American Journal of Plant Sciences
Vol.4 No.2A(2013), Article ID:28453,9 pages DOI:10.4236/ajps.2013.42A050
Generalist versus Specialist Herbivores on the Invasive Senecio inaequidens and a Native Related Species: What Makes the Difference?
![]()
1Earth and Life Institute, Catholic University of Louvain, Louvain-la-Neuve, Belgium; 2Plant Ecology and Biogeochemistry, Université libre de Bruxelles, Brussels, Belgium.
Email: *anne-laure.jacquemart@uclouvain.be
Received December 17th, 2012; revised January 18th, 2013; accepted January 25th, 2013
Keywords: Enemy Release; Biotic Resistance; Invasive Plant; Invasive Success; Pyrrolizidine Alkaloids
ABSTRACT
We compared herbivory pressure in the native Jacobaea vulgaris (formely Senecio jacobaea) and the alien invasive S. inaequidens in sites where they co-occur in Belgium. We predicted that the alien species experiences relaxed herbivory pressure by specialist herbivores (enemy release hypothesis ERH) whereas it is still attacked by generalist herbivores. Impacts of two generalist (gastropods and rabbits) and one specialist (the caterpillar Tyria jacobaeae) herbivores were assessed with field observations and exclusion experiments. The generalist herbivores had a higher impact on the biomass and survival of the seedlings of the alien S. inaequidens than on the native J. vulgaris. On the contrary, the specialist Tyria jacobaeae attacked exclusively the adults of the native species, supporting one of the main predictions of the ERH. These results are discussed in relation to differences in pyrrolizidine alkaloid profiles between the two species.
1. Introduction
Natural enemies play a key role in hypotheses explaining the invasive success of alien plant species when introduced outside their native range [1-5]. First the enemy release hypothesis (ERH) proposes that the proliferation of an introduced plant species is due to the relaxed pressure by coevolved herbivores and pathogens [6,7]. The ERH has been tested in many different ways including comparisons between native and alien congeners (community approach), or between aliens in their native and introduced range (biogeographical approach) [5,8,9]. The ERH has been confirmed in many cases, as the invasive plants were less attacked in their new range than indigenous counterparts or than in their native range [2,9-13]. In contrast, the biotic resistance hypothesis (BRH) predicts that native generalist herbivores will suppress alien plants because these are naive, i.e. have not been selected to deter those herbivores [6,14]. Alien species are in this case considered to be more vulnerable than native species to attacks by native herbivores [1,15-18]. These two hypotheses could be complementary as differences in herbivore damage can exist between generalist and specialist herbivores [19-21]. In the introduced range, generalist and specialist herbivores are expected to differently interact with a new alien plant. Generalists feed on a wide range of plants, possibly including introduced species [14]. In contrast, specialists are not expected to feed on introduced plants, except in the case of host-switching from native to introduced plant. Moreover, introduced species with congeners in the resident flora are more likely to acquire enemies from them, partly due to chemical similarity [16,22].
In the present study, we compared damage of generalist versus specialist herbivores on two closely related Senecionae species (Asteraceae): Jacobaea vulgaris Gaert. (syn. Senecio jacobaea L.) native to NW Europe and the invasive Senecio inaequidens DC. We chose these species because they occasionally co-occur in open grasslands in Europe. S. inaequidens is troublesome in Europe while the native European species is invasive in other parts of the world. They thus represent a adequate system for testing hypotheses about herbivore influence on alien species success.
Senecio inaequidens, the narrow-leaved ragwort, native to South Africa and Lesotho, was accidentally introduced at several locations in Europe at the end of the 19th century as a contaminant of sheep wool [23,24]. The species is now considered as one of the most aggressive alien invasive species in Europe [25,26]. The species is a perennial polycarpic suffrutescent herb, mostly occurring, in its introduced range, as a pioneer of well-drained soil, also occurring occasionally in closed mesic grassland [23, 24].
Jacobaea vulgaris (formerly Senecio jacobaea), the tansy ragwort, is native to Europe. It is a biennial to shortlived perennial monocarpic species which forms a rosette during the first year and a flowering shoot in the second year [27]. It is usually found in later successional stages (closed grasslands on humus-rich soil) compared to S. inaequidens, but both species can occasionally co-exist in open grasslands on dry soils [27-29].
In this paper, we assessed herbivore damage at different life stages for generalist and specialist herbivores. We focused on two types of generalist herbivores, i.e. gastropods and rabbits that are known to feed on seedlings [30, 31]. On the other hand, we assessed damages due to the specialist caterpillar Tyria jacobaeae (Lepidoptera, Arctiidae), the cinnabar moth, which attacks J. vulgaris shoots mainly at the flowering stage. We did not estimate damages from other herbivores as they remained rare with very low damages on our sites (even the specialists Botanophila seneciella, Diptera, seed-head fly or Longitarsus jacobaeae, Coleoptera, flea beetle or Aphis jacobaeae, Hemiptera, aphid).
All Senecionae species contain pyrrolizidine alkaloids (PAs) of the senecionine type as constitutive defences against herbivores [32,33]. Some adapted co-evolved insects can detoxify and even sequester and use PAs for their own defence against predators [33] like the specialist moth Tyria jacobaeae. We thus attempted to relate patterns of herbivory to the PA defences at the different life stages.
We addressed three questions: 1) Is the alien S. inaequidens less attacked by generalists and/or specialists than the native J. vulgaris? Based on the ERH and the BRH, we predict that S. inaequidens should experience lower rates of foliar damage than the native congener by specialist herbivores and equal to higher pressure by generalist herbivores; 2) Are the two species equally affected by herbivores throughout their whole life cycle? We predict that the most critical life stages (seedlings) are those affected by generalist herbivores; 3) Can differences in the pattern of herbivory between the two species be explained by their profiles of pyrrolizidine alkaloids? We predict that the concentrations of PAs are higher in the alien than in the native species.
2. Material and Methods
2.1. Study Sites
Observations and field experiments were conducted in two field sites where both species co-occurred. Such sites were chosen to ensure that the two species are subjected to the same herbivore community. The first field site was a rough mesic mown grassland on a SW-facing slope on a roadside situated in Nossegem (50˚52'18.30''N, 4˚30' 39.44''E), central Belgium. Vegetation was dominated by S. inaequidens, J. vulgaris, Bromus mollis and Daucus carota, with scattered alien shrubs (Buddleja davidii). This site was studied in 2005 and was thereafter destroyed by road works. Therefore, a second site was selected in Antwerp (51˚14'36.40''N, 4˚23'15.03''E), northern Belgium, for field observations and experiments in 2006 and 2007. There, the vegetation was a rough grassland on sandy soil dominated by S. inaequidens, J. vulgaris, Festuca rubra, Plantago lanceolata and Cirsium arvense.
2.2. Generalist Herbivores on Early Stages
We collected seeds in a total of seven populations of either species in Belgium. Seeds were pooled per species and sown in 3 L pots filled with a mixture of sand and compost (1:3, v:v) in a glasshouse. After emergence, the number of seedlings per pot was equalized to 30 and transferred to the field.
In June 2007, 80 pots were distributed in five blocks in the field site at Antwerp, separated from each other by approximately 10 m. In each block, four treatments were applied, i.e. exposed to both gastropods and rabbits, exposed to gastropods only, exposed to rabbits only and exposed to neither gastropods nor rabbits. Rabbit protection consisted of 60 cm high fences (mesh: 2.5 cm), buried to 20 cm deep, forming an exclosure of 70 × 120 cm around the pots. Gastropod protection consisted of copper tapes (Adalia®) glued around the pots, thus preventing gastropods from reaching the seedlings. The fully protected (neither gastropods nor rabbits) pots were protected both by the rabbit fence and the copper tape. The unprotected pots had no copper tape and were placed outside the fence. Surviving seedlings per pot were counted twice a week. After six weeks, surviving seedlings in each pot were cut at soil level, pooled in a paper bag, and dried at 50˚C for 6 days. The mean individual biomass was calculated as the total biomass per pot divided by the final number of surviving seedlings.
2.3. Specialist Herbivores at Flowering Stage
The number of flowering individuals of J. vulgaris and S. inaequidens attacked by Tyria jacobaeae caterpillars, and the number of caterpillars per plant, were counted from July to October, in the sites Nossegem (in 2005, 400 individuals per species) and Antwerp (2006 and 2007). In Antwerp the populations of both species were too large for an exhaustive survey. Plants were thus monitored within five permanent plots of approximately 0.75 m2 for each species. These plots initially contained a total of 50 individuals per species. Damage of T. jacobaeae was estimated (percent leaf damage) and the number of remained flowering heads per plant was counted.
2.4. Pyrrolizidine Alkaloid Analysis
We sampled both species at different life stages. The seedlings were collected twice, i.e. at 2 weeks old (one true leaf) and 2 weeks later (2 - 4 true leaves), from plants used in the generalist herbivore field experiment. For later stages, plant material was randomly sampled at the Antwerp site in early July 2008. Leaves were harvested from non flowering plants (6 plants per species) and 5 flowering heads (all florets open) on flowering plants. All samples were individually dried at 50˚C for 6 days, except for seedlings pooled in 2 bags per species.
Chemical analyses of pyrrolizidine alkaloids (PAs) were performed by Klaas Vrieling at the Institute of Biology, Leiden University, The Netherlands, using previously described method. Composition and concentration in PAs were determined by gas chromatography (HP 6890, 30 m × 0.25 µm HP-1) with split injector (1 - 30), Exterlut columns, PND detector and N2 as carrier gas at 0.9 ml/min. The temperature program was 0-22-5-250˚C and pressure 56 kPa. The total PA concentration was calculated by summing the area of all the peaks in the chromatogram.
2.5. Statistical Analyses
Survival rate and remaining biomass were tested by 4- way ANOVA with all factors fixed. The effects of species, rabbit protection, gastropod protection, block and interactions were tested. Survival rates of seedlings were arcsin transformed and seedling biomass data were logtransformed to achieve normality. PA composition was tested with 2-way ANOVA with species and life stage as main factors. All statistical analyses were performed with SAS Enterprise version 4.1. Data are shown as means ± SD.
3. Results
3.1. Generalist Herbivores
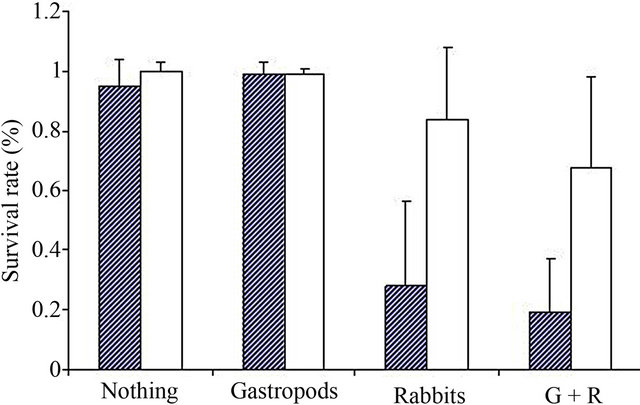
In fully protected pots and in pots exposed to gastropods only, nearly all seedlings of the two species survived. In fully exposed pots and in pots exposed to rabbits, survival rates were significantly lower (Figure 1). Rabbit grazing significantly decreased survival rate, but gastropods did not (Table 1). Rabbit grazing had a higher negative impact on the survival of S. inaequidens than on J.
 (a)
(a)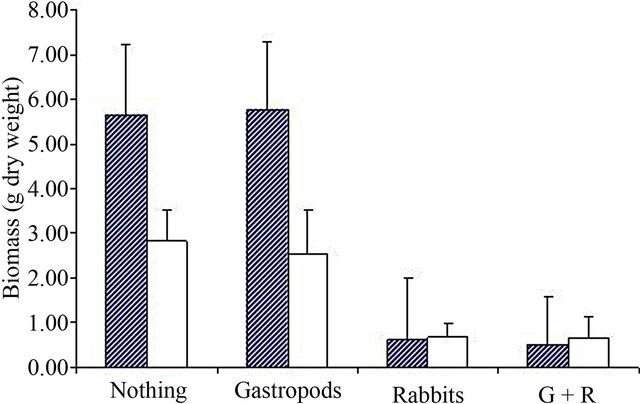 (b)
(b)
Figure 1. Survival rate (a) and biomass (b, in g dry weight) of S. inaequidens (hatched) and J. vulgaris (white) seedlings in the field experiment at Antwerp. G + R = Gastropods + Rabbits.
vulgaris (significant rabbit * species interaction; Table 1).
Gastropod grazing, obviously not very important in our study site, had no effect on the remaining biomass of both species (Figure 1 and Table 1). Rabbit grazing significantly decreased the remaining biomass, and this effect was larger on S. inaequidens than on J. vulgaris, accounting for the significant rabbit * species interaction. Biomass loss due to rabbits reached approximately 90% for S. inaequidens and 75% for J. vulgaris.
3.2. Specialist Herbivores
No caterpillar was observed feeding on S. inaequidens over the three years at both field sites. In contrast, caterpillars were often observed feeding on J. vulgaris but their impact varied highly among years and sites (Table 2). At Nossegem in 2005, caterpillars were found on only 10 individuals of J. vulgaris (i.e. 4% of the population). In Antwerp in 2006, caterpillars were mainly observed in dense patches of J. vulgaris. In several patches, the plants were completely defoliated and their flowering
Table 1. Seedling herbivory by gastropods and rabbits in the field. Results of ANOVA for effects of gastropod protection, rabbit protection, species and block on the survival rate and the remaining biomass of S. inaequidens and J. vulgaris.

Table 2. Monitoring of Tyria jacobaeae caterpillar attacks in two natural populations from 2005 to 2007.

heads have been eaten, with only stems remaining ungrazed. Approximately half of these individuals showed compensatory growth, and produced secondary flowering heads and set fruit in September, when caterpillars had pupated. In Antwerp in 2007, caterpillars were very abundant and attacked nearly all the J. vulgaris plants (Table 2), so that a very small part of the population was able to set fruit in July. Seven out of 50 monitored individuals of J. vulgaris produced on average 6 flowering heads only and set fruit in 2007. Only 10% of the grazed plants produced secondary flowering heads (1 to 4 for per plant). On average (over the three years), impacts of T. jacobaeae prevented flowering in 34% of the individuals. For S. inaequidens, all the monitored individuals produced an average of 122 flowering heads per plant and successfully set fruit.
3.3. Pyrrolizidine Alkaloids
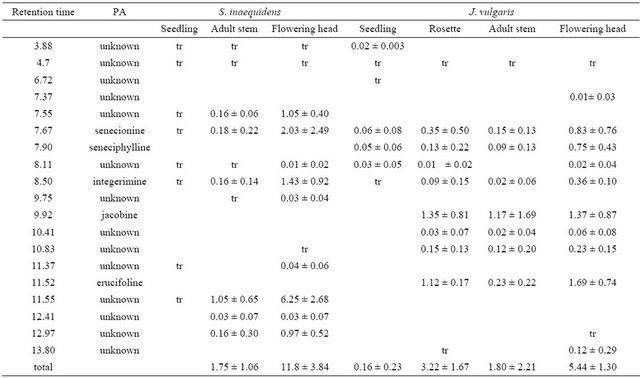
For all stages pooled, a total of 13 and 14 different PAs were detected in S. inaequidens and J. vulgaris, respectively and 7 were shared (Table 3). Only five of these PAs corresponded to known compounds, i.e., senecionine, seneciphylline, integerimine, jacobine and erucifoline. The PA profile of S. inaequidens was dominated (56%) by an unknown compound not detected in J. vulgaris. The profile of J. vulgaris included jacobine (37%), erucifoline (28%) and senecionine (14%).
The PA concentration significantly increased (F = 38.2, p < 0.001) across the successive life stages from seedlings to flowering heads, except for the rosettes of J. vulgaris which contained higher PA than adult flowering plants. The composition also varied with life stages as 7 - 8 different PAs were detected in seedlings and 12 - 13 different alkaloids were present in flowering heads (Table 3). The total concentrations were not significantly different between the two species at the adult stage (F = 2.37, p = 0.11) while they were higher in the flowering heads of S. inaequidens than in those of the native species (F = 9.64, p = 0.011).
4. Discussion
Previous work showed that the alien Senecio inaequidens does not escape herbivory in Europe, with more than 62 species of insects feeding on it in Germany [34, see also 35]. Heteropteran species and rabbits had a significant negative effect on fitness [31]. In Spain snails and slugs had impacts on the alien species too [36]. In our sites, gastropods had almost no effect whereas rabbits negatively impacted survival and biomass of seedlings. Nevertheless, gastropods had a negative impact in an experimental garden experiment with potted plants of both species [37]. Rabbits had a more pronounced negative effect on the alien S. inaequidens than on the native species.
On the contrary, the specialist caterpillar Tyria jacobaeae had no effect on the alien species while it can destroy all the flowering population of the native species, thus having a great negative impact on the reproductive
Table 3. Pyrrolizidine alkaloids (PA) composition and contents (in µg/mg dry weight) for the different life stages of S. inaequidens and J. vulgaris. tr = traces (<0.02 µg/mg).
success. These results are in agreement with some studies [31], but contradictory with others [28] where caterpillars have been sporadically observed feeding on S. inaequidens.
4.1. Influence of Alkaloids
Explanation for differences in herbivore preferences may lie in PA concentrations for flowering life stages. PA defences are synthesized in roots and translocated to shoots; as for most plant defences, reproductive organs usually show the highest concentrations [33]. The high PA concentrations in flowering heads of both species are consistent with previous results showing that 79% of PA in those species is located in inflorescences [38]. We did not detect any difference in total PA concentrations between the two species in adult leaves like in [9]. Nevertheless, differences in concentrations among populations have been reported [9,32,39,40]. Extracts of S. inaequidens are considered to stimulate oviposition of T. jacobaeae females [41]. However, larvae fed with S. inaequidens under controlled conditions did not survive [32]. The absence of some PAs (including jacobine or erucifoline for example) might explain why T. jacobaeae did not consume the alien species [19,41].
For both species, seedlings contained only traces of PA and represented the most palatable stage. Therefore, differences in rabbit damages between the two species cannot be ascribed to PA defences. Differences in seedling morphology might be more important. Seedlings of S. inaequidens possess erect leaves while those of J. vulgaris form a rosette closely pressed onto the soil, thus being less easily grazed by mammalian herbivores including rabbits [42].
4.2. Herbivore Impacts throughout the Life Cycle
Based upon assessments of herbivore damage, we tentatively calculated an overall estimate of the global effect of herbivory on the whole life cycle for both species (Figure 2). The initial number of seedlings for the estimation was fixed to 100. Germination rates are 67% for S. inaequidens and 57% for J. vulgaris [36]. Adult plants of the invasive S. inaequidens typically have several ramified flowering shoots and produce on average 122 flower heads per plant, even in the first year following germination and each flowering head bears on average 59 seeds [29,43]. The survival of J. vulgaris rosettes to the adult stage was estimated as ca. 76% [44]. There are on average 6 flowering heads per plant and 32 seeds per flowering head for J. vulgaris [29,45]. Even if the native J. vulgaris suffers from lower damage at the seedling stage and the survival rate remains high during the first year,

Figure 2. Global estimation of herbivore damage on the life cycle of S. inaequidens and J. vulgaris.
lower flowering head production, high damage due to the specialist caterpillar and lower seed set per flowering head explain the mean seed production that only reaches 3650 seeds after two years. This huge difference in seed production can explain the invasive success of S. inaequidens in Europe.
Several other studies already suggested that invasiveness may be the result of different combinations of particular functional traits such as high reproductive success [46], dispersal ability [47], increased size or competitive ability [12]. Moreover, multiple introductions, environmental preadaptation and high gene flow along invasion routes also contributed to the success of this rapid Senecio invader [48].
5. Conclusion
Senecio inaequidens suffers from herbivory mostly at the seedling stage, due to generalist herbivores, i.e. rabbits in this study. For J. vulgaris, the flowering stage was the most heavily affected, due to the attacks from the specialist moth Tyria jacobaeae. Finally, the global estimation of herbivory pressure throughout the life cycle suggests that populations of S. inaequidens can still grow faster compared to J. vulgaris, and that herbivore pressure cannot control the invasion. This conclusion is in line with the rapid expansion of S. inaequidens in Europe.
6. Acknowledgements
This work was financed by the Belgian Science Policy (framed within INPLANBEL project), the Université catholique de Louvain (FSR project) and the F.N.R.S. (FRFC contract number 2.4605.06). V. Vanparys had FRIA fellowship (Fonds pour la formation à la Recherche en Industrie et en Agriculture). We would like to acknowledge gratefully C. Dechamps, K. Wart, J. Vermander and the technical staff of the Jardin Jean Massart for their help in the construction of the experimental designs, K. Vrieling and his team for PA analysis. Finally, we thank N. Noret, C. Mayer, A. Paulet, P. V. Baret and A. Vervoort for their help in improving the manuscript.
REFERENCES
- R. I. Colautti, A. Riccardi, I. A. Grigorovich and H. J. Macisaac, “Is Invasion Success Explained by the Enemy Release Hypothesis?” Ecology Letters, Vol. 7, No. 8, 2004, pp. 721-733. HUdoi:10.1111/j.1461-0248.2004.00616.xU
- S. B. Hill and P. M. Kotanen, “Evidence That Phylogenetically Novel Non-Indigenous Plants Experience Less Herbivory,” Oecologia, Vol. 161, No. 3, 2009, pp. 581- 590. HUdoi:10.1007/s00442-009-1403-0U
- J. A. Harvey, T. Bukovinszky and W. H. van der Putten, “Interactions between Invasive Plants and Insect Herbivores: A Plea for a Multitrophic Perspective,” Biological Conservation, Vol. 143, No. 10, 2010, pp. 2251-2259. HUdoi:10.1016/j.biocon.2010.03.004U
- Y. J. Chun, M. van Kleunen and W. Dawson, “The Role of Enemy Release, Tolerance and Resistance in Plant Invasions: Linking Damage to Performance,” Ecology Letters, Vol. 13, No. 8, 2010, pp. 937-946. HUdoi:10.1111/j.1461-0248.2010.01498.xU
- A. M. O. Oduor, R. A. Lankau, S. Y. Strauss and J. M. Gomez, “Introduced Brassica nigra Populations Exhibit Greater Growth and Herbivore Resistance but Less Tolerance than Native Populations in the Native Range,” New Phytologist, Vol. 191, No. 2, 2011, pp. 536-544. HUdoi:10.1111/j.1469-8137.2011.03685.xU
- C. S. Elton, “The Ecology of Invasions by Animals and Plants,” Chapman and Hall, London, 1958. HUdoi:10.1177/0309133307087089U
- R. M. Keane and M. J. Crawley, “Exotic Plant Invasions and the Enemy Release Hypothesis,” Trends in Ecology and Evolution, Vol. 17, No. 4, 2002, pp. 164-170. HUdoi:10.1016/S0169-5347(02)02499-0U
- F. X. Sans, H. Garcia-Serrano and I. Afan, “Life-History Traits of Alien and Native Senecio Species in the Mediterranean Region,” Acta Oecologica, Vol. 26, No. 3, 2004, pp. 167-178. HUdoi:10.1016/j.actao.2004.04.001U
- L. Caño, J. Escarré, K. Vrieling and F. X. Sans, “Patability to a Generalist Herbivore, Defence and Growth of Invasive and Native Senecio Species: Testing the Evolution of Increased Competitive Ability Hypothesis,” Oecologia, Vol. 159, No. 1, 2009, pp. 95-106. HUdoi:10.1007/s00442-008-1182-zU
- C. E. Mitchell and A. G. Power, “Release of Invasive Plants from Fungal and Viral Pathogens,” Nature, Vol. 421, No. 6923, 2003, pp. 625-627. HUdoi:10.1038/nature01317U
- J. L. Funk and H. L. Throop, “Enemy Release and Plant Invasion: Patterns of Defensive Traits and Leaf Damage in Hawaii,” Oecologia, Vol. 162, No. 4, 2010, pp. 815- 823. HUdoi:10.1007/s00442-009-1497-4U
- C. V. Hawkes, A. E. Douglas and A. H. Fitter, “Origin, Local Experience, and the Impact of Biotic Interactions on Native and Introduced Senecio Species,” Biological Invasions, Vol. 12, No. 1, 2010, pp. 113-124. HUdoi:10.1007/s10530-009-9435-2U
- D. W. Tallamy, M. Ballard and V. D’Amico, “Can Alien Plants Support Generalist Insect Herbivores?” Biological Invasions, Vol. 12, No. 7, 2010, pp. 2285-2292. HUdoi:10.1007/s10530-009-9639-5U
- W. E. Morrison and M. E. Hay, “Herbivore Preference for Native vs. Exotic Plants: Generalist Herbivores from Multiple Continents Prefer Exotic Plants That Are Evolutionary Naïve,” PLoS One, Vol. 6, No. 3, 2011, Article ID: e17227. doi:10.1371/journal.pone.0017227U
- J. M. Levine, P. B. Adler and S. G. Yelenik, “A MetaAnalysis of Biotic Resistance to Exotic Plant Invasions,” Ecology Letters, Vol. 7, No. 10, 2004, pp. 975-989. HUdoi:10.1111/j.1461-0248.2004.00657.xU
- A. A. Agrawal, P. M. Kotanen, C. E. Mitchell, A. G. Power, W. Godsoe and J. Klironomos, “Enemy Release? An Experiment with Congeneric Plant Pairs and Diverse Above and Belowground Enemies,” Ecology, Vol. 86, No. 11, 2005, pp. 2979-2989. HUdoi:10.1890/05-0219U
- J. D. Parker and M. E. Hay, “Biotic Resistance to Plant Invasions? Native Herbivores Prefer Non-Native Plants,” Ecology Letters, Vol. 8, No. 9, 2005, pp. 959-967. HUdoi:10.1111/j.1461-0248.2005.00799.xU
- H. Liu and P. Stiling, “Testing the Enemy Release Hypothesis: A Review and Meta-Analysis,” Biological Invasions, Vol. 8, No. 7, 2006, pp. 1535-1545. HUdoi:10.1007/s10530-005-5845-yU
- H. Müller-Schärer, U. Schaffner and T. Steinger, “Evolution in Invasive Plants: Implications for Biological Control,” Trends in Ecology and Evolution, Vol. 19, No. 8, 2004, pp. 417-422. HUdoi:10.1016/j.tree.2004.05.010U
- J. Joshi and K. Vrieling, “The Enemy Release and EICA Hypothesis Revisited: Incorporating the Fundamental Difference between Specialist and Generalist Herbivores,” Ecology Letters, Vol. 8, No. 7, 2005, pp. 704-714. HUdoi:10.1111/j.1461-0248.2005.00769.xU
- K. J. F. Verhoeven, A. Biere, J. A. Harvey and W. H. van der Putten, “Plant Invaders and Their Novel Natural Enemies: Who Is Naïve?” Ecology Letters, Vol. 12, No. 2, 2009, pp. 107-117. HUdoi:10.1111/j.1461-0248.2008.01248.xU
- C. E. Mitchell, A. A. Agrawal, J. D. Bever, G. S. Gilbert, R. A. Hufbauer, J. N. Klironomos, J. L. Maron, W. F. Morris, I. M. Parker, A. G. Power, E. W. Seabloom, M. E. Torchin and D. P. Vazquez, “Biotic Interactions and Plant Invasions,” Ecology Letters, Vol. 9, No. 6, 2006, pp. 726- 740. HUdoi:10.1111/j.1461-0248.2006.00908.xU
- L. Lafuma and S. Maurice, “Increase in Mate Availability without Loss of Self-Incompatibility in the Invasive Species Senecio inaequidens (Asteraceae),” Oikos, Vol. 116, No. 2, 2007, pp. 201-208. HUdoi:10.1111/j.0030-1299.2007.15220.xU
- O. Bossdorf, A. Lipowsky and D. Prati, “Selection of Preadapted Populations Allowed Senecio inaequidens to Invade Central Europe,” Diversity and Distributions, Vol. 14, No. 4, 2008, pp. 676-685. HUdoi:10.1111/j.1472-4642.2008.00471.xU
- T. Heger and H. J. Boehmer, “The Invasion of Central Europe by Senecio inaequidens DC—A Complex Biogeographical Problem,” Erdkunde, Vol. 59, No. 1, 2005, pp. 34-49. HUdoi:10.3112/erdkunde.2005.01.03U
- DAISIE (Delivering Alien Invasive Species Inventories for Europe), “Handbook of Alien Species in Europe,” Invading Nature—Springer Series in Invasion Ecology, Vol. 3, Springer, Dordrecht, 2009.
- J. L. Harper and W. A. Wood, “Biological Flora of the British Isles: Senecio jacobaea L.,” Journal of Ecology, Vol. 45, No. 2, 1957, pp. 617-637. HUdoi:10.2307/2256946U
- W. H. O. Ernst, “Invasion, Dispersal and Ecology of the South African Neophyte Senecio inaequidens in the Netherlands: From Wool Alien to Railway and Road Alien,” Acta Botanica Neerlandica, Vol. 47, No. 1, 1998, pp. 131- 151.
- V. Vanparys, P. Meerts and A.-L. Jacquemart, “Plant-Pollinator Interactions: Comparison between an Invasive and a Native Congeneric Species,” Acta Oecologica, Vol. 34, No. 3, 2008, pp. 361-369. HUdoi:10.1016/j.actao.2008.06.008U
- M. E. Hanley, M. Fenner and P. J. Edwards, “Mollusc Grazing and Seedling Survivorship of Four Common Grassland Plant Species: The Role of Gap Size, Species and Season,” Acta Oecologica, Vol. 17, No. 4, 1996, pp. 331- 341.
- C. Scherber, M. J. Crawley and S. Porembski, “The Effects of Herbivory and Competition on the Invasive Alien Plant Senecio inaequidens (Asteraceae),” Diversity and Distributions, Vol. 9, No. 6, 2003, pp. 415-426. HUdoi:10.1046/j.1472-4642.2003.00049.xU
- M. Macel, P. G. L. Klinkhamer, K. Vrieling and E. Van der Mijden, “Diversity of Pyrrolizidine Alkaloids in Senecio Species Does Not Affect the Specialist Herbivore Tyria jacobaeae,” Oecologia, Vol. 133, No. 4, 2002, pp. 541-550. HUdoi:10.1007/s00442-002-1074-6U
- M. Macel, “Attract and Deter: A Dual Role for Pyrrolizidine Alkaloids in Plant-Insect Interactions,” Phytochemistry Reviews, Vol. 10, No. 1, 2011, pp. 75-82. HUdoi:10.1007/s11101-010-9181-1U
- G. Schmitz and D. J. Werner, “The Importance of the Alien Plant Senecio inaequidens DC (Asteraceae) for Phytophagous Insects,” Zeitschrift für Ökologie und Naturschutz, Vol. 9, No. 3, 2000, pp. 153-160.
- T. Engelkes, B. Wouters, T. M. Bezemer, J. A. Harvey and W. H. van der Putten, “Contrasting Patterns of Herbivore and Predator Pressure on Invasive and Native Plants,” Basic and Applied Ecology, Vol. 13, No. 8, 2012, pp. 725-734. HUdoi:10.1016/j.baae.2012.10.005U
- H. Garcia-Serrano, F. X. Sans and J. Escarré, “Interspecific Competition between Alien and Native Congeneric Species,” Acta Oecologica, Vol. 31, No. 1, 2008, pp. 69- 78. HUdoi:10.1016/j.actao.2006.09.005U
- V. Vanparys, “Ecology of an Invasive Plant, Senecio inaequidens: Interactions with Pollinators, Herbivores and Soil Fauna,” Ph.D. Dissertation, Université catholique de Louvain, Louvain-La-Neuve, 2009.
- T. Hartmann, A. Ehmke, U. Eilert, K. von Borstel and C. Theuring, “Sites of Synthesis, Translocation and Accumulation of Pyrrolizidine Alkaloid N-Oxides in Senecio vulgaris L.,” Planta, Vol. 177, No. 1, 1989, pp. 98-107. HUdoi:10.1007/BF00392159U
- L. Witte, A. Ehmke and T. Hartmann, “Interspecific Flow of Pyrrolizidine Alkaloids—From Plants via Aphids to Ladybirds,” Naturwissenschaften, Vol. 77, No. 11, 1990, pp. 540-543. HUdoi:10.1007/BF01139268U
- L. Joosten, P. P. J. Mulder, P. G. L. Klinkhamer and J. A. van Veen, “Soil-Borne Microorganisms and Soil-Type Affect Pyrrolizidine Alkaloids in Jacobaea vulgaris,” Plant and Soil, Vol. 325, No. 1-2, 2009, pp. 133-143. HUdoi:10.1007/s11104-009-9963-7U
- M. Macel and K. Vrieling, “Pyrrolizidine Alkaloids as Oviposition Stimulants for the Cinnabar Moth, Tyria jacobaeae,” Journal of Chemical Ecology, Vol. 29, No. 6, 2003, pp. 1435-1445. HUdoi:10.1023/A:1024269621284U
- I. Noy-Meir, M. Gutman and Y. Kaplan, “Responses of Mediterranean Grassland Plants to Grazing and Protection,” Journal of Ecology, Vol. 77, No. 1, 1989, pp. 290- 310. HUdoi:10.2307/2260930U
- M. C. Lopez-Garcia and J. Maillet, “Biological Characteristics of an Invasive South African Species,” Biological Invasions, Vol. 7, No. 2, 2005, pp. 181-194. HUdoi:10.1007/s10530-004-8978-5U
- D. A. Wardle, “The Ecology of Ragwort (Senecio jacobaea L.)—A Review,” New Zealand Journal of Ecology, Vol. 10, 1987, pp. 67-76.
- V. Vanparys, V. Cawoy, O. Mahaux and A.-L. Jacquemart, “Comparative Study of the Reproductive Ecology of Two Co-Occurring Related Plant Species: The Invasive Senecio inaequidens and the Native Jacobaea vulgaris,” Plant Ecology and Evolution, Vol. 144, No. 1, 2011, pp. 1-9. HUdoi:10.5091/plecevo.2011.434U
- A. Vervoort, V. Cawoy and A.-L. Jacquemart, “Comparative Reproductive Biology in Co-Occurring Invasive and Native Impatiens Species,” International Journal of Plant Sciences, Vol. 172, No. 3, 2011, pp. 366-377.
- M. Pairon, O. Chabrerie, C. Mainer-Casado and A.-L. Jacquemart, “Sexual Regeneration Traits Linked to Black Cherry (Prunus serotina Ehrh.) Invasiveness,” Acta Oecologica, Vol. 30, No. 2, 2006, pp. 238-247. HUdoi:10.1016/j.actao.2006.05.002U
- S. Lachmuth, W. Durka and F. M. Schurr, “The Making of a Rapid Plant Invader: Genetic Diversity and Differentiation in the Native and Invaded Range of Senecio inaequidens,” Molecular Ecology, Vol. 19, No. 18, 2010, pp. 3952-3967. HUdoi:10.1111/j.1365-294X.2010.04797.xU
NOTES
*Corresponding author.

