American Journal of Plant Sciences
Vol.08 No.12(2017), Article ID:80261,10 pages
10.4236/ajps.2017.812200
Description of a Novel Allelic “Thick Leafed” Mutant of Sorghum
Dennis C. Gitz III, Lan Liu-Gitz, Zhanguo Xin, Jeffrey T. Baker, Paxton Payton, Robert J. Lascano
Cropping Systems Research Laboratory, ARS-USDA1, Lubbock, TX, USA
Copyright © 2017 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY 4.0).
http://creativecommons.org/licenses/by/4.0/



Received: September 30, 2017; Accepted: November 7, 2017; Published: November 13, 2017
ABSTRACT
An allelic sorghum [Sorghum bicolor (L.) Moench] mutant with thick and narrow erect leaves (Thl) and reduced adaxial stomatal density was isolated from the Annotated Individually pedigreed Mutagenized Sorghum mutant library developed at the Plant Stress and Germplasm Development Unit at Lubbock TX. The mutant, Thl, was isolated from a pedigreed M3 family generated by ethyl methanesulfonate mutagenization from an elite inbred sorghum line, BTx623, which had been used to sequence the sorghum genome. The mutant has been backcrossed to the wild-type BTx623 confirming that the trait results derive from a stable recessive nuclear gene mutation. Herein, we briefly described morphological and selected physiological characteristics of this mutant sorghum.
Keywords:
Sorghum, Stoma, Stomatal Density, Stomatal Ratio, Leaf Thickness

1. Introduction
Herein we described an allelic erect leafed [1] sorghum with thick and narrow leaves developed by methane sulfonate induced mutagenesis [2] [3] of BTx623 [4] . This “thick leaf” (Thl) allelic mutant may help to identify genes influencing leaf development through mutant mapping, taking advantage of the already sequenced uniform genetic background of BTx623 in which the mutation is embedded [5] . Together with the previously reported leaf architecture mutants [1] , this allelic thick leafed mutant may be used to investigate and improve sorghum canopy architecture with the goal of increasing light penetration through the canopy and reducing water use while maintaining or increasing biomass, forage, or grain production. The trait might also be useful in conferring aphid resistance by increasing the distance between the phloem and epidermis.
2. Materials and Methods
2.1. Mutant Generation and Selection
A sorghum mutant library was generated as previously described [1] [2] . Briefly, air-dried BTx623 seeds were soaked with gentle agitation in concentrations (0.10, 0.15, 0.20, or 0.25%; w/v) of aqueous ethyl methane sulfonate, for 16 h at 25˚C, thoroughly rinsed with distilled water and dried. These seeds were designated as first generation mutant (M1) seeds. These M1 seeds were planted, the plants allowed to develop, and to self pollinate by bagging the panicles with 400-weight rainproof paper pollination bags (Lawson Pollinating Bags2, Northfield IL; http://www.lawsonbags.com/) obtained from a distributor (Seedburo Equipment Co., Des Plains IL; http://www.seedburo.com/) after heading and before anthesis to prevent cross-pollination. Panicles setting seeds were manually harvested, individually threshed, planted as M2 plots, allowed to self pollinate with bagging, and a single fertile panicle selected as a source of M3 seeds. Each M3 family of seeds was subsequently planted as a single plot for phenotype evaluation and selection.
The subsequently identified mutant displayed an erect, narrow, thickened leaf in both greenhouse and field. Individual plants within the M3 plots were examined for leaf erectness as described earlier [1] . Leaves that did not droop and maintained erectness as a more acute angle between the leaf and the shoot were identified, but in addition to the erect leafed phenotype, plants with narrower leaves that subjectively felt “thicker” than the wild type (WT) BTx623 were selected.
2.2. Plant Culture
Sorghum isolines BTx623 or Thl were either container or field grown during the 2017-growing season. Container grown plants were grown in a polyhouse at the ARS-USDA facility in Lubbock TX, in twenty 8-L thermoplastic pots containing a soil-less rooting medium consisting of a proprietary mix of peat moss, vermiculite and perlite (Sunshine Traditional Loose Fill mix; Sungro Horticultural Products, Bellevue WA; http://www.sungro.com/). About 35 g of fertilizer (Osmocote® 14-14-14, Scotts LLC, Marysville OH; http://www.scotts.com/) was incorporated into the upper 10 cm of the potting mixture, the pots irrigated to runoff several times over two hours and 5 seeds were planted in the center of each container at a depth of 2 cm on Day Of Year (DOY) 145. Emergence (>50%) occurred on DOY 149 or DOY 152 (BTx623 and Thl, respectively). After allowing the first leaf to fully expand, plants were selected for uniformity and the remaining plants eliminated by severing the shoots just beneath the surface of the rooting medium leaving a single plant in each pot. After thinning, a disk of aluminized reflective aluminized polyethylene bubble insulation (Reflectix® Double Reflective Insulation; Reflectix Inc., Markleville IN) was cut to fit inside the pot and reduce water evaporation from the surface of the rooting medium. A 4 cm diameter hole was cut in the center of each disk to accommodate the plant in the center of each pot. As the plants developed, the containers were frequently irrigated past the drip point in an attempt to ensure that the plants were developing under uniform conditions of minimal water stress.
Plants were also grown in plots at the USDA facility (33˚35'38.20" N, 101˚54'11.07"W) and on DOY 180 of the 2017 growing season, Btx-623 and Thl seeds were planted in plots at a depth of 1.5 cm into North-South oriented rows on raised beds spaced 1 m at a rate of 20 seeds/m. The soil at the USDA location is classified as an Amarillo fine sandy loam (fine-loamy, mixed, superactive, thermic Aridic Paleustalfs). After planting, the plots were furrow irrigated several times to induce emergence and ensure an even stand. After the plants reached the fifth to eighth leaf growth stage no more irrigation water was applied. Environmental conditions were recorded by a weather station located 100 m east from the plots (http://www.lbk.ars.usda.gov/WEWC/weather-pswc-data.aspx).
2.3. Morphometric Analysis
Leaf thickness was measured with a digital micrometer (Pittsburgh® Tool Item # 47257, Harbor Freight Tools, Calabasas CA) with a claimed accuracy of ± 0.03 mm. To minimize error and to increase reproducibility between measurements that could arise by crushing leaf tissues between the micrometer jaws, the instrument was fitted with two polished zinc disks mounted to the jaws with polyacrylate cement. This distributed the compressive force across the surface of the leaf and prevented crushing soft tissues resulting in more consistent, reproducible, and accurate measurements. It was also thought that this would additionally integrate thickness measurement across a larger sampling area across the surface of the leaf. The second leaf below the flag leaf was selected for leaf thickness measurement at anthesis [6] .
Leaf area, plant height and above ground (shoot) biomass of greenhouse grown plants at selected growth stages was determined. Plants were moved from the greenhouse to the laboratory where leaves were detached from the plant and leaf area determined by passing each leaf through a leaf area meter (Model LI-3100C, Li-Cor Corp., Lincoln NE). Leaves stems and panicles (when present) were separated, dried at 60˚C over three days and the biomass gravimetrically measured.
2.4. Microscopy
Microscopy of selected leaf anatomical features and characteristics of field grown plants was performed on the first leaf immediately below the flag leaf at the boot growth stage. Stomatal density was determined from epidermal impressions produced essentially as described by [7] . Leaves were collected from field grown plants, taken to the laboratory, washed, rinsed, blotted dry, dewaxed with an electronic parts cleaner composed of halogenated solvents, allowed to dry, coated with several coats of an acrylic finish (Minwax® Polycrylic™ Protective Finish, Minwax Corp., Upper Saddle River NJ; http://www.minwax.com), and allowed to dry. The resulting epidermal casts were removed with pieces of clear urethane packing tape, mounted on 50 mm × 75 mm glass slides, viewed under bright field microscopy, and the stomatal densities determined from digital micrographs of known dimensions collected through a 10× objective [8] .
Scanning electron microscopy (SEM) micrographs were made of leaf surfaces and leaf cross sections. Leaves were collected, washed, and dewaxed as for epidermal impressions but were then trimmed with scissors and the upper and lower surfaces viewed with a Hitachi S-4300 field emission environmental scanning electron microscope at 15 KeV accelerating voltage and 30 - 90 Pa pressure. Micrographs of cross sections were produced similarly except that leaves were sectioned with a razor blade, frozen under liquid nitrogen, and the sections held on the electron microscope’s cold stage to keep the samples frozen during imaging.
2.5. Gas Exchange
Gas exchange measurements were made with portable photosynthesis systems (Model LI-6400, LiCor Inc., Lincoln NE) fitted with LI-6400-02B LED light sources using mixed LED’s delivering both red and blue light to leaves within a 2 cm × 3 cm (6 cm2) sample cuvette. Measurements were made during the grain filling growth stage on the first leaf below the flag leaf during a temporal window beginning 4 hours before solar noon and ending no later than 2 hours after solar noon. Inlet air was passed through a 4-L chamber to buffer rapid changes in [CO2] before entering the system.
Apparent quantum yield as net CO2 assimilation (Anet) from 0 to 2000 µmol m−2・s−1 of photosynthetically active radiation (PAR, 400 - 700 nm) was determined at 28˚C block temperature, ambient [CO2], ambient [H2O], and a flow rate through the leaf cuvette of 500 mL・min−1. Data were fitted to a 3 parameter rectangular hyperbolic function of the form f = y0 + (a * x)/(b + x) in SigmaPlot (SysStat Software Inc., San Jose CA).
The response of Anet to substomatal [CO2] was determined at a block temperature of 28˚C and a constant light level of 1800 µmol・m−2・s−1 PAR. Cuvette [CO2] was scrubbed down in steps from 400 µmol・mol−1 [CO2] to near nil, and then increased as follows: 400, 300, 200, 100, 50, 0, 75, 150, 250, 350, 450, 900, 1250, 1500, 1750, 2000 µmol・mol−1. Data were expressed as Anet as a function of calculated sub-stomatal [CO2].
3. Results
Growth and development: Field grown plants at the grain filling growth stage placed against black velvet and photographed in the laboratory are shown in Figure 1. The Thl leaves adopted a horizontal orientation as the plants approached the reproductive growth stage. During the vegetative stages of growth the leaves were much more erect (not shown). Panicles appeared to be similarly sized in this image but grain yield under field conditions was not rigorously tested. Leaf thickness of both greenhouse grown and field grown mutant Thl plants was about double that of Btx-623 as measured with the leaf micrometer. Leaf thickness of the Thl line was 0.43 ± 0.01 mm while the BTx leaves were only 0.20 ± 0.006 mm (Pt 0.0001, n = 20), i.e., twice as thick. No clear difference in leaf midrib thickness was noted. Specific leaf mass (dried leaf mass/unit area) of Thl was about double that of the wild type. Leaf area, leaf mass, stem mass, shoot mass and whole shoot biomass of the mutant was one third that of the wild type in vegetative stages through anthesis, but grain yield was only reduced by about 50%. Seed yield of greenhouse grown Thl plants was 62-g/plant ± 10 g and that of the wild type was 133-g/plant ± 6.5 g/plant (Pt 0.001, n = 7).
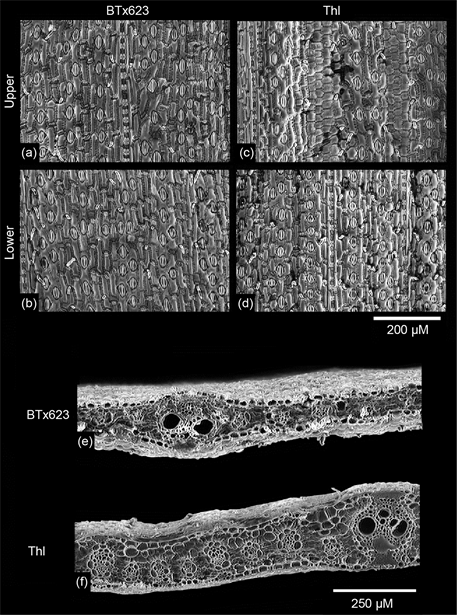
Scanning electron micrographs of representative leaf surfaces are shown in Figures 2(a)-2(d). No obvious changes in architecture of individual stoma were observed. However, visual inspection of the images suggested a reduction in
Figure 1. Color image of field grown Btx-623 (left) and Thl (right) shoots. Representative leaves, the second leaf descending from the flag leaf, are shown in the lower portion of the image. Bar is 25 cm.
Figure 2. Scanning electron micrographs of Btx-623 and Thl leaf surfaces (a) through (d) and cross sections ((e) and (f)).
adaxial stomatal density resulting from fewer files of cells differentiating into stoma. Scanning electron micrographs of leaf cross sections are shown in Figure 2(e) and Figure 2(f). Leaf anatomy was unremarkable except for the increase in leaf thickness. Vascular bundles appeared to be of similar size so that the increased thickness resulted from other tissues such as thicker leaf chlorenchyma. That is, similarly sized vasculature appeared to be embedded deeper within the photosynthetically active leaf chlorenchyma. Stomatal distribution as density, i.e., numbers mm−2 and stomatal ratio (upper density/lower density) is shown in Figure 3. The lower leaf stomatal density and upper leaf stomatal densities are “stacked” so that lower, upper, and their contribution to total stomatal densities may be more easily seen (Figure 3(a)). Error bars associated with the measure-
Figure 3. Stomatal distribution as stomatal density (a) and stomatal ratio (b) of BTx-623 and Thl. Bars are S.E., n = 4.
ments in Figure 3(a) are for individual surfaces, not for the whole leaf. The stomatal density of the upper (adaxial) leaf surface was substantially reduced by about 50% in the mutant Thl line as compared to the wild type, but this was offset somewhat by an increase in stomatal density of the lower (abaxial) leaf surface (Figure 3(a)). This resulted in a very slight reduction in mean total leaf stomatal density of only modest statistical significance (Pt = 0.10, n = 4 leaves). The resulting effect upon the stomatal distribution as stomatal ratio is shown in Figure 3(b).
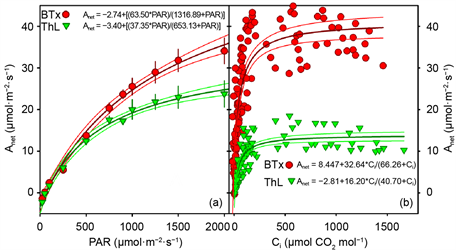
Results of leaf gas exchange characterizations of apparent quantum yield as net assimilation (Anet) to PAR and of responses of Anet to sub-stomatal [CO2] concentration (Ci) are shown in Figure 4(a) and Figure 4(b), respectively. Quantum yield and compensation points at low light intensities were nearly identical in the two lines. At higher PAR levels Anet of Thl diverged and was only 70% that of Btx-623. The maximal PAR used in this experiment was 2000 µmol・m−2・s−1 PAR. The fitted equations of the quantum yield response suggested that the maximal assimilation rate may have been approached at the highest PAR level in the Thl leaves, but the wild type had apparently not yet reached the PAR saturated Anet at ambient [CO2]. The maximal photosynthetic rate at 1800 µmol・m−2・s−1 PAR was greatly reduced in Thl as compared to the wild type. Maximal photosynthetic rates occurred at about the same Ci levels (Figure 4(b)).
Discussion
It appears that the differences in gas exchanges between the two isolines might be due to a combination of two anatomical features, the increase in leaf thickness and the reduction in the upper stomatal densities. The decrease in quantum
Figure 4. (a) Response of net assimilation (Anet) to photosynthetically active radiation (PAR) of greenhouse grown Btx-623 (Red, circles) and Thl (Green, triangles). Lines are fitted functions (shown) and 95% confidence interval. Bars are S.E. of individual measurements, n = 5. (b) Response of net assimilation (Anet) to sub-stomatal [CO2] of field grown Btx-623 (Red, circles) and Thl (Green, triangles). Lines are fitted functions (shown) and 95% confidence interval (n = 5).
yield seems consistent at least in part, with the associated reduction in the upper stomatal density. However, the reasons for the extremely reduced ACi response remained unclear and must await further work. We propose that increased mesophyll resistance and the lower light levels in the lower regions of the Thl leaves may have led to the apparently reduced ACi response. Leaf internal sub-stomatal [CO2] is derived from the water vapor conductance calculated from transpiration rates and from Anet [9] . Lower leaf surfaces that are actively transpiring but not actively assimilating might explain, at least in part, the reduced ACi response of Thl as compared to Btx623. This suggests that the mutant might be less water use efficient than the wild type from which it was derived, though extrapolating results obtained from a 2 × 3 cm leaf cuvette to the agronomic level is fraught with assumption. Again, this was beyond the purview of the current work, which was to briefly describe the “thick leafed” allelic mutant, Thl.
Another factor that might have affected the observed ACi response is the distance through which carbon must travel through the mesophyll before reaching the bundle sheathes [10] . Visual examination of the SEM micrographs Figure 2(e) and Figure 2(f)) reveal considerable increase in the distance between the epidermis and the vasculature. While not of direct relevance to the gas exchange rates, it was also noted that the phloem tissues within the vascular bundles were considerably more removed from the lower epidermis in the Thl plants. It was observed that field grown Thl plants were considerably less affected by sugar cane aphids as compared to the Btx623 plants. This was of particular interest because sugar cane aphids are an emerging problem in sorghum production, and because a clear mechanism for sugar cane aphid resistance can be hypothesized. An aphid proboscis must be at least as long as the distance from the lower epidermis to the phloem bundle to feed. It might simply require considerably less effort for aphids to feed on BTx623 vs. Thl. At this time this remains an observation, but one which could be important, and so it is included herein.
The morphological differences between Thl and BTx623 were more pronounced when plants were field grown or when polyhouse grown under higher light conditions experienced in the months near the summer solstice (not shown). When plants were grown in the winter and early spring, morphological differences between the mutant and the wild type were subtle, though these characteristics were not quantified. The physiological characteristics of the plants grown under these lower light conditions were not examined because we were interested in how the morphological responses would affect the physiology. The assumption here was that the morphological differences of Thl as compared to BTx623 were developmental, possibly photomorpogenic, pleiotropic responses to the environmental cues. If so, the lack of gross morphological differences between the two lines (such as thicker narrower leaves) would be associated with a lack of anatomical differences associated with gas exchange (such as stomatal distribution). However, a systematic approach investigating these hypotheses was not undertaken, primarily because it was beyond the purview of the present work. Moreover, growth under low light levels characteristic of the months outside of the growing season is of limited immediate relevance to the applied nature of such studies. This also points to the importance of agronomically relevant conditions in applied studies evaluating the potential utility of plant traits. Nevertheless, this mutant might be a useful tool for longer term studies investigating leaf developmental processes in sorghum, perhaps with the goal of manipulating traits that would increase light penetration through the canopy, reduce water use while maintaining or increasing forage biomass and grain production, and conferring resistance to sugar cane aphids.
4. Conclusion
The Thl mutant sorghum line exhibits altered stomatal distribution via increased abaxialization of stomatal numbers and thicker and narrower leaves. These are associated with altered gas exchange. Increased leaf thickness might be a useful trait in conferring sugar cane aphid resistance.
Acknowledgements
The authors thank Ryan Mounce for his excellent technical support and Bo Zhao from the College of Arts & Sciences Microscopy Facility at Texas Tech University for assistance with SEM. This work was supported by the United Sorghum Checkoff Program and USDA-ARS CRIS projects 6208-21000-017-00D and 3096-13000-007-00D.
Cite this paper
Gitz III, D.C., Liu-Gitz, L., Xin, Z.G., Baker, J.T., Payton, P. and Lascano, R.J. (2017) Description of a Novel Allelic “Thick Leafed” Mutant of Sorghum. American Journal of Plant Sciences, 8, 2956-2965. https://doi.org/10.4236/ajps.2017.812200
References
- 1. Xin, Z., Gitz, D., Burow, G., Hayes, C. and Burke, J.J. (2015) Registration of Two Allelic Erect Leaf Mutants of Sorghum. Journal of Plant Registration, 9, 254-257. https://doi.org/10.3198/jpr2014.09.0060crgs
- 2. Xin, Z., Wang, M.L., Barkley, N.A., Burow, G., Franks, C., Pederson, G. and Burke, J. (2008) Applying Genotyping (TILLING) and Phenotyping Analyses to Elucidate Gene Function in a Chemically Induced Sorghum Mutant Population. BMC Plant Biology, 8, 103-116. https://doi.org/10.1186/1471-2229-8-103
- 3. Xin, Z. Wang, M., Burow, G. and Burke, J. (2009) An Induced Sorghum Mutant Population Suitable for Bioenergy Research. BioEnergy Research, 2, 10-16. https://doi.org/10.1007/s12155-008-9029-3
- 4. Frederiksen, R.A. and Miller, F. (1972) Proposal for Release and Increase ATx622, BTx622, ATx623, BTx623, ATx624, BTx624. TAES Form 96-72: Seed Release Committee of the Texas Agricultural Experiment Station, College Station TX.
- 5. Abe, A., Kosugi, S., Yoshida, K., Natsume, S., Takagi, H., Kanzaki, H., Matsumura, H., Mitsuoka, C., Tamiru, M., Innan, H., Cano, L., Kamoun, S. and Terauchi, R. (2012) Genome Sequencing Reveals Agronomically Important Loci in Rice Using MutMap. Nature Biotechnology, 30, 174-178. https://doi.org/10.1038/nbt.2095
- 6. Roozeboom, K.L. and Prasad, P.V.V. (2016) Sorghum Growth and Development. In: Ciampitti, I. and Prasad, V., Eds., Sorghum: State of the Art and Future Perspectives, Agronomy Monographs 58. ASA and CSSA, Madison. https://doi.org/10.2134/agronmonogr58.2014.0062
- 7. Gitz III, D.C. and Baker, J.T. (2009) Methods for Creating Stomatal Impressions Directly onto Archivable Slides. Agronomy Journal, 101, 232-236. https://doi.org/10.2134/agronj2008.0143N
- 8. Gitz III, D., Baker, J.T., Echevarria-Laza, H., Payton, P., Mahan, J. and Lascano, R.J. (2017) CO2 and Chamber Effects on Epidermal Development in Field-Grown Peanut (Arachis hypogaea L.). American Journal of Plant Science, 8, 349-362. https://doi.org/10.4236/ajps.2017.83025
- 9. LICOR, Inc. (2012) Using the LI-6400XT, OPEN Software Version 6.2.x. https://www.licor.com/documents/s8zyqu2vwndny903qutg http://sites.middlebury.edu/biol323/files/2011/01/6400MAN.pdf
- 10. von Caemmerer, S. and Furbank, R.T. (2003) The C4 Pathway: An Efficient CO2 Pump. Photosynthesis Research, 77, 191-207. https://doi.org/10.1023/A:1025830019591
NOTES
1The US Department of Agriculture (USDA) prohibits discrimination in all its programs and activities on the basis of race, color, national origin, age disability, and where applicable, sex, marital status, familial status, parental status, religion, sexual orientation, genetic information, political beliefs, reprisal, or because all or part of an individual's income is derived from any public assistance program (Not all prohibited bases apply to all programs.) Persons with disabilities who required alternative means for communication of program information (Braille, large print, audiotape, etc.) should contact USDA’s TARGET Center at (202) 720-2600 (voice and TDD). To file a complaint of discrimination, write to USDA, Office of Civil Rights, 1400 Independence Avenue, S.W., Washington, D.C. 20250-9410, or call (800) 795-3272 (voice) or (202) 720-6382 (TDD). USDA is an equal opportunity provider and employer.
2Mention of this or other proprietary products is for the convenience of the readers only, and does not constitute endorsement or preferential treatment of these products by USDA-ARS.