American Journal of Plant Sciences
Vol.5 No.18(2014), Article
ID:48580,6
pages
DOI:10.4236/ajps.2014.518276
Environmental Factors Undermine Genetic Expression of Tiller Dynamics in Wild Rice Oryza nivara and Oryza rufipogon
Ekamber Kariali
School of Life Sciences, Sambalpur University, Sambalpur, India
Email: ekamberk@rediffmail.com
Copyright © 2014 by author and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/



Received 10 June 2014; revised 12 July 2014; accepted 30 July 2014
ABSTRACT
Tillering is a principal trait for the study of branching and production of more number of panicles for increased grain production in many cereal crops. Most of the semi-dwarf high yielding rice cultivars exhibit a remarkable degree of stability with respect to their tillering ability and maintain almost a constant tiller number which is genetically fixed for a particular cultivar. However, tiller production in wild species of rice is largely determined by environmental parameters, which supersede genetic features for expression of complete tillering ability. Two species of wild rice like Oryza nivara and Oryza rufipogon were tested for influence of manipulated growing conditions on tiller dynamics by comparing their growth in natural habitats and cemented pots filled with manure added soil. The results revealed a significant enhancement in the number of tiller production as well as biomass accumulation of each tiller with more grain yield in the cultivated conditions in both the species in comparison to the wild situations. The dryland inhabitant O. nivara became mono-tillering and deep water species O. rufipogon produced as many as five tillers with relatively lesser grain yield in their natural environments compared to their cultivated counterparts. From these observations, it is concluded that expression of genetic potential for tiller production is amenable to fluctuation of environmental factors in the wild species of rice and their capacity for adaption to inclement growth conditions.
Keywords:Oryza nivara, Oryza rufipogon, Tillering, Spikelets

1. Introduction
Each phenotypic trait of a living organism is controlled at the molecular or gene level located in the genome of the cell. However, compared to animals and microbes, plants possess more flexibility to alter their growth, development, morphology, physiology and life cycle in response to environmental cues and such responses ultimately determine the morphological traits. Changes acquiesced within the genotypes in course of adaptation to sub-optimal environments provide infrastructure for evolution of new cultivars or species. Unlike other cereals, diversity of rice is very high because the plant has the potential for adaptation to a wide spectrum of ecological conditions. In course of its domestication and evolution thereafter, rice plant has adapted into many more contrasting environments than that of other important cereals like wheat, barley, maize and oat. Exceptionally high biodiversity, with cultivars numbering 12,000 worldwide, has enabled rice cultivation in habitats varying from the deep water marsh land of Bangladesh to some of the deserts of North Africa, as well as altitudes extending from sea level of many south-east Asian countries to the high altitudes of Nepal. It is now known that cultivated rice, Oryza sativa acquired the extra-ordinary capacity for genetic diversity because of its origin from two wild species of the genus Oryza, namely Oryza nivara and Oryza rufipogon, Griff. [1] . These two species belong to AA genome complex [2] similar to Oryza sativa and possess inherent genotypic permissibility to cross bred with the latter. Apart from growth in natural conditions, these wild relatives of cultivated species also share habitat intended for cultivated rice and exhibit sympatric association. Morphological and physiological plasticity of the wild rice species increases amplitude of fluctuation in genotypic permissibility allowing the plant to imbibe strategies desired for adaptation into diversified environments. Foremost among such strategies are the measures undertaken for ensuring reproduction and dissemination of the progenies. This has been achieved by 1) initiation of large numbers of reproductive structures (spikelets) and/or 2) production of excess photosynthetic surfaces by formation of more number of tillers for catering to the demand of the reproductive structures in case of exigencies [3] [4] . Tiller production in most of the high yielding rice cultivars is a stable character and genetically determined. However, tillers formation in different species of wild rice is distinctly regulated by environmental parameters and genetic limitations are down-regulated under fluctuating environments.
Tillers are produced from vegetative shoot branching and tillering of rice plant is an important agronomic trait for more grain production to achieve higher yield and also a model system for the study of branching in cereals [5] [6] . Tillering can also be considered as a prime yield component due to the production of more numbers of panicles per unit area and often contributes to phenotypic plasticity in small-grain cereals [7] like wheat and rice especially under inclement climatic conditions. Tillering is initiated on the main shoot at five-leaf stage. During early vegetative growth, rice plant continuously forms new leaves in regular spatial and temporal patterns [8] . The emerging tillers develop from axils of these leaves on the un-extended nodes of the main shoot. The earlyinitiated tillers on the main shoot also give rise to secondary tillers and secondary tillers produce tertiary tillers on their stem nodes [3] (Figure 1). Morphogenetic processes in sequentially growing leaves and tiller buds are highly synchronized in rice and the appearance of successive leaves in the main culm acts as the “pace maker”
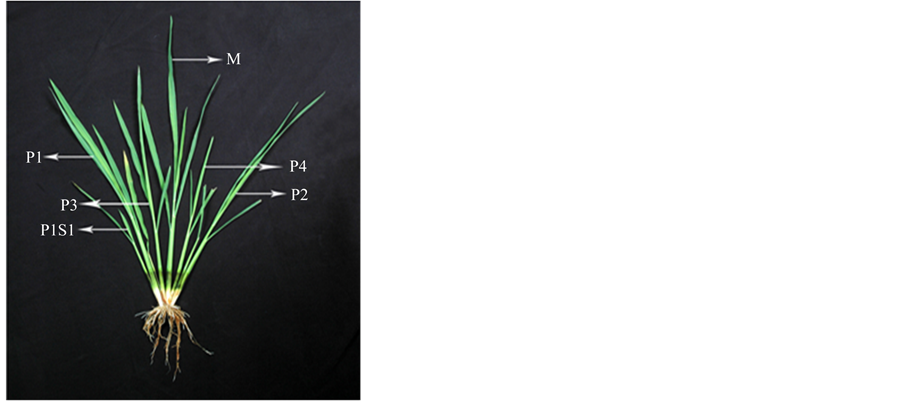
Figure 1. Tillering pattern in rice few days after transplanting. M, mother shoot; P1, first primary tiller; P2, second primary tiller; P3, third primary tiller; P4, fourth primary tiller; P1S1, first secondary tiller emerged from P1 and so on.
for the whole shoot system development [9] . Tillering continues up to the panicle initiation stage and thereafter some of the late-formed tillers die because of premature senescence. Tiller geometry follows a particular pattern in rice plant. On the main shoot, the coleoptile leaf and the first leaf do not produce any tiller. There is a definite relationship between tiller and leaf emergence on a rice culm. Emergence of fifth leaf coincides with that of the tiller at the axil of second leaf [3] ; this is the first tiller of the plant called P1 (Figure 1). Emergence of subsequent tillers follows the same spatial synchrony; emergence of nth leaf of a culm coincides with that of the tiller of n − 3 leaf. Tillers are also produced on the early initiated primary and secondary tillers on a free-tillering cultivar and spatial synchrony as evident in the main shoot is also retained on the tillers. Tillers have been classified in to different groups according to their spatial origin on successive internodes. The tillers of the main shoot are called primary tillers whereas those produced from primary tillers are named secondary and tillers emerged out of secondary are called tertiary tillers [10] .
Tiller formation on rice plant is a dynamic process; both environmental and genetic factors regulate tiller development and its number. Kawano and Tanaka [11] found that tiller number is positively or negatively correlated to grain yield depending on cultivar and environment. Traditional rice cultivars possess strong apical dominance and produce a limited number of tillers [12] . In contrast, semi-dwarf rice cultivars are free-tillering and produce a large number of tillers, most of them in a homogeneous pattern. Apart from the genotypic permissibility, environmental factors are extremely important for regulation of tiller dynamics; plants growing under submerged soil are mono-tillering, whereas many tillers are produced when surface soil moisture regime is moderate. This feature became most evident in wild species of rice like O. nivara and O. rufipogon (Figure 2). Apart from this, tillering is also affected by irradiance, nutrients, depth of planting, population density and temperature [3] . Tillering is poor at high density of population and deep seeded conditions. In contrast, tillering is encouraged by application of mineral nutrients; tillering rate increases linearly with increase of essential elements like
 (a)
(a) (b)
(b)
Figure 2. Tillering under cultivated condition (a), natural habitat (b) in O. nivara (upper) and under cultivated condition (c) and natural habitat (d) in O. rufipogon (lower).
nitrogen, phosphorus and potassium levels in the growing medium. Tiller bud initiation and subsequent developments are also regulated by the interplay of genetic programs with the environment [13] . In the present experiment, attempts have been made to assess the impact of environmental factors on the tillering capacity of two wild species of rice in contrasting growth habitats.
2. Material and Methods
2.1. Plant Materials and Experimental Sites
In the experiment, two species of wild rice O. nivara and O. rufipogon were studied in their natural habitats around Jyotivihar and also in agronomically manipulated growth conditions of cemented pots under open field conditions in the botanical garden of the School of Life Sciences, Sambalpur University, Odisha, India (21.25˚N; 83.52˚E; altitude 160 m) during the wet season of 2009. O. nivara and O. rufipogon grew naturally in dryland aerobic conditions and aerobic swampy-lowland habitats (Figure 2) respectively around the university campus.
2.2. Plant Cultivation and Field Maintenance
Thirty days old seedlings of both the species having uniform growth and development were carefully uprooted from their natural habitats and transplanted in the cemented pots (330 × 330 × 260 mm) containing 42 Kg of sandy loam soil well mixed with farm yard manure (3:1). The pots were arranged in a randomized block design with three replicates (plant density of 36 m−2) and kept in open field conditions in the botanical garden of the School of Life Sciences. Recommended doses of chemical fertilizers were applied at the time of transplanting, tillering and panicle initiation stages and the water level in the pot was maintained at 5.0 ± 2.0 cm during the entire period of plant growth except during transplanting and maturity time. Similarly plants were observed carefully in the natural habitats with no interference from other animals or human beings.
2.3. Sampling
Careful observations were made regularly through field visit for determination of tiller number per hill both in the pot and natural habitats from the time of tiller emergence to maturity. Plants with uniform growth and development were tagged both in the natural habitats and pot conditions for both the species. Sampling was carried out randomly from three different locations in the field and one plant from each pot in three replicates in the cultivated condition. The plants were uprooted and brought to the laboratory and dissected into different types of tillers like mother shoot, primary, secondary and tertiary tillers and their numbers were counted and recorded. After recording the tiller numbers, the shoots and panicles of different tillers were separated and dried in side an oven at 90˚C for one hr and subsequently at 37˚C for 48 h till constant weight for the measurement of biomass of samples by an electronic balance.
3. Results
3.1. Tiller Number
The dryland species O. nivara grew early and completed its life cycle quickly and produced only one tiller in the natural habitat. On the other hand, the deep-water species O. rufipogon had an extended period of growth in the low land habitat and produced as many as five tillers (Table 1). When the plants were grown in the manipulated conditions of the cemented pots, O. nivara produced almost 11 primaries, 21 secondary and 12 tertiary tillers whereas the number of tillers in O. rufipon was 13 primaries, 25 secondary and 21 tertiary. O. nivara is annual type and mono-tillering in its natural habitat compared to O. rufipon which is perennial and multi-tillered. However, both the species became profusely tillering in the cultivated conditions due to suitable manipulation of growing environment (Table 1 and Figure 2).
3.2. Biomass Accumulation
The biomass accumulation in the panicle and shoot of different type of tillers exhibited significant variation both in the natural habitats and agronomically manipulated pot conditions. The shoot and panicles of different tillers including mother plant of both the species accumulated lower biomass in the natural habitats compared to their
Table 1 . Tiller number and biomass accumulation in different types of tillers in the wild rice O. nivara and O. rufipogon under cultivated and natural conditions. ± values represent SD of three replicates.
respective tiller counterparts in the cultivated conditions. Mother tiller accumulated maximum biomass in the panicle and shoot followed by primary, secondary and tertiary tillers in a sequence in both the species and same sequence was also retained when the plants were grown in the pots.
4. Discussion
The limits of genotypic permissibility for the change in the phenotypic expression of important traits like tillering in diversified environmental conditions have not been studied extensively [2] . The dryland species O. nivara bereft of water accumulation in the natural habitat was unable to sustain essential nutrients in soil because of loss with running water during rainy season. The nutrient deprived soil condition might curtail biomass production leading to formation of only one tiller. The initiation of a signal in the form of ethylene [2] generated due to water deficit might have been responsible for dictating the plant to grow quickly; produce low biomass and fold up its life cycle quite earlier in order to escape from the expected water deficit stress during the later part of the life cycle. On the contrary, the other species of wild rice O. rufipogon, was evolved in a relatively stable environment, i.e. deep water habitats and the plants grew comfortably well in the water logged lowland habitat for extended period of life cycle and produced more biomass and many tillers (Table 1).
Tiller number in rice is dynamic and adjustable [14] and tillering ability is one of the important morphological traits that are responsible for adaptation to a particular habitat. Production of more tillers during early stage of growth might be due to the abundance of resources [12] and tiller number could be reduced significantly because of limitation of resources. In the present experiment, both the species exhibited considerable variation in their tiller numbers when grown in the natural habitats whereas tillering was profuse and identical when cultivated under manipulated environment of the cemented pots which might be due to adequate supply of water, nutrients and other resources. These responses were indicative of a close phylogenetic relationship between these two species of wild rice. From this study it can be concluded that tillering capacity in the two species of wild rice is largely controlled by environmental factors and genetic features are undermined in a manipulated growth condition to a large extent.
References
- Cook, M.G. and Evans, L.T. (1983) Some Physiological Aspects of the Domestication and Improvement of Rice (Oryza spp.). Field Crops Research, 6, 219-238. http://dx.doi.org/10.1016/0378-4290(83)90062-X
- Mohapatra, P.K., Panda, B.B. and Kariali, E. (2011) Plasticity of Tiller Dynamics in Wild Rice Oryza rufipogon Griff., a Strategy for Resilience in Sub-Optimal Environments. International Journal of Agronomy, 2011, Article ID: 543237.http://dx.doi.org/10.1155/2011/543237
- Yoshida, S. (1981) Fundamentals of Rice Crop Science. International Rice Research Institute, Philippines.
- Paul, M.J. and Foyer, C.H. (2001) Sink Regulation of Photosynthesis. Journal of Experimental Botany, 52, 1383-1400. http://dx.doi.org/10.1093/jexbot/52.360.1383
- Li, X., Qian, Q., Fu, Z., Wang, Y., Xiong, G., Zeng, D., Wang, X., Liu, X., Teng, S., Hiroshi, F., Yuan, M., Luo, D., Han, B. and Li, J. (2003). Control of Tillering in Rice. Nature, 422, 618-621. http://dx.doi.org/10.1038/nature01518
- Chen, F., Jiang, L., Zheng, J., Huang, R., Wang, H., Hong, Z. and Huang, Y. (2014) Identification of Differentially Expressed Proteins and Phosphorylated Proteins in Rice Seedlings in Response to Strigolactone Treatment. PLoS ONE 9, Article ID: e93947. http://dx.doi.org/10.1371/journal.pone.0093947
- Dreccer, M.F., Chapman, S.C., Rattey, A.R., Neal, J., Song, Y., Christopher, J.T. and Reynolds, M. (2013) Developmental and Growth Controls of Tillering and Water-Soluble Carbohydrate Accumulation in Contrasting Wheat (Triticum aestivum L.) Genotypes: Can We Dissect Them? Journal of Experimental Botany, 64, 143-160. http://dx.doi.org/10.1093/jxb/ers317
- Xiong, G.S., Hu, X.M., Jiao, Y.Q., Yu, Y.C., Chu, C.C., Li, J.Y., Qian, Q. and Wang, Y.H. (2006) LEAFY HEAD2, Which Encodes a Putative RNA-Binding Protein, Regulates Shoot Development of Rice. Cell Research, 16, 267-276. http://dx.doi.org/10.1038/sj.cr.7310034
- Miyamoto, N., Goto, Y., Matsui, M., Ukai, Y., Morita, M. and Nemoto, K. (2004) Quantitative Trait Loci for Phyllochron and Tillering in Rice. Theoretical and Applied Genetics, 109, 700-706. http://dx.doi.org/10.1007/s00122-004-1690-0
- Kariali, E., Kuanar, S. and Mohapatra, P.K. (2008) Individual Tiller Dynamics of Two Wild Oryza Species in Contrasting Habitats. Plant Production Science, 11, 355-360. http://dx.doi.org/10.1626/pps.11.355
- Kawano, K. and Tanaka, A. (1968) Studies on the Inter-Relationships among Plant Characters in Rice. I. Effect of Varietal Difference and Environmental Condition on the Correlation between Characters. Japanese Journal of Breeding, 18, 75-79. http://dx.doi.org/10.1270/jsbbs1951.18.75
- Dingkuhn, M. and Kropff, M. (1996) Rice. Photo-Assimilate Distribution in Plants and Crops: Source-Sink Relationships. Zamski, E. and Schaffer, A.A. (Eds.), Marcel Dekker Inc., New York, 519-547.
- Mc Steen, P. and Leyser, O. (2005) Shoot Branching. Annual Review of Plant Biology, 56, 353-374. http://dx.doi.org/10.1146/annurev.arplant.56.032604.144122
- Kariali, E. and Mohapatra, P.K. (2007) Hormonal Regulation of Tiller Dynamics in Differentially-Tillering Rice Cultivars. Plant Growth Regulation, 53, 215-223. http://dx.doi.org/10.1007/s10725-007-9221-z


