Modern Research in Catalysis
Vol.2 No.2(2013), Article ID:30186,4 pages DOI:10.4236/mrc.2013.22005
Kinetics of the Oxidation of Benzhydrols with Permanganate under Phase Transfer Catalysis in Organic Solvents
1Department of Chemistry, Farook College, Feroke, India
2Department of Chemistry, St. Joseph’s College, Devagiri, India
3School of Chemical Sciences, Payyanur Campus, Kannur University, Payyanur, India
Email: *tdrnair@rediffmail.com
Copyright © 2013 K. Muhammad Basheer et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received December 21, 2012; revised January 28, 2013; accepted February 6, 2013
Keywords: Phase Transfer Catalysis; Kinetics; Oxidation; Benzhydrols; Permanganate
ABSTRACT
Kinetic studies of the oxidation of benzhydrol and some of its para-substituted derivatives using phase transferred permanganate in organic solvents that are immiscible with water have been carried out. The products of oxidation were the corresponding benzophenones. Kinetically first order dependence on the concentrations of substrate as well as the oxidant has been observed. The reaction rate was found to decrease with an increase in the dielectric constant of the organic solvent employed. The electronic effects of the substituents were found to be interesting. Both electron withdrawing and electron releasing groups at the para position of benzhydrol increased rate of the oxidation. The effects of different phase transfer catalysts (PTC) were also compared. The activation parameters have been evaluated and a mechanism consistent with the kinetic results has been proposed.
1. Introduction
Permanganate is an inexpensive oxidant being widely used in organic synthesis. It is not soluble in organic solvents and hence, oxidations have traditionally been conducted in the aqueous medium. The discovery that materials known as phase transfer catalysts such as crown ethers or quaternary ammonium salts can transport permanganate ions into organic solvents across the aqueousorganic solvent boundary helped to extend its use in organic syntheses to a great extent when the organic substrates are soluble in organic solvents only [1,2]. Kinetics of the permanganate oxidation of some other organic compounds, have been investigated under phase transfer catalysis and the results reported elsewhere [3-5]. We have reported the kinetics of the monochromate oxidation of benzhydrols in non-polar media [6]. In the present kinetic study, we report the results of the kinetic study of the PTC catalysed oxidation of benzhydrols with permanganate along with its p-chloro, p-methyl and p-methoxy derivatives in benzene, chlorobenzene, chloroform and methylene chloride as the organic solvents used.
2. Experimental
Benzhydrol (BH), para-chlorobenzhydrol p-(ClBH), paramthylbenzhydrol (p-MeBH) and para-methoxy-benzhydrol (p-MeOBH) used were Lancaster make. The organic solvents were purified by methods known and refluxed with PTC and KMnO4 for three hours and then distilled before use. Analar grade potassium permanganate (Fluka) was used and its solution was prepared in doubly distilled water. Tetrabutylammonium bromide (TBAB) and tetrabutylammoniumhydrogen sulphate (TBAHS) were SRL make and tetrabutylphosphonium bromide (TBPB) was Merck quality and were used as such without further purification.
Solutions of permanganate ions in organic solvents were prepared by shaking an aqueous solution of KMnO4 (0.005 M) with an equal volume of PTC solution (0.01 M) in the organic solvent. Transports of the permanganate ions were almost quantitative. The organic layer was separated and dried over anhydrous Na2SO4 and the concentration of the ions ascertained.
The stoichiometry of the reaction was determined by treating a known amount of benzhydrol with a known excess of phase transferred permanganate and by estimating the permanganate remaining after completion of the reaction. Formation of benzophenone as the only product was qualitatively and quantitatively ascertained by its conversion into the 2, 4-DNP derivative.
Kinetic measurements were carried out under pseudofirst order conditions where, [substrate]  [oxidant] at all the four different temperatures employed. Progress of the reaction was monitored through spectral measurements at definite intervals of time by measuring the absorbance (OD) at 528 nm using the Systronics double beam uv-vis spectrophotometer (model 2201). The pseudo-first order rate constants, kobs, were calculated from the slope of the linear plots of log Absorbance versus time. The second order rate constant, k2 was obtained from the relation.
[oxidant] at all the four different temperatures employed. Progress of the reaction was monitored through spectral measurements at definite intervals of time by measuring the absorbance (OD) at 528 nm using the Systronics double beam uv-vis spectrophotometer (model 2201). The pseudo-first order rate constants, kobs, were calculated from the slope of the linear plots of log Absorbance versus time. The second order rate constant, k2 was obtained from the relation.
k2 = kobs/[substrate]. Successive scan of the absorption spectra taken at definite time intervals yielded isosbestic points at 498 nm and 582 nm. Experiments were carried out at various concentrations of oxidant and substrate, at different temperatures and in various organic solvents. Different PTCs were also used.
3. Results and Discussion
The stoichiometry of the oxidation was found to be in the molar ratio benzhydrol: permanganate as 3:2. The reaction may be represented by the equation:
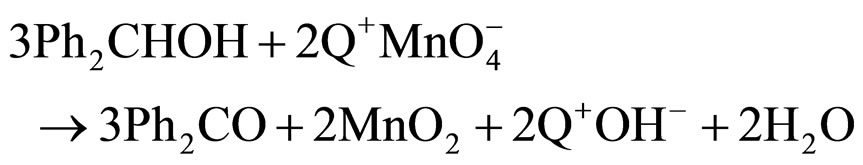
The successive scans of the absorption spectra which showed isosbestic points at 498 nm and 582 nm, suggests formation of a single product. This oxidation reaction failed to induce any polymerization of added acrylonitrile, which rules out involvement of any radical intermediate during the course of the reaction (Q+ represents the quarternary ammonium cation).
The values of rate constants determined at different substrate concentrations and oxidant concentrations are given in Table 1. The plots of log absorbance due to the [oxidant] versus time were linear, indicating first order dependence on the [oxidant]. This is further supported by the fact that kobs values are constant for different initial concentrations of  when the substrate concentration was fixed. The second order rate constant, k2 values are reasonably constant indicating first order dependence on the [substrate] also. This is further supported by the linearity of the log kobs versus log [substrate] plots with a slope of almost unity (slope = 0.9113) and good correlation coefficient (r = 0.9960).
when the substrate concentration was fixed. The second order rate constant, k2 values are reasonably constant indicating first order dependence on the [substrate] also. This is further supported by the linearity of the log kobs versus log [substrate] plots with a slope of almost unity (slope = 0.9113) and good correlation coefficient (r = 0.9960).
The rates of oxidation of benzhydrol in different organic solvents were found to decrease with increase in the dielectric constant (D) of the medium (Table 2). The plot of log k2 vs 1/D is linear. The decrease in rate of oxidation in solvents with high dielectric constant can be attributed to larger stabilization by solvation extended to the organic part of the quaternary catalyst-oxidant moiety. Moreover, solvents with low dielectric constant exert larger relative desolvation effect on the inorganic 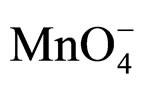 ion part and make it more reactive so that the reaction proceeds faster in solvents with lower D-values.
ion part and make it more reactive so that the reaction proceeds faster in solvents with lower D-values.
The experimental activation energy for the oxidation of benzhydrol in chlorobenzene using TBAB as PTC has been found to be 44.04 kJ∙mol−1, that with TBPB is 38.26 kJ∙mol−1 and that with TBAHS is 32.5 kJ∙mol−1. The other thermo dynamical parameters such as enthalpy, entropy and free energy of activation values for these different catalysts are given (Table 3). The entropies of activation values being largely negative suggests formation of an ordered transition state, possibly with involvement of internal bonding, both inter nuclear and intra-nuclear. The free energies of activation values remain practically constant at 86 kJ∙mol−1 which is indicative of the operation of similar mechanistic modes for the oxidations.
The same trend is kept up with the oxidation of the para substituted derivatives also (Table 4).
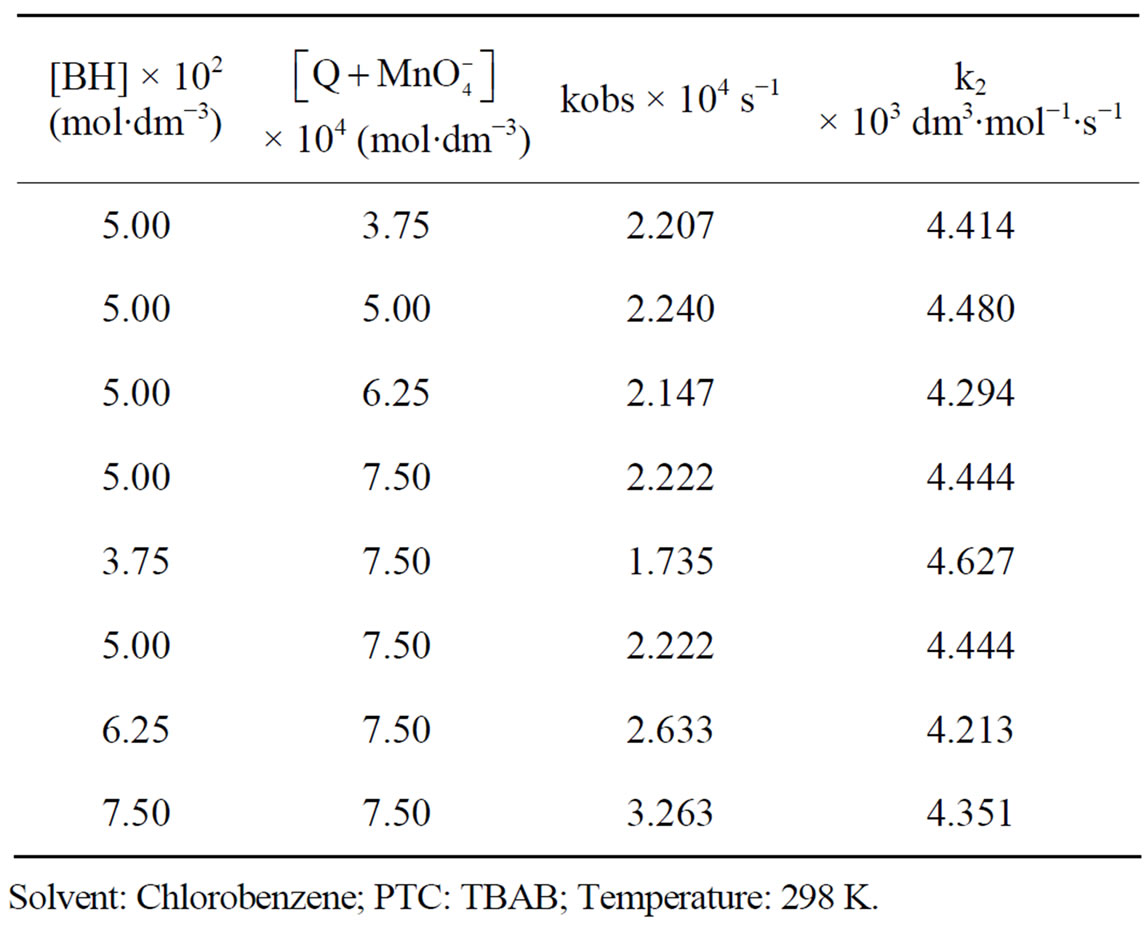
Table 1. Effect of change in [oxidant] and [substrate] on the rate of oxidation of benzhydrol.
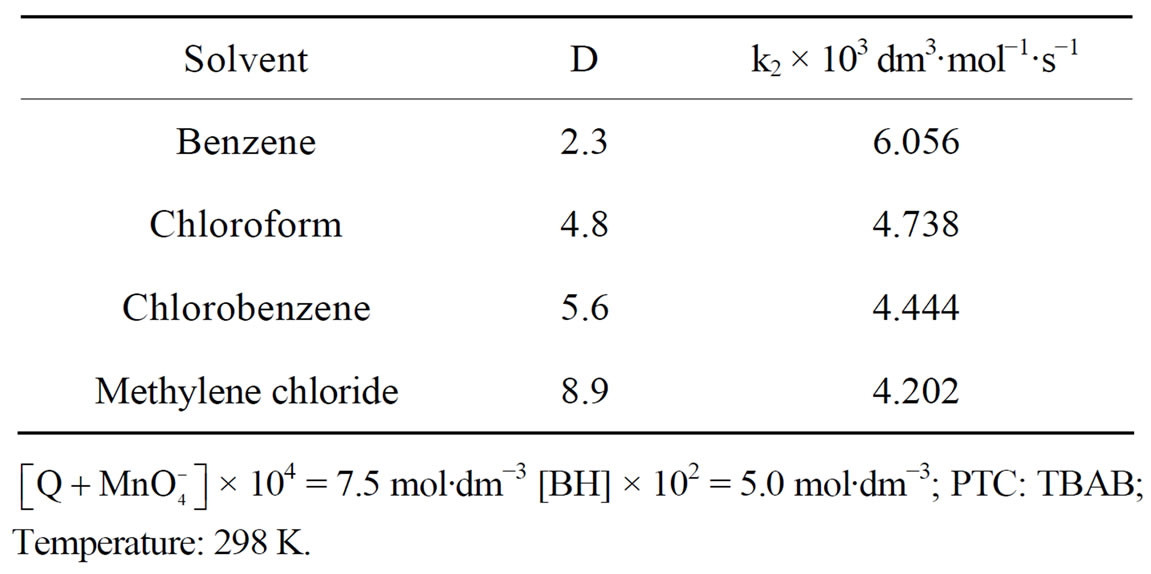
Table 2. Effect of dielectric constant (D) of the medium on the rate of oxidation of BH by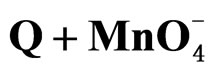 .
.
Table 3. Activation parameters for the oxidation of BH by 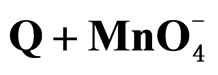 assisted by different PTCs in chlorobenzene.
assisted by different PTCs in chlorobenzene.
Table 4. Reaction rates at different temperatures and activation parameters for the oxidation of parasubstituted benzhydrols by  in chlorobenzene.
in chlorobenzene.
The rates of oxidation of BH at different temperatures were determined using , phase transferred using different PTCs, and the activation parameters were computed (Table 3). The efficiency of different PTCs were found to be in the order TBAHS > TBPB > TBAB.
, phase transferred using different PTCs, and the activation parameters were computed (Table 3). The efficiency of different PTCs were found to be in the order TBAHS > TBPB > TBAB.
The higher rate with TBPB in comparison with TBAB may be due to the stronger cation-anion interaction of the ion-pair with TBAB which makes the anion less available for oxidation. The higher rate with TBAHS compared to TBPB may be attributed to the easy ion-pair formation between catalyst cation and reagent nucleophile in non-polar solvent. Moreover, the  anion of TBAHS is highly hydrophilic compared to bromide and readily get partitioned into the aqueous phase, making the transfer of
anion of TBAHS is highly hydrophilic compared to bromide and readily get partitioned into the aqueous phase, making the transfer of 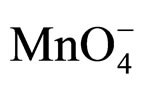 ions into the organic phase with better ease. Further, the values of AG# are very close to each other (~85 kJ∙mol−1) indicating that same mechanism operates with different PTCs.
ions into the organic phase with better ease. Further, the values of AG# are very close to each other (~85 kJ∙mol−1) indicating that same mechanism operates with different PTCs.
Study of the effect of substituents at the para position of one of the benzene rings showed that, both electron donating and electron withdrawing groups enhance the rate (Table 4).
The log k2 vs σ plot is non-linear which was concave upwards. The activation enthalpies and entropies are linearly related. The linear isokinetic correlation implies that all the substituted benzhydrols under investigation are oxidised by the same mechanism. This is further confirmed by the in AG# values.
According to the Frontier Molecular orbital approach the reaction would be initiated by an interaction of the HOMO of the substrate (an oxygen 2P-orbital containing a lone pair of electrons) with the LUMO of the oxidant (an antibonding orbital located primarily on Mn) resulting in the formation of a permanganate ester [7]. This is followed by the rate limiting slow decomposition of the ester via a five membered cyclic transition state. The large negative entropy of activation is consistent with the formation of a transition state having greater degree of order in its structure.
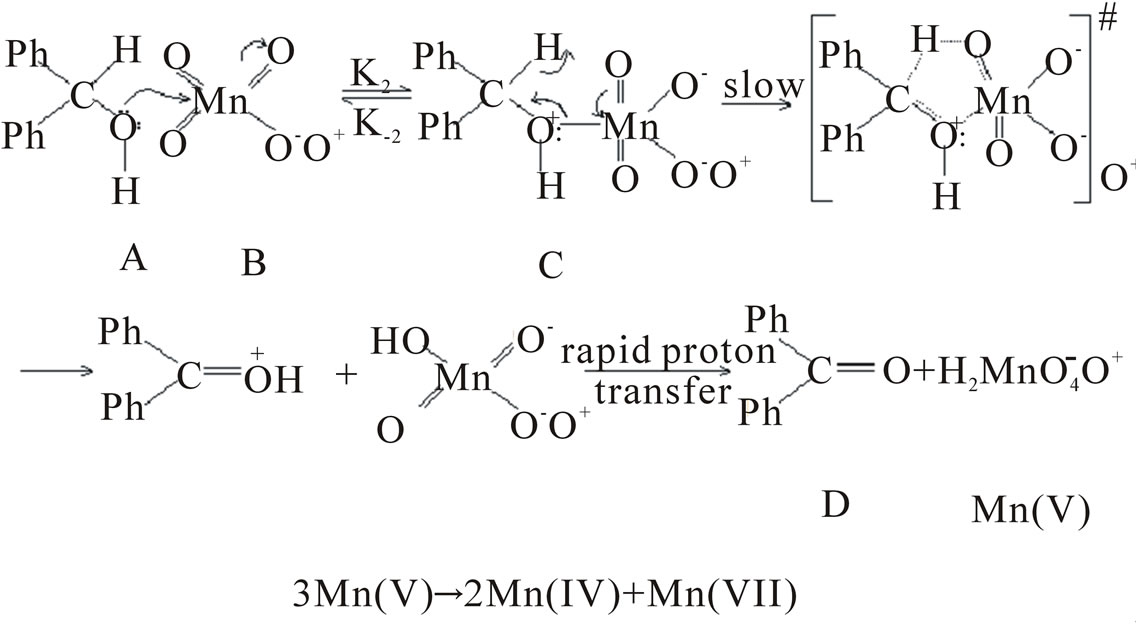
A rate expression consistent with the above mechanism can be given from the equilibrium, [C] = Keq [A] [B]
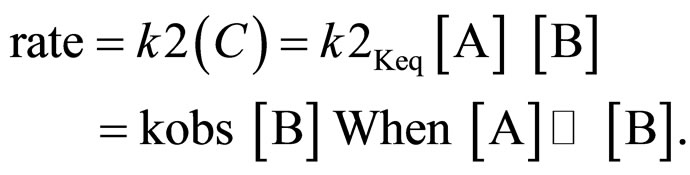
In the rate determining slow decomposition of the permanganate ester, breaking away of the αC–H bond and formation of the carbon-oxygen double occurs. It is probable that electron donating groups enhance the rate by facilitating the cleavage of αC–H bond, while electron withdrawing groups enhance the rate by facilitating the formation of carbon-oxygen double bond.
4. Conclusion
The kinetics of the permanganate oxidation of benzydrols under PTC in water immiscible organic solvents have been made. The oxidation proceeds faster in solvents with low dielectric constants. Of the three catalysts used TBAHS gave maximum rate. It was interesting to see that both electron donating and electron withdrawing substituents increased the rate. The mechanism explained with necessary kinetic parameters.
5. Acknowledgements
Two of the authors (KMB and JJ) thank the HOD, Chemistry Department, and Calicut University for providing facilities.
REFERENCES
- D. J. Sam and H. E. Simmon, “Crown Polyether Chemistry—Potassium Permanganate Oxidation in Benzene,” Journal of American Chemistry Society, Vol. 94, No. 11, 1972, pp. 4024-4025. doi:10.1021/ja00766a069
- A. W. Herriot and D. Picker, “Purple Benzene: Permanganate Oxidations Using Quartenary Ammonium Ions,” Tetrahedron Letters, Vol. 15, No. 16, 1974, pp. 1511-1514. doi:10.1016/S0040-4039(01)93123-5
- R. Sumichrast and V. Helba, “Knetic Studies of the Oxidation of Alcohols by Cetyltrimethylammonium Permanganate,” Reaction Kinetics and Catalysis Letters, Vol. 48, No. 1, 1992, pp. 93-98.
- N. T. D. Radhakrishnan and P. Rajendran, “Phase Transfer Catalysis and Kinetics of Oxidation of Some Secondary Aromatic Alcohols by Permanganate in Organic Media,” Indian Journal of Chemistry, Vol. 41A, No. 1, 2002, pp. 64-67.
- K. Bijudas and N. T. D. Radhakrishnan, “Selective Oxidation of Benzyl Alcohol with Monochrmate in Non Polar Solvents,” Indian Journal of Chemistry, Vol. 43A, 2004, pp. 1216-1218.
- K. M. Basheer, J. Joseph and T. D. N. Radhakrishnan, “Catalysis Onium Salts on the Monchromate Oxidation of Benzydrols in Non Plar Media—A Kinetic Study,” Indian Journal of Chemistry, Vol. 46A, 2007, pp. 273-275.
- K. N. Rankin, Q. Liu, J. Hendry, H. Yee, N. Noureldin and D. G. Lee, “Mechanisms for the Oxidation of Para-Substituted Benzyl Alcohols and Benzyl Ethers by Permanganate,” Tetrahedron Letters, Vol. 39, No. 10, 1998, pp. 1095-1098. doi:10.1016/S0040-4039(97)10788-2
NOTES
*Corresponding author.

