American Journal of Plant Sciences
Vol.3 No.4(2012), Article ID:18691,6 pages DOI:10.4236/ajps.2012.34053
Rapid in Vitro Multiplication of an Endangered Medicinal Plant Sarpgandha (Rauwolfia serpentina)
![]()
Department of Agricultural Biotechnology, Orissa University of Agriculture and Technology, Bhubaneswar, India.
Email: *samalkc@rediffmail.com
Received October 11th, 2011; revised December 15th, 2011; accepted December 30th, 2011
Keywords: Rauwolfia serpentine; In Vitro Propagation; Medicinal Plant; Endangered Species
ABSTRACT
An efficient and reproducible procedure for clonal multiplication through in vitro culture of Rauwolfia serpentina (L.) Benth. (Sarpagandha) is standardized. The different explants like leaf and stem were used for callus induction and regeneration of the complete plantlets. High-frequency callusing was induced in leaf and stem explant of Rauwolfia serpentina on modified Murashige and Skoog (MS) medium supplemented with 2.5 mg/l 2,4-D. Maximum regeneration of shoots from callus (90%) was observed in MS medium supplemented with 0.2 mg/l NAA and 1.5 mg/l BA. However direct regeneration (96%) was recorded best in MS medium supplemented with BAP 2.5 mg/l. The maximum number of shoots per explant (6.5) was also highest in this phyto-hormone combination. Higher induction of root (100%) was observed in MS medium supplemented with NAA 0.5 mg/l. The rooted plantlets were successfully established in the field.
1. Introduction
Rauwolfia serpentina L. Benth. (family: Apocynaceae) is a woody perennial shrub, commonly known with different names; sarpagandha, snake root plant, chotachand, chandrika etc. The roots of this shrub have been used for centuries in ayurvedic medicines. This shrub is highly effective in the treatment of high blood pressure. It is also very useful in mental disorders like insanity, mental illness and traumas. It is equally effective in treating insomnia because of its sedative properties. It has found also useful for Hysteria and Urticaria. According to Ayurveda it is the best among all anti-hypertensive drugs. It has been stated that the drug is useful in mental disease, epilepsy, sleeplessness and several other ailments [1]. But due to rapid growth of world population, increasing anthropogenic activities, rapidly eroding natural ecosystem etc, the natural habitat of this important shrub Rauwolfia is dwindling. It is now an endangered species in India due to indiscriminate collection and over exploitation of natural resources for commercial purposes to meet the requirements of pharmaceutical industry, coupled with limited cultivation.
The propagation of R. serpentina through seeds is difficult due to less viability and very low germination percentage. Propagation by direct sowing of seeds in the field has not been found successful. Sun-dried and stored seeds generally gave a low rate of germination and seeds store more than 7 - 8 months practically did not germinate. The germination percentage of seed is very poor and variable (25% - 50%) and is often as low as 10 percent that is due to the presence of cinnamic acidderivatives [2]. To cope up with alarming situation, the recent exciting developments in biotechnology have come as a boon. One of them is the use of plant tissue culture technique. In this method, explants such as nodal segments, auxiliary bud, shoot meristem are sub-cultured in tissue culture medium supplemented with growth regulators cytokinin and or auxin and cytokinin combinations to produce multiple shoots. Shoot number increases logarithmically with each subculture to give greatly enhanced multiplication rates. As this method involves only organized meristem, hence it allows recovery of genetically stable and true to type progenies [3,4]. The objective of the present study is to develop a reproducible and efficient regeneration system for production of uniform, elite, true to the type plantlets for large scale in situ and ex situ plantation to conserve this endangered medicinal plant for commercial exploitation.
2. Materials and Methods
2.1. Plant Materials and Culture Establishment
The nodes and internodes and leaves of Rauwolfia grown in the medicinal plant garden of Orissa University of Agriculture and Technology, Bhubaneswar, India were collected. They were washed with running tap water for 30 min, soaked in 70% (v/v) ethanol for 1 min and then immersed in solution containing 1% (v/v) sodium hypochlorite and 5 - 10 drops/l of Tween-20 for 20 min. After surface sterilization, they were rinsed six times with sterile distilled water. Under laminar flow cabinet, explants were excised and cultured in the Murashige and Skoog (MS) [5] basal medium containing 3.0% sucrose (w/v), 0.7% agar and different concentration and combination of plant growth regulators like 2,4-dichlophenolxyacetic acid (2,4-D), 6-benzylaminopurine (BAP), N6- benzyladenine (BA), indole-3-butyric acid (IBA), indole- 3-acetic acid (IAA) and naphthalene acetic acid (NAA). The pH of medium was adjusted to 5.8 before autoclaving at 121˚C for 20 min. The stock culture was sub-cultured at 20 - 30 days intervals. In this culturing stage, we evaluated plant growth regulators (PGRs) types and concentrations, basal medium composition for culture establishment, callusing, shoot proliferation and root regeneration.
2.2. Culture Conditions
Stock culture and leaf explants were initially incubated in darkness in a culture chamber at 25˚C ± 2˚C. Subsequently, explants were incubated under a 16/8-h (light/ dark) photoperiod with light supplied by cool-white fluorescent lighting at an intensity of 60 μE∙m–2∙s–1 intensity at a constant temperature of 25˚C ± 2˚C.
2.3. Rooting and Acclimatization
Regenerated shoots were excised and transferred to vessels containing 30 ml MS basal medium for root growth. MS basal medium with 3 different concentrations of NAA (0.2, 0.5 and 2.0 mg/l) and IAA (0.2, 0.5 and 2.0 mg/l) alone or in combination supplemented with 3% sucrose and 0.7% agar were tested for initiation, regeneration and growth of roots from cultured shoots. For acclimatization in vitro plantlets, roots of the regenerated plantlets were washed well with sterile distilled water to remove the adhered agar and dipped in 0.2% bavistin for 5 - 10 min. Plantlets were transferred to plastic cups containing sterile soil, sand, vermicompost (1:1:1). The pots were covered with plastic film for 2 weeks with frequent sprinkling of water to have higher humidity condition. Then the plantlets were moved to a greenhouse with watering at 2 - 3 days intervals. After 2 months, plantlets were ready for field transplantation
2.4. Alkaloid Extraction
The alkaloid content was estimated by adopting the standard procedure [6]. One gram of the freeze dried material of callus, shoot or root was grinded with 10% ammonia (NH3) solution for about one hour. The powder was mixed with methanol and left over night. The content was filtered and the residue was again extracted 2 times with methanol. The methanol phase was evaporated under vacuum. The alkaloids were separated through thin layer chromatography following the method described by [7]. The alkaloids are eluted from the plate and then estimated spectrometrically.
2.5. Data Analysis
Each in vitro experiment was replicated thrice with 20 explants per treatment. The frequency of shoot organogenesis and mean number of shoots per explant were determined 50 days after inoculating explants on different media. All data were analyzed by SAS v. 8.0. Analysis of variance (ANOVA) was used to test the statistical significance [8]. The difference between significant treatments means were tested against critical difference (C.D.) at 5% (p ≤ 0.05).
3. Results and Discussion
3.1. Callogenesis
Aseptic stem nodal and leaf explants of Rauwolfia serpentina were cultured aseptically under in vitro condition. MS medium containing 3.0% sucrose (w/v) and different concentration and combination of plant growth regulators like 2,4-D, BAP, IBA, IAA were tested for callus initiation (Table 1). After six weeks of culture, a shinny cream coloured callus got induced on the explant. The rate of callus formation was slow in the first two weeks. However, the callus exhibited good growth and covered the entire explant within four weeks. Shoot primordia also got developed on the callus, which revealed its organogenic nature. To sustain the continuous growth of this callus, further subcultures were made onto MS containing at the same level of phyto-hormones. After six weeks of culture, an excellent loose textured friable callus got developed on the pre-existing callus. The major portion of the callus was creamy white, while some portion of the callus showed signs of browning, which may be due to longer culture period No shoot formation occurred in this combination of the growth regulators used. Out of different combination of phyto-hormone tested, 2,4-D (2.5 mg/l) was found to be best for callus induction percentage and days to callus induction (Figure 1(A)). This combination resulted 89% of callus induction in leaf and 70% callus induction in stem in 25 days. Excellent organogenic callus along with meristemoid-like structures was achieved under the influence of 2.5 mg/l 2,4-D. Similar to our results, Ilahi & Akram [9] obtained good
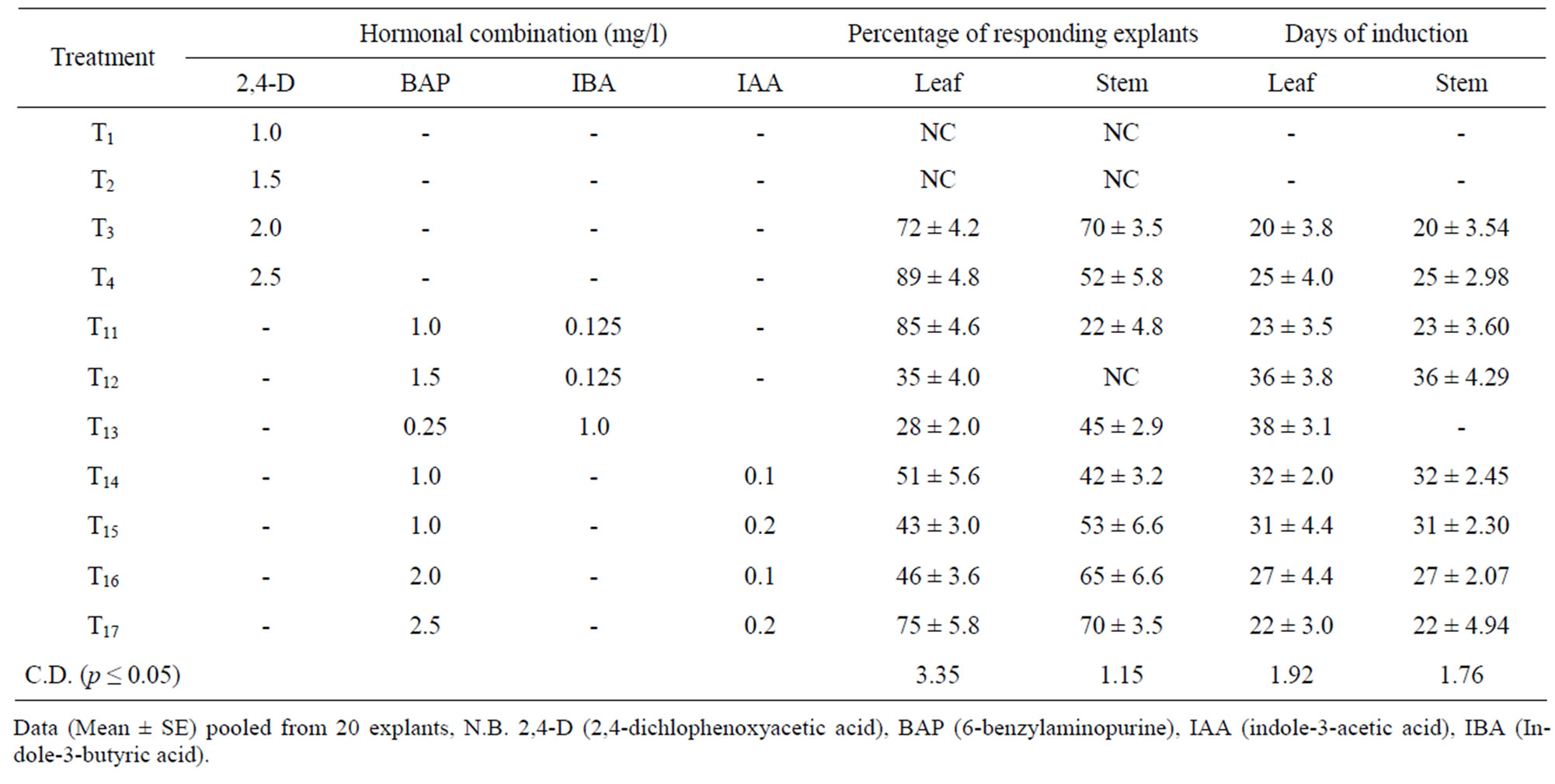
Table 1. Callus induction on stem nodal and leaf explant of Rauwolfia serpentina under the influence of different concentrations of phytohormones.
callus growth on Rauwolfia serpentina explants using a combination of NAA (1.0 mg/l), kinetin (0.5 mg/l) in addition to 10% coconut water.
3.2. Shoot Regeneration on Callus
Regeneration of adventitious shoots was obtained on MS medium supplemented through different sets of hormonal combinations as detailed in Table 2. High level of shoot regeneration was observed when MS medium supplemented with 0.2 mg/l of NAA and 1.5 mg/l of BA. Higher level of BAP (4 mg/l) in combination with 0.5 mg/l NAA also resulted better percentage of shoot formation (85%) and number of shoots per explant (7.5) with mean shoot length (4 cm) followed by the same medium supplemented with BAP (3 mg/l) and NAA (0.5 mg/l) (Figures 1(B) and 1(C)). The better shoot length (4.5 cm) and higher number of shoots per explant (3.5) were also recorded in this combination. Thus it reveals that for shoot regeneration, a low concentration of auxin and high concentration of cytokinin is necessary. The present result is in congruent with the findings reported by [10-12]. They reported that the medium containing BAP alone (5 - 25 mg/l) or in combination with NAA (0.05 - 1.0 mg/l) favour better shoot formation from callus.
3.3. Direct Shoot Regeneration
The in vitro multiplication of Rauwolfia serpentina shoots through the sprouting of axillary buds is the most commercially viable means of micro-propagation. It is also useful for increasing number of shoots, which originally differentiated in vitro. Best growth of axillary shoots was obtained on MS supplemented with 2.5 mg/l BAP followed by MS supplemented with 2 mg/l BAP (Table 3, Figure 1(D)). Four to six vigorously growing shoots were developed on the explant, which was due to the multiplication of the original bud. Similarly, multiple shoots on nodal segments from in vitro shoots of Rauwolfia serpentina, were induced when cultured on MS medium containing 1.0 mg/l BAP and 0.1 mg/l NAA. Thus the propagation of plants from axillary buds has proved to be the most generally applicable and reliable method of in vitro propagation in Rauwolfia serpentina, as the regeneration of the plant is very difficult from seeds and other sources. The seeds are mostly non-viable due to abortive embryos [2,13].
3.4. Root Regeneration and Aclimatization
Rooting ability of micro shoots obtained in the present studies was satisfactory. Highest rooting frequency was obtained in presence of NAA. Out of different hormone combination tested, NAA (0.5 mg/l) was found to be best for root induction percentage (100%), total number. of root per explant (3.5) (Table 4, Figure 1(E)). MS medium supplemented with IBA (0.5 mg/l) also induced better rooting (85%) with total number. of roots per explant (2.8). The present result is in accordance with the result reported by Rahman et al. [14]. They reported that the adventitious shoots were best rooted on half strength MS medium supplemented with 1.0 mg/l each of IBA and IAA. NAA and IBA has been reported to have a
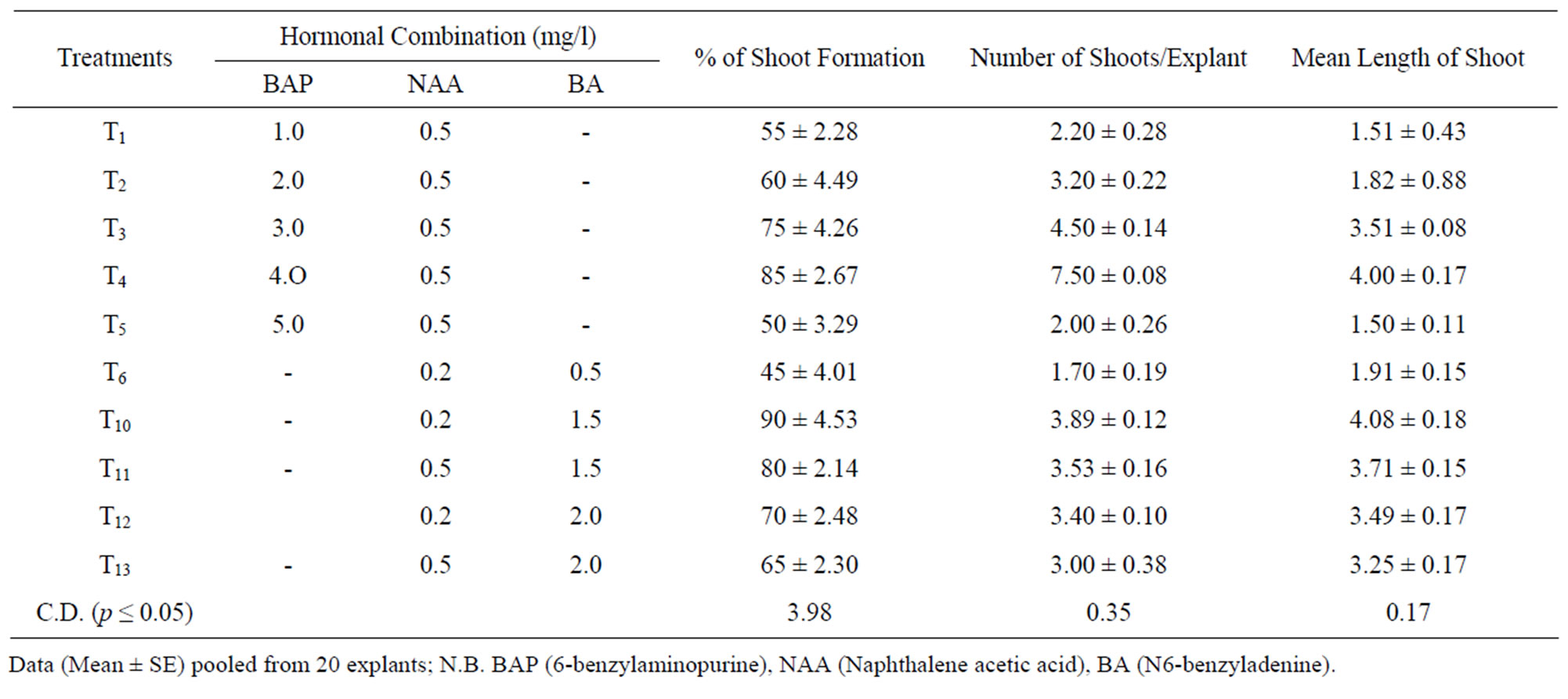
Table 2. Shoot regeneration from callus under the influence of different combination of phyto-hormones supplied through MS medium.
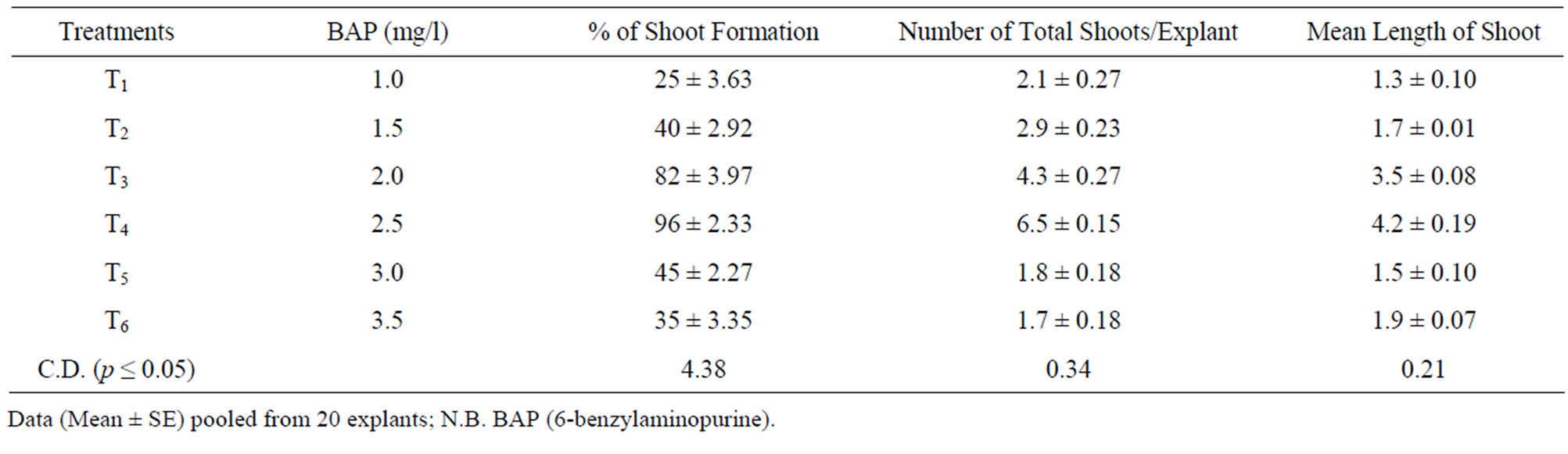
Table 3. Direct shoot proliferation from stem nodal explant under the influence of different concentrations of phytohormones.
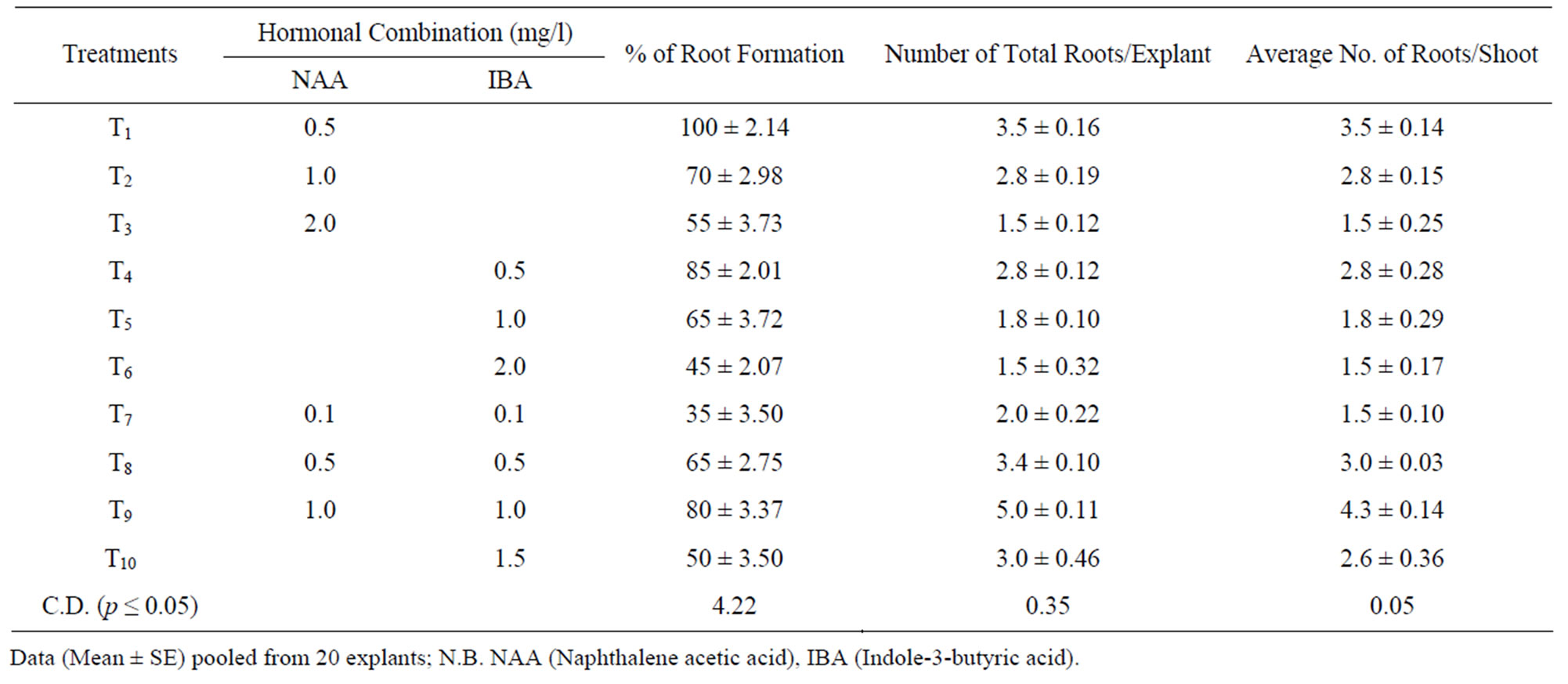
Table 4. Rooting of the in vitro plantlets under the influence of different combination of phyto-hormones supplied through MS medium.
Data (Mean ± SE) pooled from 20 explants; N.B. NAA (Naphthalene acetic acid), IBA (Indole-3-butyric acid).
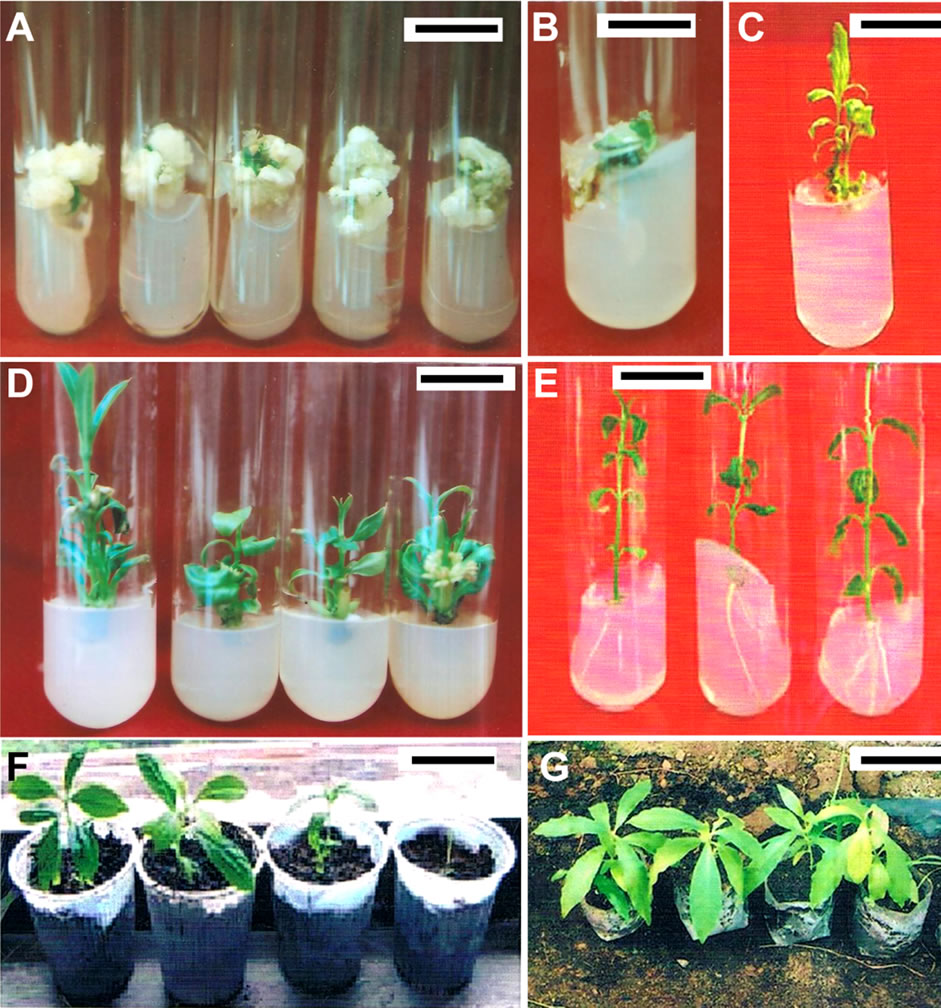
Figure 1. In vitro regeneration of complete plantlets of R. serpentina (A) = callus initiation from leaf explant on MS media supplemented with 2.5 mg/l 2.4-D mg/l IAA (bar = 25 mm); (B) = Organogenic callus and initiation of shoot formation on MS media supplemented with 4 mg/l of BAP NAA and 0.5 mg/l of NAA (bar = 18 mm); (C) = Shoot regeneration from callus on MS media supplemented with 0.2 mg/l of NAA and 1.5 mg/l of BA (bar = 25 mm); (D) = Formation of multiple shoots on nodal explants when cultured on MS medium supplemented with 2.5 mg/l BAP (bar = 20 mm); (E) = root induction on MS media supplemented NAA (0.5 mg/l) NAA (bar = 25 mm); (F) = hardening of plantlet in mixture of sterile soil, sand and vermicompost (bar = 25 cm); (G) = completely acclimatized in vitro plantlets (bar = 40 cm)
stimulatory effect on root induction in many tree species [15]. Finally, the developed plantlets were kept in polyhouse and greenhouse for hardening, and then transferred to field. About 85% of the plants transferred to field survived and did not show any detectable variation in morphology or growth characteristics when compared to the mother plants (Figures 1(F) and 1(G)).
The use of clonal propagation of Rauwolfia serpentine through in vitro culture offers many advantages. This method produces a large number of disease free, uniform, elite, true to the type plantlets throughout the year which will simplify large scale cultivation of this endangered important shrub for commercial extraction of alkaloid for therapeutic use. This micropropagation protocol will also be useful for conservation and further improvement of this important shrub.
3.5. Alkoloids
As evident from the Table 5, the alkaloid such as reser-
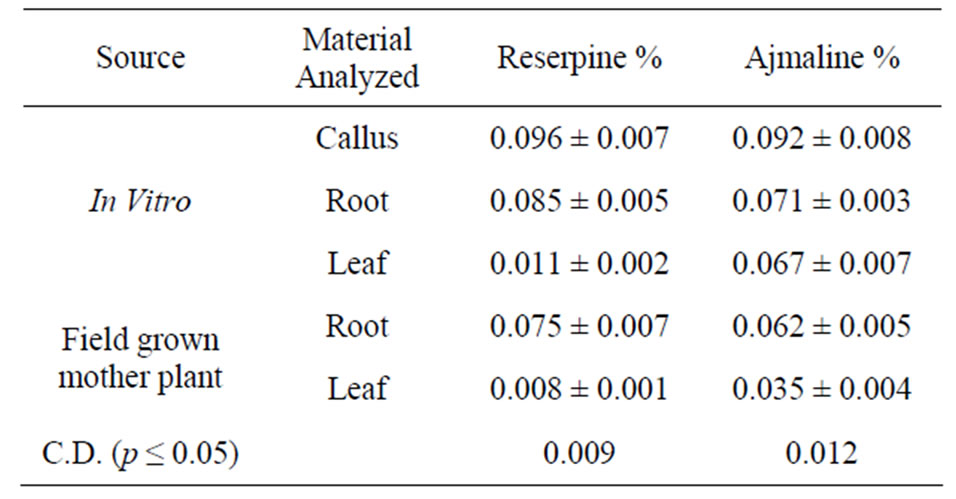
Table 5. Percentage of indole alkaloids in root, callus and shoot cultures of Rauwolfia serpentine.
pine and ajmaline were detected in higher percentage compared to the field grown mother plant. However, percentage of these alkaloids varied when callus, regenerated shoots or roots were analyzed. High level of alkaloid such as reserpine (0.096%) and ajmaline (0.092%) were recorded in callus followed by the roots of in vitro plantlets. Bohm [16] reported that in vitro cultures are capable of synthesizing alkaloids and contain a spectrum of these substances which is identical to that found in the whole plant.
4. Acknowledgements
Authors are grateful to the Department of Biotechnology, Ministry of Science and Technology, Government of India, New Delhi, India for financial support.
REFERENCES
- J. Ojha and U. Mishra, “Dhanvantari Nighantuh, with Hindi Translation and Commentary,” IST Ed., Department of Dravyaguna, Institute of Medical Sciences, BHU, Varanasi, 1985, p. 204.
- G. C. Mitra, “Studies on the Formation of Viable and Non-Viable Seeds in Rauwolfia serpentina Benth,” Indian Journal of Experimental Biology, Vol. 14, No. 1, 1976, pp. 54-56.
- T. Murashige, “Plant Propagation through Tissue Cultures,” Annual Review of Plant Physiology, Vol. 25, No. 1, 1974, pp. 135-166. doi:10.1146/annurev.pp.25.060174.001031
- C. Y. Hu and P. J. Wang, “Meristem Shoot Tip and Bud Cultures,” In: D. A. Evans, W. R. Sharp, P. V. Ammirato and Y. Yamada, Eds., Handbook of Plant Cell Culture, Vol. 1, Macmillan, New York, 1983, pp. 177-227.
- T. Murashige and F. Skoog, “A Revised Medium for Rapid Growth and Bioassays with Tobacco Tissue Cultures,” Physiologia Plant, Vol. 15, No. 3, 1962, pp. 473- 497. doi:10.1111/j.1399-3054.1962.tb08052.x
- H. Wangwagner, S. Bladt and E. M. Zginski, “Plant Drugs Analysis. A Thin Layer Chromatography Atlas,” Springer-Verlag, Heidelbug, 1984.
- AOAC, “Official Methods of Analysis. The Association of Official Analytical Chemists,” 15th Edition, Arlington, West Virginia, Washington DC, 1990, 1400 Pages.
- V. G. Panse and P. V. Sukhatme, “Statistical Methods for Agricultural Workers,” Indian Council of Agricultural Research, New Delhi, 1967, 381 Pages.
- I. Ilahi and M. Akram, “Enhanced Propagation, Root Production and Alkaloid Biosynthesis by Cultures of Rauwolfia serpentine,” In: N. T. Quynh and N. Van Hyen, Eds., Adapted Propagation Techniques for Commercial Crops of the Tropics, Agriculture Publishing House, Hochi Minh City, 1993.
- Q. Z. Wang, J. B. Zhao and B. H. Zhao, “Fast Propagation of Superior Clones of Robinia pseudoacacia through Tissue Culture,” Forest Science Technology, (Linye Keji Tongxun), Vol. 8, 1985, pp. 8-9.
- K. H. Han and D. E. Keathley, “Regeneration of Whole Plants from Seedling-Derived Callus of Black Locust Tree (Robinia pseudoacacia L.),” Nitrogen Fixing Tree Research Report, Vol. 7, 1989, pp. 112-114.
- K. H. Han, J. M. Davis and D. E. Keathley, “Differential Responses Persist in Shoot Explants Which Have Been Regenerated from Callus of Two Mature Black Locust (Robinia pseudoacacia L.),” Tree Physiology, Vol. 6, No. 2, 1990, pp. 235-240.
- M. Akram and I. Ilahi, “Plantlets Formation in Root Callus of Rauwolfia serpentine,” Pakistan Journal of Botany, Vol. 18, 1986, pp. 15-19.
- M. S. M. Rahman, S. Islam, N. Haque, T. A. Jubair, A. K. M. F. Haque and I. J. Mukti, “The Influence of Different Hormone Concentration and Combination on Callus Induction and Regeneration of Rauwolfia serpentina L.,” Benth Pakistan Journal of Biological Sciences, Vol. 11, No. 12, 2008, pp. 1638-1641. doi:10.3923/pjbs.2008.1638.1641
- P. K. Channd, Y. Sahoo, S. K. Pattnaik and S. N. Patnaik, “In Vitro Meristem Culture—An Efficient ex Situ Conservation Strategy for Elite Mulberry Germplasm,” In: R. C. Mohanty, Ed., Environment Change and Management, Kamla Raj Enterprises, New Delhi, 1995, pp. 127-133
- H. Bohm, “The Formation of Secondary Metabolites in Plant Tissue and Cell Cultures,” International Review of Cytology, Suppl. 11B, 1980, pp. 183-208.
Abbreviations
2,4-D: 2,4-dichlophenoxyacetic acid
BA: 6-benzylaminopurine (N6-benzyladenine)
BAP: 6-benzylaminopurine
IAA: indole-3-acetic acid
IBA: Indole-3-butyric acid
mg/l: milligram/litre
min: minute
MS: Murashige and Skoog medium
NAA: Naphthalene acetic acid
h: hour
NOTES
*Corresponding author.

