American Journal of Plant Sciences
Vol.4 No.1(2013), Article ID:27576,6 pages DOI:10.4236/ajps.2013.41003
Water Stress in Beta vulgaris: Osmotic Adjustment Response and Gene Expression Analysis in ssp. vulgaris and maritima
![]()
Consiglio per la Ricerca e la Sperimentazione in Agricoltura, Centro di Ricerca per le Colture Industriali, Bologna, Italy.
Email: giuseppe.mandolino@entecra.it
Received October 20th, 2012; revised November 28th, 2012; accepted December 6th, 2012
Keywords: Beta vulgaris; Beta maritime; Drought; Osmotic Adjustment; Gene Expression
ABSTRACT
Beta vulgaris genus comprises wild and cultivated subspecies. The “maritima” subspecies is formed by wild or weedy accessions, well adapted to low-water potential environments; it was previously shown that B. vulgaris ssp. maritima has mechanisms of osmotic adjustment more effective than the cultivated B. vulgaris ssp. vulgaris. The response to a progressive lowering of soil potential was compared in two Beta accessions, a cultivated and a wild one. Throughout the 4-months experiment under rain shelters, osmotic potential and relative water content were measured and total RNA was extracted to test the expression of six target genes known in sugar beet or in other plants to be modulated by water shortage. The mild occurrence of drought was paralleled by slow increase in transcription for sucrose synthase 1 and choline monoxygenase, in a way that was in some cases accession-dependent, e.g. the gene for choline monoxygenase was found to be up-regulated at the later stages of growth in stressed plants compared to control ones, and showed a higher constitutive transcription in sea beet compared to sugar beet. Transcription factor DREB2A also was slowly induced during the growth season and upon onset of water shortage, and this induction was stronger in sea beet than in sugar beet. In control plants, the transcription of all genes tested except DREB2A were significantly higher in maritima accession compared to vulgaris one.
1. Introduction
Beta is an economically important genus, and it includes, besides the cultivated forms of beets (Beta vulgaris ssp. vulgaris), wild or weedy forms like the subspecies B. vulgaris ssp. maritima, source of agronomically important traits such as cercospora and rhizomania resistance [1]. Abiotic stresses are major causes of crop yield reduction; particularly, low water availability is a increasingly severe environmental stress affecting agricultural production and quality [2]. Water resources are declining not only in dry areas, but also in temperate ones; as water for agronomic use becomes limiting, the development of drought tolerant cultivars gains in importance also in sugar beet genetic improvement [3,4]. Beta genus is known to be relatively tolerant to low soil water potentials, compared to other genera of high economic importance. Different mechanisms can contribute to an improved drought tolerance, including morphological characteristics like deep rooting [5] and metabolic regulatory mechanisms like osmotic adjustment [6,7]. The Beta vulgaris ssp. maritima germplasm includes accessions able to develop mechanisms of escape from water shortage (e.g. osmotic adjustment) more effectively than the cultivated ssp. vulgaris. Many of these accessions are phenotypically similar to sugar beet, and are probably derived from hybridization of “bolters”—i.e. sugar beet plants flowered during their first year—with truly wild sea beets; these hybrids are known as “weedy” beets. The comparison of the response to limited water supply of these accessions with cultivated forms can give useful informations for the future exploitation of wild or weedy germplasm in sugar beet breeding [8].
In experiments with Beta accessions raised in pots up to different growth stages and medium to heavy stress conditions, a close negative relationship has been found between the concentration of compatible solutes and sucrose content of the root. It is therefore concluded that the accumulation of compatible solutes in the storage root of beets under drought might be a physiological constraint limiting sucrose accumulation [9]. However, plant response to water deficit depends on the length and severity of water deficit experienced by the plants, on the species or genotype, and on the age and stage of development. In previous experiments [10], run under controlled conditions, it was shown that B. vulgaris ssp. maritima accession IDBBNR7268 (International Database for Beta number), had a significantly higher (0.95 MPa) osmotic adjustment compared to the cultivated diploid variety Bianca (0.81 MPa) and to other Beta accessions (leaf beet, garden beet, B. patellaris).
The aim of the present work was to compare the response to a progressive water shortage applied gradually throughout the growth season to well developed plants of B vulgaris ssp. vulgaris and B vulgaris ssp. maritima grown outdoor in pots under rain shelters. The aim was the physiological and molecular analysis of representative accessions of both cultivated and wild/weedy germplasm, for the identification of possible variations in the gene expression—either constitutive or induced by low soil potentials—of some genes selected among those known to be influenced by a low water potential; therefore, the responses to a progressive water shortagemeasured as an increase in soil potential in the treated plantsof the two subspecies were compared throughout a 4-month period, by periodically measuring the osmotic potential of leaf tissue and the expression levels of the selected genes.
2. Materials and Methods
2.1. Experimental Setup
Twelve plants for each accession (B. vulgaris ssp. maritima IDBBNR7268 and B. vulgaris ssp. vulgaris cv. Bianca) and treatment (lowand high-soil potential) were germinated in paper pots and transplanted at the second-leaf stage (in March) in 100 liter pots, and arranged in alternate rows under two rain shelters. Water delivery was controlled through a computerized system, on the basis of daily readings of soil potential made on six pots per treatment, in which water tensiometers were installed.
The time-course of average soil potential of the two groups of plants (controls and stressed) is shown in Figure 1.
At the end of May, after a two-month growth period under optimal water availability, a differentiated watering regime was applied to the control and stressed plant groups. Following this regime, the soil potential of the watered plants remained around −10 cbar until the end of the season, while the soil potential of the stressed plants slowly reached an average of −70/−80 cbar at mid-August (Figure 1).
During the growth season, leaf discs were sampled from control and stressed plants of both accessions, for the measurements of water status, gene expression analysis and sugar determination.
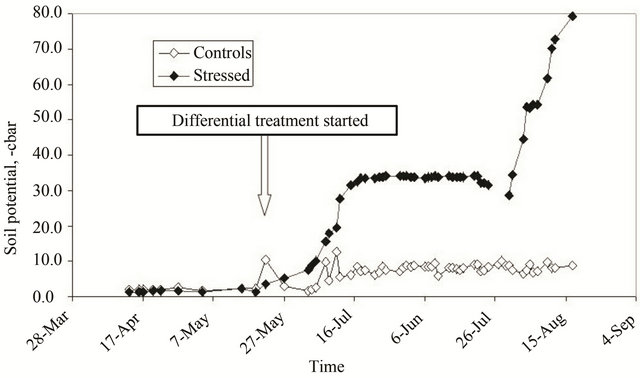
Figure 1. Variation of soil potential during the growth season in control (dark gray dots) and treated (light gray dots) plants.
2.2. Osmotic Potential and RWC Measurements
Leaf osmotic potential (OP) was measured on two 15 mm leaf discs of each plants, frozen in liquid nitrogen and stored at −80˚C until the measurement. Once thawed, the discs were centrifuged for 5 min. at 3000 g; the measurement of OP was carried out on 10 μL aliquots of the sap collected at the bottom of the tube. The osmolarity was measured with a Wescor 5520 VAPRO (Logan, USA) vapour pressure osmometer; the conversion from mosmol/kg to MPa was made as in [10]. Relative water content of leaf samples taken during the growth period were measured as described in [10]. Progressive water limitation was applied to plants of both accessions starting from May 28 (60 d.a.p.), until the leaf OP was different in the stressed (av. −2.0 MPa) and control plants (av. −1.4 MPa), at the end of August.
2.3. RNA Isolation and qPCR
RNA was extracted using the RNeasy plant mini kit (Qiagen); cDNA was synthesized from 5 μg RNA using High-Capacity Reverse Transcription Kits (Applied Biosystems). Real time-PCR was carried out by TaqMan assay (Applied Biosystems) including 5’FAM labelled probes MGB, on a Rotorgene 6000 System (Corbett Life Science). For each gene, specific intron-spanning primers and probes were designed by software File Builder 3.0. The sequences of the genes selected were obtained by screening of the Tentative Consenus (TC) sequences in the Sugar Beet EST Database curated by USDA and in the BvGI (Beta vulgaris Gene Index) database maintained by TIGR. A master mix for each qPCR run was prepared with TaqMan Universal PCR Master Mix (Applied Biosystems). 18S RNA transcription levels were very uniform among the growth periods analysed (May, August and September), among the suspecies (ssp. vulgaris and ssp. maritima) and the treatments (low and high soil potentials, data not shown), with a Ct average of 9.80 ± 0.54 (corresponding to a variation coefficient of 5.5%) among all the samples tested (2 replicates × 3 periods × 2 subspecies × 2 treatments); therefore, 18S RNA amounts were always taken as reference to measure the transcript levels of the target genes The only exception was the quantitation of DREB2A transcripts; due to the low abundance of this target gene, tubulin gene was used instead of 18S RNA as reference [11].
Fifty ng of cDNA were used as template for real time PCR. All the samples were amplified in triplicate, and run twice in separate plates. Negative controls, RT minus (absence of reverse transcription) and NTC (no template), were always added to each plate. The reproducibility was measured by comparing the efficiencies in order to compare different assays; all efficiencies resulted between 86% and 100% with an inter-assay variation always less than 5%.
The target genes selected were the following: sucrose synthase 1 (SBSS1), sucrose phosphate synthase 2 (SPS2), choline monoxygenase (CMO), subunit 1 of plasma membrane aquaporin (PIP1), dehydration responsive element binding protein (DREB2A), and RuBisCo small subunit 1 (ss1RuBisCo). The primer design for real time assays was carried out as described in [11]. The transcript levels of the target genes were normalized to the most suitable reference genes, and compared in target versus control samples by the comparative Ct method. According to [12], the fold change in gene expression was calculated by relative quantitation method of comparative Ct (2−ΔΔCt). The calibrators used for relative quantification were: May for comparison between growth periods; ssp. vulgaris for subspecies comparison; full watering (i.e. low soil water potentials and higher leaf osmotic potentials) for treatments’ comparison.
3. Results and Discussion
The slow and progressive drying of the soil in the treated group of plants of the two accessions, produced an effective adaptation, as no significant biomass loss in both accessions tested was observed (data not shown); this capacity of adaptation of Beta vulgaris had already been observed previously in the capacity of the plants not to loose biomass and sugar yield when a salinity stress was progressively applied in experimental conditions similar to those of the field (large pots under rain shelters, [13]. The ability of Beta species to maintain high RWC, turgor and growth despite the decrease in soil potential had been proposed as deriving from the response of osmotic adjustment rather than stomata closure [7]; indeed, in our experiments the average leaf OP in both subspecies decreased from an initial −1.0 MPa (on May 25) up to a final −2.1 MPa in treated plants, concordingly with the change of soil potential from −10 to −70 cbars, while in the control plants OP remained constant (Figure 2). This slow decrease in the cellular OP value, however, was not due to water loss, as foliar RWC determined on fully expanded leaves, remained unchanged between the two accessions and treatments over a wide range of soil potential values, confirming that mechanisms of osmotic adjustment are actively operating, able to accumulate solutes in order to retain tissutal water and sustain growth [10]. Also the final fresh weight of control and stressed plants of both accessions was not significantly different (data not shown).
Gene expression levels were compared between the growth periods for control plants (May-August-September, section A of Table 1), between the treatments for each accession (control and stressed, section B of Table 1) or between accessions in control plants. (B. vulgaris and B. maritima, section C of Table 1); in these comparisons, the effects of the time after planting, of the treatment and of the genotype on the expression of 6 target genes were estimated.18S RNA was the reference genes, as it showed an extremely low level of variation between genotypes, harvest date of samples, and treatments (data not shown); however, as the level of expression of one of the target genes tested, DREB2A, was
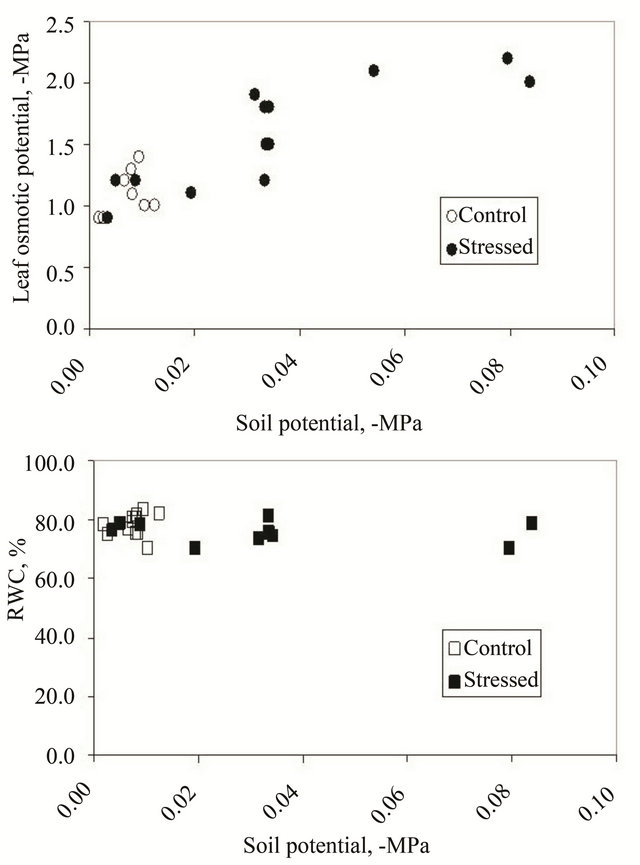
Figure 2. Dependance of cellular osmotic potential (top) and relative water content (bottom) of leaf samples on different soil potentials during the growth season. Each point is the average of four or six data from leaf discs excised from plants of both accessions.
Table 1. Relative gene expression levels for the six target genes in different comparisons. For each comparison, values taken as calibrators are indicated in bold: (a) Comparison between growth stages (control plants); (b) Comparison between treatments (for each accessions); (c) Comparison between accessions (control plants).
(a)

(b)
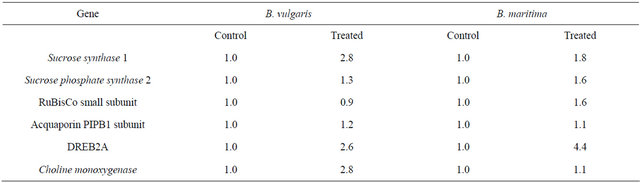
(c)

very low (i.e. average Ct values around 43,0) compared to the high abundance of 18S RNA gene, for the quantification of DREB2A, tubulin, another well-established housekeeping gene, was used as reference.
Sucrose is synthetized in the leaves mainly by sucrose phosphate synthases (SPS), while sucrose synthases (SBSS) are engaged in the cleavage of sucrose; the relative rate of transcription of the isoform 2 of SPS and of the isoform 1 of SBSS were quantified and are shown in Table 1. Transcription rate of the cleaving enzyme SBSS1 increased during the season, peaking in August (Table 1(a)), and was also enhanced by the lowering of soil potential during application of the stress (Table 1(b)) in both accessions. The basal transcription level of SBSS1 is slightly higher in sea beet than in sugar beet during all season (Table 1(c)).
The transcription of the synthetizing enzyme sucrose phosphate synthase 2, showed on the contrary very small changes in all conditions tested, and again, it was constitutively slightly higher in control plants of the wild beet compared to the cultivated one (Tables 1(a)-(c)); however, no modulation associable to variation in leaf osmotic potential was observed.
The rate of transcription in the leaves of these two key enzymes of the sugars metabolism were not immediately related with the levels of sugars substrate or products of their activities (sucrose, glucose, fructose), as detected by HPLC in the leaves (data not shown).
In response to drought stress, expression of a large number of genes is up or down-regulated. Drought stress is known to decrease the amounts of Rubisco small subunit transcripts [14]. However in sugar beet leaves, slow stress deriving from lowering soil potential did not induce changes in Rubisco small subunit transcription, while a slight increase was recorded in sea beet (Table 1(b)). In untreated leaves, the expression of Rubisco small subunit was at higher levels (4 - 5×) in B. maritima than B. vulgaris during growth (Table 1(c)); its transcription showed a decline in untreated plants throughout the growth, in a pattern that was the same for both accessions (Table 1(a)).
According to [7,15], Beta species appear to be anisohydric plants, reacting to a mild water stress by osmotic adjustment rather than by closing stomata. The lack of observed growth limitation and the capacity to increase tissutal osmotic pressure not associated to a significant foliar water loss, shown by our data, seem to confirm this fact. It has been reported that anisohydric behaviour does not require modulation of acquaporins, but rather their constitutive expression [15,16]. PIP1 subunit of plasma membrane acquaporin indeed did not show any regulation following onset of water shortage (Table 1(b)), and was found only slightly and transiently higher in Beta vulgaris ssp. maritima compared to ssp. vulgaris (Table 1(c)).
DREB2A is a transcription factor known in Arabidopsis to be activated during early exposition to drought stress, through interaction with cis-acting dehydrationresponsive elements. Under normal conditions, during the season a slight increase in DREB2A transcription was observed in B. vulgaris leaves (2.3× in September), while in B. maritima this increase was much stronger, up to 15× in September compared to May (Table 1(a)); the response to the lowering soil potential was evident in both accessions, as treated plants transcribed DREB2A almost 3× (B. vulgaris) or 4.4× (B. maritima) compared to controls (Table 1(b)). Finally, the comparison of the two accessions in control plants showed a lower transcription of DREB2A in May in sea beet, but a slightly higher one in September (Table 1(c)).
Choline monoxygenase is an enzyme involved in the synthesis of the compatible solute glycin betaine, and it was demonstrated in sugar beet to be up-regulated by water stress [17]. During the season, in control plants a transient increase in transcription levels in sugar beet was observed, while in sea beet a steady decline from May to September (Table 1(a)) was observed; the drying of soil induced a 2.4x increase in B. vulgaris, while transcription levels remained constant in B. maritima (Table 1(b)); this failure in a stimulation of CMO expression during water-limiting growth conditions in wild beet, however, can be explained by a much higher basal transcription rate in sea beet compared to sugar beet, ranging from over 8× in May to about 3× late in the season (Table 1(c)).
In conclusion, both wild and cultivated accessions were able to stand increasingly dry soil conditions without significant loss of water and growth delay, probably due to osmotic adjustment mechanisms, especially effecttive during slow onset of water shortage. The adaptation showed by the two beet accessions to slow decrease of soil potential was probably not mediated by ABA, as it is known that Beta is a genus not responding to drought by closing stomata [7]; this limited dependence from ABAmediated response could explain both the lack of aquaporins induction and the slow and small increase in DREB2A transcription. However, an enhancement of transcription of enzymes involved in other water-shortage responses (CMO) and in the mobilization of sucrose (SBSS1) was observed, while no changes in the transcription of the sucrose-synthetizing enzyme SPS2 was recorded.
Interesting differences in the gene expression levels between the wild and the cultivated accession were observed either in control and in treated plants, especially for choline monoxygenase gene and for DREB2A transcription factor, suggesting that the differential responses of the two subspecies to a rapidly-occurring water shortage, previously described [10], but also to a slow adaptation, might involve a long-term transcriptional regulation also for these genes.
REFERENCES
- M. J. C. Asher and S. A. Francis, “Exploiting Disease Resistance in Beta Germplasm,” Report of the Second Joint Meeting of the Working Group on Beta and World Beta Network, Bologna, 23-26 October 2002, p. 111.
- J. S. Boyer, “Plant Productivity and Environment,” Science, Vol. 218, No. 4571, 1982, pp. 443-448. doi:10.1126/science.218.4571.443
- E. S. Ober, C. J. A. Clark, M. Le Bloa, A. Royal, K. W. Jaggard and J. D. Pidgeon, “Assessing the Genetic Resources to Improve Drought Tolerance in Sugar Beet: Agronomic Traits of Diverse Genotypes under Droughted and Irrigated Conditions,” Field Crops Research, Vol. 90, No. 2-3, 2004, pp. 213-234. doi:10.1016/j.fcr.2004.03.004
- E. S. Ober, M. Le Bloa, C. J. A.Clark, A. Royal, K. W. Jaggard and J. D. Pidgeon, “Evaluation of Physiological Traits as Indirect Selection Criteria for Drought Tolerance in Sugarbeet,” Field Crops Research, Vol. 91, No. 2-3, 2005, pp. 231-249. doi:10.1016/j.fcr.2004.07.012
- E. Biancardi, G. Mandolino and W. Boschetti, “A Study of the Sugar Beet Root System by Endoscopic Techniques,” Proceedings of the 29th General Meeting of American Society of Sugar Beet Technologists, Phoenix, 2-5 March 1997, pp. 73-81.
- P. V. Biscoe, “The Diffusion Resistance and Water Status of Leaves of Beta vulgaris,” Journal of Experimental Botany, Vol. 23, No. 4, 1972, pp. 930-940. doi:10.1093/jxb/23.4.930
- K. J. McCrea and S. G. Richardson, “Stomatal Closure vs. Osmotic Adjustment: A Comparison of Stress Responses,” Crop Science, Vol. 27, No. 3, 1987, pp. 539-543.
- E. Biancardi, L. W. Panella and R. T. Llewellen, “Beta maritima. The Origin of Beets,” Springer, Berlin, 2012. doi:10.1007/978-1-4614-0842-0
- C. M. Hoffmann, “Sucrose Accumulation in Sugar Beet under Drought Stress,” Journal of Agronomy and Crop Science, Vol. 196, No. 4, 2010, pp. 243-252.
- M. Bagatta, D. Pacifico and G. Mandolino, “Evaluation of the Osmotic Adjustment Response within the Genus beta,” Journal of Sugar Beet Research, Vol. 45, No. 3-4, 2008, pp. 119-133. doi:10.5274/jsbr.45.3.119
- D. Pacifico, C. Onofri and G. Mandolino, “Cold-Modulated Expression of Genes Encoding for Key Enzymes of the Sugar Metabolism in Spring and Autumn cvs. of Beta vulgaris L.,” Plant Genetic Resources: Characterization and Utilization, Vol. 9, No. 2, 2011, pp. 268-271. doi:10.1017/S1479262111000438
- K. J. Livak and T. D. Schmittgen, “Analysis of Relative Gene Expression Data Using Real Time Quantitative PCR and the 2–ΔΔCt Method,” Methods, Vol. 25, No. 4, 2001, pp. 402-408. doi:10.1006/meth.2001.1262
- N. Katerij, J. W. van Hoorn, A. Hamdy, M. Mas-Trorilli and E. Mou Karzel, “Osmotic Adjustment of Sugar Beets in Response to Salinity and Its Influence on Stomatal Conductance, Growth and Yield,” Agricultural Water Management, Vol. 34, No. 1, 1997, pp. 57-69. doi:10.1016/S0378-3774(96)01294-2
- D. C. Dreesmann, C. Harn and J. Daie, “Expression of Genes Encoding Rubisco in Sugarbeet (Beta vulgaris L) Plants Subjected to Gradual Desiccation,” Plant and Cell Physiology, Vol. 35, No. 4, 1994, pp. 645-653.
- N. Sade, A. Gebremedhin and M. Moshelion, “RiskTaking Plants. Anisohydric Behaviour as a Stress-Resistance Trait,” Plant Signaling and Behavior, Vol. 7, No. 7, 2012, pp. 767-770. doi:10.4161/psb.20505
- A. Pou, H. Medrano, J. Flexas and S. D. Tyerman, “A Putative Role for TIP and PIP Acquaporins in Dynamics of Leaf Hydraulic and Stomatal Conductances in Grapevine under Water Stress and Re-Watering,” Plant, Cell & Environment, 2012, in Press. doi:10.1111/pce.12019
- B. L. Russell, B. Rathinasabapathi and A. D. Han-Son, “Osmotic Stress Induces Expression of Choline Monooxygenase in Sugar Beet and Amaranth,” Plant Physiology, Vol. 116, No. 2, 1998, pp. 859-865. doi:10.1104/pp.116.2.859

