American Journal of Plant Sciences
Vol.05 No.10(2014), Article ID:45664,8 pages
10.4236/ajps.2014.510157
Relationship between leaf micro- and macro-nutrients in top canopy trees in a mixed forest in the upper Rio Negro in the Amazon region
M. A. Sobrado
Laboratorio de Biología Ambiental de Plantas, Departamento de Biología de Organismos, Universidad Simón Bolívar, Apartado 89.000, Caracas, Venezuela
Email: msobrado@usb.ve
Copyright © 2014 by author and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/



Received 3 March 2014; revised 5 April 2014; accepted 17 April 2014
ABSTRACT
The mixed forests of the upper Rio Negro at the northern of the Amazon basin grow in oxisol soils that are extremely infertile. These areas exhibit deficiencies in several macro-nutrients, and may also be characterized by the shortage or toxic excess of some micronutrients. The overall goal of this research is to collect more comprehensive information regarding the micronutrient composi- tion of the upper Rio Negro forests as well as discern the relationship between leaf micro- and macro-nutrients that may contribute to the homeostasis and balance of the ionome. Firstly, the nutrient composition within the oxisol soil and leaf tissues of two top canopy tree species from the mixed forests was determined. We then analyzed the relationship between leaf micronutrient composition with N and P levels of the two species and that of species inhabiting the Amazon caatinga. Extractable soil Zn, B, Mn and Cu were very low in the mixed forest. In contrast, Fe and Al levels were potentially toxic. The analysis of leaf N/P ratios revealed for the first time the co-limi- tation of N and P in the mixed forest. This contrasts with species from the adjacent Amazon caat- inga toposequence that are characterized by strong N limitation. All micronutrients within leaves of species inhabiting the mixed forest were also found to have low concentrations. Moreover, Fe and Al were detected at concentrations well below those reported for accumulator species. This suggested that leaf ion homeostasis was maintained under potentially toxic soil Fe and Al conditions. Leaf micronutrient (Fe, Zn and B) contents mirrored that of leaf N and P contents, and comparable Fe/N, Fe/P, Zn/N, Zn/P, B/N as well as B/P ratios were found across species and forest types. Therefore, forest species exhibited the capability to maintain leaf nutrient balances under soil conditions with deficient or toxic levels of micronutrients.
Keywords:
Amazon forests, Amazon caatinga, leaf ionome homeostasis, leaf N-P and micronutrients, micro-nutrient deficiency, micronutrients toxicity, mixed forest, N-P co-limitation, plant nutrient balance, oxisol, podzol

1. Introduction
Many soils located in tropical rainforests are formed from materials that are highly weathered and strongly leached. These areas exhibit deficiencies in several macro-nutrients, and may also be characterized by the shortage or toxic excess of some micronutrients [1] [2] . In general, this issue has been widely analyzed and discussed within the context of agronomic constrains and management of fertilization strategies [3] . In contrast, few studies have investigated the role of micronutrients in nutrient cycles, litter decomposition and possible interactions with macronutrients in natural tropical ecosystems [4] [5] . A first approach would be to analyze the plant ionome and to identify possible interactions between elements in species inhabiting these environments. Comparative elemental profiles will yield valuable insight into how the plant ionome responds to the environment and provides clues to explore the genetics that control the homeostasis of the ionome [6] [7] . Physiological mechanisms that enable plants to respond to environmental conditions through the flexible modification of key regulators of cellular physiology may be highly selected in order to maintain ionic homeostasis [8] .
A large portion of Amazonia is characterized by lowland evergreen and highly diverse forests thriving on non-flooded oxisol soils. The mixed forests of the upper Rio Negro at the northern tip of the Amazon basin grow in oxisol soils that are extremely infertile. The poor soils typical of this area result from a lack of geological activity and sand deposition from Precambrian formations of the Guiana Shield [9] . This mixed forest has high species diversity and occupies the rolling hills rising up to 50 m above river level, whereas the lowland areas comprise the Amazon caatinga continuum whose soils are largely podzolized sands [10] [11] . Historically, the mixed forest has been considered limiting in P, Ca and Mg as compared to the strongly N-limited Amazon caatinga [12] . However, a recent analysis of soil and leaf nitrogen isotopic signatures (δ15N) in both forests types showed that the mixed forest is poor in N as well, and consequently nitrogen cycling is tight [13] . Indeed, the oldest and/or the most chemically weathered forest soils of the Amazon support ecosystems with limitations in their productivity due to low levels of nitrogen availability [14] .
Although macronutrient levels have been studied intensively and compared across species in the mixed forest [12] [15] , the micronutrient composition has not been analyzed. The overall goal of this research is to collect more comprehensive information regarding the micronutrient composition of the upper Rio Negro forests as well as discern the relationship between leaf micro- and macro-nutrients that may contribute to the homeostasis of the ionome. Firstly, the nutrient composition within the oxisol soil and leaf tissues of two top canopy tree species from the mixed forests was determined. We then analyzed the relationship between leaf micronutrient composition with N and P levels in two species residing in the mixed forest as well as species inhabiting the Amazon caatinga [16] . This approach allowed us to test the hypothesis that leaf micronutrient levels would be coupled to the macronutrient levels, particularly N and P, in order to maintain the balance between nutrients under environmental conditions characterized by either deficient or toxic levels of micronutrients.
2. Material and Methods
2.1. Study site
The study site is located in southern Venezuela near the town of San Carlos de Rio Negro (1˚54'N, 67˚3˚W, 119 m ASL), located in the margin of the upper Rio Negro in the Amazon region. The climatic conditions at this site are typical of tropical rainforest areas, with a mean annual temperature of 26˚C and a mean annual rainfall of 3600 mm . This area has been described in a number of other studies [9] - [11] .
2.2. Tree species
This study focused on the nutrient composition of leaves sampled from two canopy trees species dominant in the mixed forest on oxisol soil: Caryocar glabrum (Aubl.) Pers. (Caryocaraceae) and Ocotea aciphylla (Nees & Mart. Ex Nees) Mez (Lauraceae). Subsequently, the data collected were compared with similar data from species occupying the Amazon caatinga toposequence on podzolized sands [16] . Details regarding the ecophysiology of species growing in both types of forest as previously published are presented in Table 1 [13] [17] - [21] . C. glabrum and O. aciphylla, inhabiting the mixed forest, and Eperua leucantha Benth. (Caesalpiniaceae), inhabiting the Amazon caatinga valley, possess thinner and less dense leaf blades [13] [17] . These species also exhibit relatively low fiber to protein ratios (sclerophylly index) and carbon to nitrogen ratios (C/N) [13] . In addition, these three species present with the highest, albeit negative, δ15N signatures, which is consistent with a very tight nitrogen cycle (Table 1) [13] . Conversely, species sampled from the slopes of the Amazon caatinga toposequence (Micranda sprucei Müll. Arg. (R. E. Schultes); Euphorbiaceae) and top mounds ((Pachira sordida (R.E. Schult.) W.S. Alverson (syn. Rodognaphalopsis discolor A. Robyns); Malvaceae and Remijia morilloi Steyerm; Rubiaceae)) tend to have thicker leaf blades with a higher sclerophyll index [13] [18] . The Amazon caatinga species exhibit a more negative δ15N, suggesting a stronger N limitation than the mixed forest and a very tight nitrogen cycle as well (Table 1) [13] [19] . Furthermore, species thriving in the drought-prone top mounds of the Amazon caatinga support lower leaf water potentials (Y) and still maintain turgor (
2.3. Leaf and soil sampling
For both mixed forest species, three mature trees with a fully exposed top canopy were selected and tagged for soil and plant collection during August of 2011. The entire forest floor was covered by a dense root mat covered with leaf litter [22] . Four soil samples consisting mostly of mineral soil and organic matter were collected at a depth from 0 - 5 cm from the bare soil under the root mat of each tree. After all visible particles of plant material were removed by hand, the four samples were pooled in the field. The resulting soil samples were dehydrated at room temperature, ground, homogenized, and sieved though 2 mm mesh. For analysis of leaf nutrients, three top canopy branches were detached in each tagged tree and adult leaves with a healthy appearance were collected and pooled. Leaf blade samples, excluding major veins, were oven dried to a constant weight at 60˚C and ground prior to performing further analyses. A total of three pooled leaf blade samples were analyzed for each plant species.
2.4. Soil analyses
The soil analyses were performed following previously described procedures [23] . Soil subsamples were used
Table 1. Summary of leaf characteristics of mixed and caatinga forest species.
Species denoted as Cg (C. glabrum) and Oa (O. aciphylla) inhabit the mixed forest. El (E. leucantha), Ms (M. sprucei), Ps (P. sordida) and Rm (R. morilloi) inhabit the Amazon caatinga toposequence. The parameters are: leaf carbon to nitrogen ratio (C/N), leaf nitrogen isotopic signature (δ15N), leaf water potential (Y) at zero turgor (Y(0)) from leaf tissue analysis, minimum leaf Y measured in the field (Y(field)) and leaf carbon isotopic signature (δ13C) as reported previously in several studies [13] [17] - [21] .
for the following analyses: soil pH was measured from a mixture of 5 g of soil in distilled water at a ratio of 1:1. Organic content (OM) was determined by weight loss after combusting samples of approximately 5 - 7 g . Soil exchangeable micronutrients (Fe, Mn, Zn, and Cu) as well as Al were extracted according to the Mehlich 3 soil test, and hot water was used to extract B. Subsequently, the micronutrient composition of the extracts was measured using an Inductively Coupled Plasma-Atomic Emission Spectrometer (ICP-AES). The soil N and C composition analysis was performed by mass spectrometry.
2.5. Leaf analyses
Leaf samples were prepared for micronutrient measurements using the ICP-AES following procedures described previously [24] : Samples of 0.2 g were muffled and ashed, dissolved in 2 ml of 5 N HCL, heated at 200˚C for 2 min, and then brought to a 10 ml volume with de-ionized water. Prior to taking measurements, residual carbon was eliminated by centrifugation at 2000 rpm for 4 minutes. The leaf N composition was measured by mass spectrometry.
2.6. Statistical analysis
Measurements were performed on randomly selected independent replicates. For each parameter, normality of the sampling data was assessed using the Kolmogorov-Smirnov test and equal variance of the data was tested using Levene’s test. These tests showed that data were normally distributed and had equal variance. Comparisons between both species studied were made by using paired t-tests. Pearson moment correlation coefficients were used to quantify the strength of association between leaf micronutrient content with N and P content. The level of significance for statistical tests was set at p < 0.05 and all analyses were performed using Sigma Plot version 11.1 software (Systat Software, Inc., Chicago, USA).
3. Results and Discussion
3.1. Soil analysis
The results of soil analysis are shown in Table 2. The mixed forest soil was acidic with very little organic matter, C and N, as well as extremely low extractable Mn, Zn, B and Cu (Table 2). The C/N ratio was approximately 19 kg∙kg−1 which is slightly lower than that observed for soils harboring the caatinga species (Table 2). In the oxisol soils of the mixed forest, most of the organic matter is confined above the root mat, which explains the low levels of organic matter detected in this study [22] . Soil Mn, Zn and B were within the range found in the adjacent Amazon caatinga toposequence (Table 2) [16] . Previously published data for crop soils set critical values for Mn, Zn, B and Cu at 1, 3.3, 2.4 and 1 mg∙kg−1, respectively [25] . Conversely, levels of Fe and Al were 10 - and 2.5-fold higher than the highest values found in the caatinga toposequence (Table 2). These results suggest that Fe and Al are potentially toxic in the oxisol soils where the mixed forest thrives. Indeed, oxisol soils are very unproductive given the soil acidity and low native fertility [3] . In this mixed forest, it is well documented that macronutrients are obtained from leaf litter and organic matter covering the root mat on the soil floor [22] . Whether this is also the case for micronutrients is unknown, but the entire forest floor is potentially exposed to high Fe and Al, and very low inputs for the other micronutrients can be expected from parental material.
3.2. Leaf analysis
The results of leaf nutrient analyses are shown in Table 3. Concentrations of Fe and Al were low in leaf tissues, despite the toxic levels of these metals in the soil, and were comparable to levels observed for species of the Amazon caatinga toposequence (Table 3). The fact that mixed forest species did not accumulate Al or Fe in leaves implicates a very efficient mechanism for excluding these metals in photosynthetic tissue. Reference values for plant hyperaccumulation of Al are the range of 2.3 - 3.9 g∙kg−1 [26] , and for Fe, the reported accumulation is approximately 10 g∙kg−1 [27] . Notably, these values are well above those found in this study (Table 3). The leaf Mn, Zn and B concentrations were low but comparable to those described for the nearby caatinga trees (Table 3). However, the leaf Cu concentration in C. glabrum was higher than the level reported for the caatinga trees. Overall, the concentrations of Fe, Zn, B and Cu in the mixed forest trees were lower than sufficiency levels as defined for tropical crops [28] . This finding highlights that the mechanisms of nutrient acquisition,
Table 2. Soil analysis.
Parameters measured in this study were soil pH in water, organic matter (OM) content, extractable micronutrient content as well as total C, N, and C/N). Data for the mixed forest species are expressed as means ± SE. Range values in the adjacent Amazon caatinga toposequence were taken from a previous report [16] .
Table 3. Leaf nutrient composition.
Parameters are expressed as means ± SE. Statistically significant differences between the means of both species in the mixed forest are denoted as *(p < 0.05) and **(p < 0.01). The range of leaf nutrients in the adjacent Amazon caatinga toposequence species was taken from a previous report [16] .
transport and homeostasis employed by non-crop species are still poorly understood.
The concentrations of N and P macronutrients in the mixed forest trees were low and overlapped with those reported for leaves of the caatinga toposequence species (Table 3). However, the leaf N/P ratio was higher than in the caatinga species. Leaf N/P was 18.92 ± 0.62 kg∙kg−1 in the mixed forest, which was significantly higher (p < 0.001) than 12.90 ± 0.93 kg∙kg−1 in the Amazon caatinga species. Furthermore, leaf N/P in the mixed forest species was lower than that found in habitats where P limitations override that of N (24 - 27 kg∙kg−1), but was higher than that found in species mostly limited by N (9 - 16 kg∙kg−1), as previously described [29] . These results indicate for first time that N and P are co-limiting macronutrients in these mixed forest species growing in oxisol soil (Table 3).
3.3. Micro- and macro-nutrients interrelations
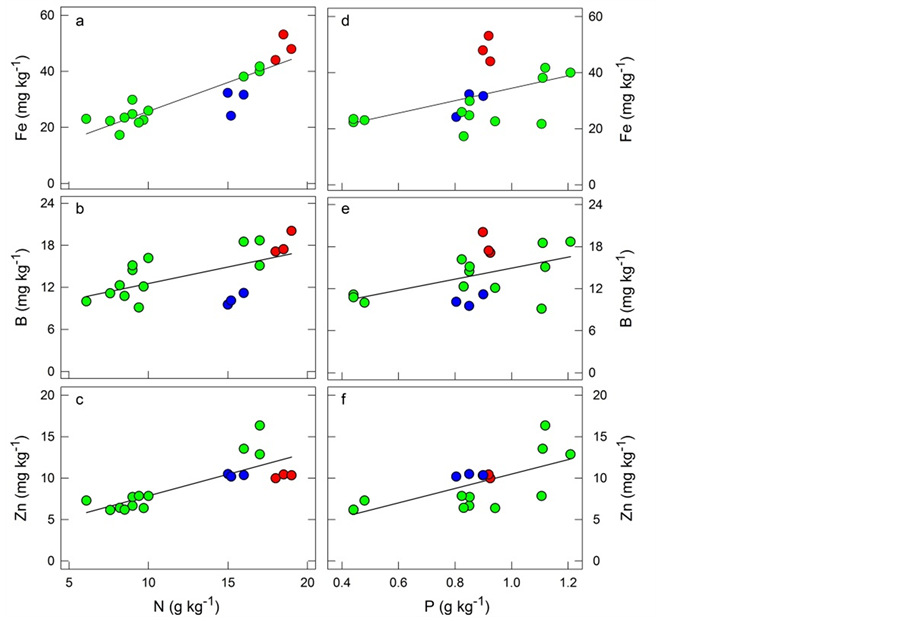
Determining ranges of “sufficiency” for individual nutrients has been recognized as a non-optimal method to establish or diagnose the nutritional needs of crops. Instead, it has been recommended that important pairs of nutrients are evaluated as part of a diagnosis and recommendation integrated system (DRIS) [30] . In natural for- ests inhabiting poor tropical soils, nutrient sufficiency ranges may also be of limited value. In the case of natural forest ecosystems, it seems equally insightful to evaluate the relationships between nutrients. Implementation of this method focuses on plant demand, and allows the assessment of nutritional balance between the nutrients in a leaf sample. Indeed, species thriving in habitats with deficient nutrient supply tend to have a constrained growth rate without apparent dysfunction [31] . This may require very tight homeostasis of each nutrient as well as a balanced macro- and micro-nutrient composition. In this study, we further explored the relationships between leaf N and P as major limiting macronutrients in this Amazon mixed forest and the somewhat limiting micronutrients (Mn, B, Zn, Cu) and those present in toxic excess (Fe). The evaluation of essential nutrients together recognizes the effect of one element (macronutrients) on the other (micronutrients) for optimal leaf physiology. Thus, we assessed the correlations between each leaf micronutrient level (Fe, Mn, B, Zn and Cu) with those of N and P. We detected linear and statistically significant correlations between leaf N and P with leaf Fe, B and Zn (Figure 1). These relationships between pairs of nutrients suggested that micronutrients (Fe, B and Zn) mirrored the leaf concentrations of N and P.
Iron is an integral part of chlorophyll that is involved in photosynthesis [28] . Chlorophyll synthesis, including the activation of several enzymes and oxido-reduction reactions requires Zn. Zinc deficiencies are associated with low total concentrations of zinc in soils featuring highly weathered parental material and low pH such as in tropical areas [32] . Boron is a metalloid with properties intermediate between metals and non-metals. B shortage affects a number of metabolic processes such as protein, carbohydrate and nucleic acid metabolism, cell wall synthesis and membrane integrity, which ultimately impacts photosynthetic function [33] [34] . Boron-depleted soils are usually created under conditions of high rainfall and strong weathering typical of the oxisols and podzols [35] . The Fe/N, B/N and Zn/N and ratios were 2.54 ± 0.12, 1.17 ± 0.08 and 0.76 ± 0.04 g∙kg−1, respectively, and the Fe/P, B/P and Zn/P ratios were 37.59 ± 2.73, 16.78 ± 1.10 and 11.00 ± 0.61 g∙kg−1, respectively. These values reflect pooled data from six species growing in two contrasting forest types (Figure 1). These results point to a comparable nutrient balance across species and forest types, despite the fact that both forests feature different soil conditions and micro-nutrient levels (Table 2).
Figure 1. Leaf Fe (a,d), B (b,e) and Zn (c,f) as a function of leaf N and P of C. glabrum (red), O. aciphylla (blue) used in this study, and that of four species of the Amazon caatinga toposequence (green) reported elsewhere [16] . Each point represents values found in individual trees for a total of three trees per species. Linear regressions are shown with the following equations for each micronutrient correlated: (a) Fe (mg∙kg−1) = (2.06 * N (g∙kg−1)) + 5.12; r = 0.88; p < 0.001; (b) B (mg∙kg−1) = (0.47 * N (g∙kg−1)) + 7.83; r = 0.59; p < 0.01; (c) Zn (mg∙kg−1) = (0.52 * N (g∙kg−1)) + 2.66; r = 0.80; p < 0.001; (d) Fe (mg∙kg−1) = (22.14 * P (g∙kg−1)) + 12.28; r = 0.47, p < 0.05; (e) B (mg∙kg−1) = (7.86 * P (g∙kg−1)) + 7.07); r = 0.49; p < 0.05; (f) Zn (mg∙kg−1) = (8.71 * P (g∙kg−1)) + 1.78; r = 0.67; p < 0.01.
Understanding the mechanisms underlying the homeostasis of the ionome and the balance of ion acquisition has been the subject of recent research [36] . This requires knowledge of how nutrient levels are sensed by plants and how nutrients control gene expression. The Zinc- and Iron-regulated transport Protein(ZIP) gene family encodes transporters for divalent metal ion nutrients [37] [38] . Studies suggest that uptake, translocation, and homeostasis of Zn, Fe, and Mn is controlled by the number of active transporters embedded in cell membranes. Ubiquitin (Ub) as well as other small Ub-like proteins able to conjugate with target proteins have important roles in uptake, trafficking, and maintenance of many plant essential nutrients [8] . Ubiquitination involves the conjugation of ubiquitin (Ub) onto lysine residues of acceptor proteins. This modification controls the localization and fate of many plasma membrane proteins, and appears to be a critical mechanism to maintain ion homeostasis in plants [8] . Specifically, ubiquitination is a mechanism to maintain iron and boron homeostasis in plants [39] [40] . Regulatory mechanisms that maintain N and P balance with Fe, Zn and B levels, such as those reported in this study, are currently unknown. Nonetheless, we do not preclude the possibility that an ubiquitination-like system could regulate such balances. Indeed, a novel regulatory mechanism involving an ubiquitination system has been discussed for primary carbon and nitrogen responses and metabolism as well as for maintaining the C/N balance [41] . Future research will advance our understanding of the underlying physiological mechanisms enabling plant species to thrive in native habitats characterized by soils with unusual nutrient compositions.
4. Conclusions
The conclusions of this study are:
1) Extractable soil Zn, B, Mn and Cu were very low in the mixed forest. In contrast, Fe and Al levels were potentially toxic.
2) The analysis of leaf N/P ratios revealed co-limitation of N and P macronutrients in the mixed forest species. This contrasts with species from the adjacent Amazon caatinga toposequence that are characterized by only strong N limitation.
3) Within leaves of species inhabiting the mixed forest, all micronutrients were also found in low concentrations. Moreover, Fe and Al were detected at concentrations well below those reported for accumulator species. This suggested that leaf ion homeostasis was maintained under potentially toxic soil Fe and Al conditions.
4) Leaf micronutrient (Fe, Zn and B) contents mirrored that of leaf N and P contents, and comparable Fe/N, Fe/P, Zn/N, Zn/P, B/N as well as B/P ratios were found across species and forest types. Therefore, forest species exhibited the capability to maintain leaf nutrient balances under soil conditions with deficient or toxic levels of micronutrients.
Acknowledgements
Did-USB provides partial financial support. To Pedro Maquirino for his invaluable help during field work. To the Editorial team of AJPS for suggestions to improve the manuscript.
References
- Davies, B.E. (1997) Deficiencies and Toxicities of Trace Elements and Micronutrients in Tropical Soils: Limitations of Knowledge and Future Research Needs. Environmental Toxicology and Chemistry, 16, 75-83. http://dx.doi.org/10.1002/etc.5620160108
- Kaufman, S., Sombroek, W.G. and Mantel, S. (1998) Forest Soils in the Humid Tropics: Characteristics and Classification. In: Schulte, A. and Ruhiyat, D., Eds., Soils of Tropical Forest Ecosystems: Characteristics, Ecology, and Management, Springer, Berlin, 9-20.
- Fageria, N.K., Baligar, V.C. and Clark, R.B. (2002) Micronutrients in Crop Production. In: Sparks, D.L., Ed., Advances in Agronomy, Academic Press, San Diego, 185-268.
- Kaspari, M., Garcia, M.N., Harms, K.H., Santana, M., Wright, S.J. and Yavitt, J.B. (2008) Multiple Nutrients Limit Litterfall and Decomposition in Tropical Forests. Ecology Letters, 11, 35-43. http://dx.doi.org/10.1111/j.1461-0248.2007.01124.x
- Powers, J.S. and Salute, S. (2011) Macro- and Micronutrients Effects on Decomposition of Leaf Litter from Two Tropical Tree Species: Inferences from a Short-Term Laboratory Incubation. Plant and Soil, 346, 245-257. http://dx.doi.org/10.1007/S11104-011-0815-x
- Baxter, I. (2009) Ionomics: Studying the Social Network of Mineral Nutrients. Current Opinion in Plant Biology, 12, 381-386. http://dx.doi.org/10.1016/j.pbi.2009.05.002
- Satismruti, K., Senthil, N., Vellaikumar, S.R., Ranjani, V. and Raveendran, M. (2013) Plant Ionomics: A Platform for Identifying Novel Gene Regulating Plant Mineral Nutrition. American Journal of Plant Sciences, 4, 1309-1315. http://dx.doi.org/10.4236/ajps.2013.47162
- Yates, G. and Sadanandom, A. (2013) Ubiquitination in Plant Nutrient Utilization. Frontiers in Plant Science, 4, Article ID: 452. http://dx.doi.org/10.3389/fpls.2013.00452
- Jordan, C.F. and Herrera, R. (1981) Tropical Rain Forests: Are Nutrients Really Critical? The American Naturalist, 117, 167-180. http://dx.doi.org/10.1086/283696
- Herrera, R. (1977) Soil and Terrain Condition in the San Carlos de Rio Negro proJect (Venezuela MAB-1) Study Site; Correlation with Vegetation Types. In: Brunig, E.F., Ed., Transactions of the Second International MAB-IUFRO Work- shop on Tropical Rainforest Ecosystems Research (Jakarta), World Chair of Forestry, Hamburg-Reinbek, 182-188.
- Breimer, R.F. (1985) Some Observations on Soils in Relation to Forest Types in San Carlos de Rio Negro, Venezuela. In: Breimer, R.F., van Kekem, A.J. and van Reuler, H., Eds., Guidelines for Soil Survey in Ecological Research, UNESCO, Paris, 108-110.
- Cuevas, E. and Medina, E. (1986) Nutrient Dynamics within Amazonian Forest Ecosystems. Oecologia, 68, 466-472. http://dx.doi.org/10.1007/BF01036756
- Sobrado, M.A. (2010) Leaf Characteristics, Wood Anatomy and Hydraulic Properties in Tree Species from Contrasting Habitats within Upper Rio Negro Forests in the Amazon Region. Journal of Tropical Ecology, 26, 215-226. http://dx.doi.org/10.1017/S0266467409990538
- Quesada, C.A., Lloyd, J., Anderson, L.O., Fyllas, N.M., Schwarz, M. and Czimczik, C.I. (2011) Soils of Amazonia with Particular Reference to the RAINFOR Sites. Biogeosciences, 8, 1415-1440. http://dx.doi.org/10.5194/bg-8-1415-2011
- Jordan, C.F. (1982) The Nutrient Balance of an Amazonian Rain Forest. Ecology, 63, 647-654. http://dx.doi.org/10.2307/1936784
- Sobrado, M.A. (2013) Soil and Leaf Micronutrient Composition in Contrasting Habitats in Podzolized Sands of the Amazon Region. American Journal of Plant Sciences, 4, 1918-1923. http://dx.doi.org/10.4236/ajps.2013.410235
- Sobrado, M.A. (2011) Leaf Pigment Composition and Fluorescence Signatures of Top Canopy Leaves in Species of the Upper Rio Negro Forests. Research Journal of Botany, 6, 141-149. http://dx.doi.org/10.3923/rjb.2011.141.149
- Sobrado, M.A. (2009) Cost-Benefit Relationships in Sclerophyllous Leaves of the “Bana” Vegetation in the Amazon Region. Trees-Structure and Function, 23, 429-437. http://link.springer.com/article/10.1007%2Fs00468-008-0292-x#page-1
- Sobrado, M.A. (2008) Leaf Characteristics and Diurnal Variation of Chlorophyll Fluorescence in Leaves of the “Bana” Vegetation of the Amazon Region. Photosynthetica, 46, 202-207. http://dx.doi.org/10.1007/s11099-008-0033-9
- Sobrado, M.A. (2009) Leaf Tissue Water Relations and Hydraulic Properties of Sclerophyllous Vegetation on White Sands of the Upper Rio Negro in the Amazon Region. Journal of Tropical Ecology, 25, 271-280. http://dx.doi.org/10.1017/S026646740900604X
- Sobrado, M.A. (2012) Leaf Tissue Water Relations in Tree Species from Contrasting Habitats within the Upper Rio Negro Forests of the Amazon Region. Journal of Tropical Ecology, 28, 519-522. http://dx.doi.org/10.1017/S0266467412000454
- Stark, N.M. and Jordan, C.F. (1978) Nutrient Retention by the Root Mat of an Amazonian Rain Forest. Ecology, 59, 434-437. http://dx.doi.org/10.2307/1936571
- Sims, T. and Wolfe, A. (1995) Recommended Soil Testing Procedures for the Northeastern United States. Northeast Regional Bulletin No. 493, Agricultural Experiment Station, University of Delaware, Delaware.
- Miller, R.O. (1988) High-Temperature Oxidation: Dry Ashing. In: Kaira, Y.P., Ed., Handbook of Reference Methods for Plant Analysis, CRC, Boca Raton, 53-56.
- Oyinlola, E.Y. and Chude, V.O. (2010) Status of Available Micronutrients of the Basement Complex Rock-Derived Alfisols in Northern Nigeria Savanna. Tropical and Subtropical Agroecosystems, 12, 229-237. http://www.veterinaria.uady.mx/ojs/index.php/TSA/article/view/226/345
- Metali, F., Salim, K.A. and Burslem, D.F.R.P. (2012) Evidence of Foliar Aluminium Accumulation in Local, Regional and Global Datasets of Wild Plants. New Phytologist, 193, 637-649. http://dx.doi.org/10.1111/j.1469-8137.2011.03965.x
- Rodríguez, N., Menéndez, N., Tornero, J., Amils, R. and De La Fuente, V. (2005) Internal Iron Biomineralization in Imperata cylindrica, a Perennial Grass: Chemical Composition, Speciation and Plant Localization. New Phytologist, 165, 781-780. http://dx.doi.org/10.1111/j.1469-8137.2004.01264.x
- Marschner, H. (1995) Mineral Nutrition of Higher Plants. Academic Press, London.
- Lambers, H., Brundrett, M.C., Raven, J.A. and Hopper, S.D. (2010) Plant Mineral Nutrition in Ancient Landscapes: High Plant Species Diversity on Infertile Soils Is Linked to Functional Diversity for Nutritional Strategies. Plant and Soil, 334, 11-31. http://dx.doi.org/10.1007/s11104-010-0444-9
- Pereira Serra, A., Marchetti, M.E., Bungenstab, D.J., da Silva, M.A.G., Serra, R.P., Guimarães, F.C.N., Conrad, V.D.A. and de Morais, H.S. (2013) Diagnosis and Recommendation Integrated System (DRIS) to Assess the Nutritional State of Plants. In: Matovi, D.M., Ed., Biomass Now: Sustainable Growth and Use, Intech Open Science/Open Mind, Published Online, 129-136. http://dx.doi.org/10.5772/54576
- Chapin, F.S. (1980) The Mineral Nutrition of Wild Plants. Annual Review of Ecology and Systematics, 11, 233-260. http://dx.doi.org/10.1146/annurev.es.11.110180.001313
- Alloways, B.J. (2008) Zinc in Soils and Crop Nutrition. International Zinc Association/International Fertilizer Industry Association, Brussels/Paris.
- Blevins, D.L. and Lukaszewski, K.M. (1998) Boron in Plant Structure and Function. Annual Review of Plant Physiology and Molecular Biology, 49, 481-500. http://dx.doi.org/10.1146/annurev.arplant.49.1.481
- Sharma, P.N. and Ramchandra, T. (1990) Water Relations and Photosynthesis in Mustard Plants Subjected to Boron Deficiency. Indian Journal of Plant Physiology, 33, 150-154. http://www.cabdirect.org/abstracts/19910747235.html;jsessionid=EE59A21528ED19A063FBCFFD7FE6A2E0.
- Shorrocks, V. (1997) The Occurrence and Correction of Boron Deficiency. Plant and Soil, 193, 121-148. http://dx.doi.org/10.1023/A:1004216126069
- Cuypers, A., Remans, T., Weyens, N., Colpaert, J.,Vassilev, A. and Vangronsveld, J. (2013) Soil-Plant Relationships of Heavy Metals and Metalloids. In: Alloway, B.J., Ed., Heavy-Metals in Soils: Trace Metals and Metalloids in Soils and Their Bioavailability, Springer Dordrecht Heidelberg, New York, London, 161-194. http://dx.doi.org/10.1007/978-94-007-4470-7_6
- Guerinot, M.L. (2000) The ZIP Family of Metal Transporters. Biochimica et Biophysica Acta (BBA)-Biomembranes, 1465, 190-198. http://dx.doi.org/10.1016/S0005-2736(00)00138-3
- Milner, M.J., Seamon, J., Craft, E. and Kochian, L.V. (2013) Transport Properties of Members of the ZIP Family in Plants and their Role in Zn and Mn Homeostasis. Journal of Experimental Botany, 64, 369-381. http://oxfordindex.oup.com/view/10.1093/jxb/ers315
- Kobayashy, T., Nagasaka, S., Senoura, T., Itai, R.N., Nakanishi, H. and Mishizawa, N.K. (2013) Iron-Binding Haeme- rythrin Ring Ubiquitin Ligases Regulate Plant Iron Responses and Accumulation. Nature Communications, 4, 2792. http://www.nature.com/ncomms/2013/131120/ncomms3792/full/ncomms3792.html
- Zelazny, E., Barberon, M., Curie, C. and Vert, G. (2011) Ubiquitination of Transporters at the Forefront of Plant Nutrition. Plant Signal and Behavior, 6, 1597-1599. http://dx.doi.org/10.4161/psb.6.10.17134
- Sato, T., Maekawa, S., Yasuda, S., Sonoda, Y., Katoh, E., Ichikawa, T., Nakasawa, M., Seki, M., Shinozaki, K., Matusi, M. and Goto, D.B. (2009) CNI1/ATL31, a RING-Type Ubiquitin Ligase that Functions in the Carbon/Nitrogen Response for Growth Phase Transition in Arabidopsis Seedlings. The Plant Journal, 60, 852-864. http://dx.doi.org/10.1111/j.1365-313X.2009.04006.x