and Graja, A. (2006) Modification of Electronic Structure in Supramolecular Fullerene-Porphyrin Systems Studied by Fluorescence, Photoacoustic and Photothermal Spectroscopy. Journal of Photochemistry and Photobiology A: Chemistry, 183, 79-88. http://dx.doi.org/10.1016/j.jphotochem.2006.02.024
- Datta, K. and Mukherjee, A.K. (2006) Study of Quenching of Anthracene Fluorescence by [60]Fullerene. Spectrochimica Acta Part A, 65, 261-264. http://dx.doi.org/10.1016/j.saa.2005.10.040
- Forster, Th. (1948) Zwischenmolekulare Energiewanderung und Fluoreszenz. Annalen der Physik, 437, 55-75. http://dx.doi.org/10.1002/andp.19484370105
NOTES

*Corresponding author.

