Pharmacology & Pharmacy
Vol.06 No.04(2015), Article ID:55972,10 pages
10.4236/pp.2015.64024
Evaluation Efficacy and Safety of Vortioxetine 20 mg/d versus Placebo for Treatment Major Depressive Disorder: A Systematic Review and Meta-Analysis of Randomized Controlled Trials
Masoud Behzadifar1,2, Hamidreza Dehghan1, Korush Saki3, Meysam Behzadifar4, Abouzar Keshavarzi1, Maryam Saran5, Ali Akbari Sari6*
1Department of Health, Yazd University of Medical Sciences, Yazd, Iran
2Health Management and Economics Research Center, Iran University of Medical Sciences, Tehran, Iran
3Department of Medicine, Shahid Beheshti University of Medical Sciences, Tehran, Iran
4Department of Medicine, Ilam University of Medical Sciences, Ilam, Iran
5Department of Medicine, Tehran University of Medical Sciences, Tehran, Iran
6Department of Health Management and Economics, Tehran University of Medical Sciences, Tehran, Iran
Email: *akbarisari@tums.ac.ir
Copyright © 2015 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/



Received 17 March 2015; accepted 24 April 2015; published 27 April 2015
ABSTRACT
Major depressive disorder, a common debilitating illness, is one of the leading causes of disability and disease worldwide. Different drugs for the treatment of patients with major depression can be used. Vortioxetine for the treatment of major depressive disorder was approved by the Food and Drug Administration (FDA) in 2013. This study aimed to evaluation efficacy and safety Vortioxetine 20 mg/d compared placebo in major depressive disorder. To conduct this study, we searched Pub Med, Cochrane library, Scopus, and Central Register of Controlled Trials. This study by including randomized controlled trials (RCTs) that evaluated this study by including randomized controlled trials (RCTs) that evaluated Vortioxetine 20 mg/d in patients with major depressive disorder. Data analysis was conducted by standard mean different ratios (SMD) with 95% confidence intervals (CIs), P values and odds ratios (ORs) for adverse events with 95% confidence intervals (CIs) and P values; heterogeneity testing and sensitivity analysis was also performed in this study. We found that 4 articles met the inclusion criteria and were finally used for this meta-analysis. Results showed statistical significance in the MADRS (Montgomery-Åsberg Depression Rating Scale), SMD = −4.75 with 95% CI [−6.84, −2.65] and P value < 0.00001), for Clinical Global Impression Scale-Impro- vement (CGI-I) SMD was −4.34 with 95% CI [−6.41, −2.27] and P value < 0.00001, and for Sheehan Disability Scale (SDS) SMD was −2.62 with 95% CI [−3.99, −1.25] and P value < 0.00001. The pooled analysis for safety demonstrated for diarrhea OR = 0.92 with 95% CI [0.46, 1.83] , P value = 0.09, for dry mouth OR = 1.74 with 95% CI [1.07, 2.83] , P value = 0.80, for dizziness OR = 1.62 with 95% CI [0.72, 3.66] , P value = 0.05, for fatigue OR = 1.17 with 95% CI [0.34, 4.08], P value = 0.07, for headache OR = 1.28 with 95% CI [0.91, 1.79], P value = 0.60 and for nausea OR = 4.78 with 95% CI [3.43, 6.67], P value = 0.61. Vortioxetine 20 mg/d versus placebo showed a significant difference for nausea and dry mouth, but no significant differences were observed for the four adverse effects. In several studies of the drug Vortioxetine 20 mg/d, the treatment of major depressive illness has been more effective for evaluating the effectiveness of this drug, which must be more clinical studies of sound.
Keywords:
Major Depressive Disorder, Vortioxetine 20 mg, Systematic Review, Meta-Analysis

1. Introduction
Major depressive disorder (MDD), a common debilitating illness, is one of the leading causes of disability and disease worldwide [1] . The quality of life of patients suffering from major depression diminishes. This disease causes impairment of physical, mental, and social functions and can be patient [2] . In addition, people with major depressive illness spend a lot to pay for treatment [3] . According to the World Health Organization reports, about 350 million people worldwide suffer from major depressive illness [2] . Given that most antidepressants are available to patients, the evidence shows that about 60 to 70 percent of these people make these drugs an appropriate response to health [4] . Patients with major depression often have such symptoms or signs: depressed mood, low of interest, low pleasure usual activities, changes in eating or sleeping, difficulty concentrating, suicidal thoughts and fatigue. Many treatment options for drug for major depressive disorder, antidepressants can often cause adverse effects. It is estimated that 15% of patients with major depression relapsed disease 35% of them are [5] . Major depression in physical diseases diagnosed has been proven in many studies. Expected depression is to be the second largest contributor to the world’s disease burden by 2020 [3] [4] . In patients with major depression reported: COPD, multiple sclerosis, Parkinson’s disease, diabetes mellitus, asthma, rheumatic arthritis, migraine, inflammatory bowel disease, cancer, stroke, heart disease, back problems and epilepsy [6] . Different drugs for the treatment of patients with major depression can be used. Vortioxetine for the treatment of major depressive disorder was approved by the Food and Drug Administration (FDA) in 2013. Vortioxetine is a selective serotonin reuptake inhibitor (SSRI) that binds to the presynaptic serotonin reuptake site, increasing the level of serotonin (5-HT) in the neuronal synapse and selectively binding to a variety of other serotonin receptors. It selectively binds to and acts as an antagonist of 5-HT3, 5-HT1D and 5-HT7 receptors, as a partial agonist to 5-HT1B receptors, and as an agonist of 5-HT1A receptors [7] - [10] . The objective of this article is systematic review and meta-analysis evaluation efficacy and safety of Vortioxetine 20 mg/d compared placebo in patients with major depressive disorder in randomized clinical trials.
2. Materials and Methods
2.1. Search Strategy
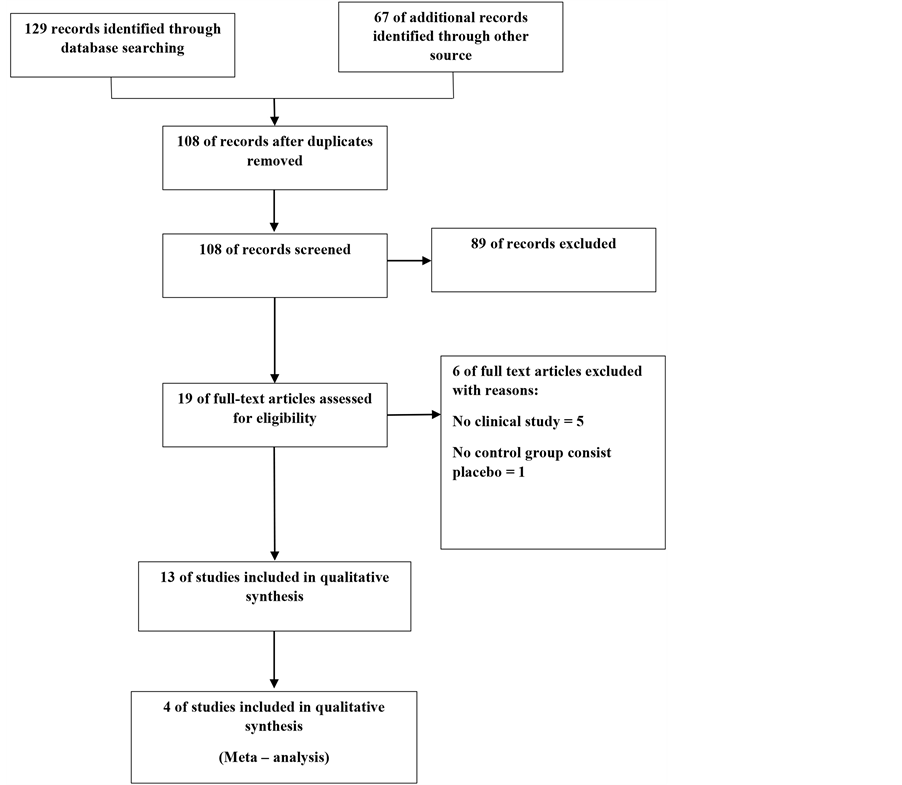
We searched Pub Med, Cochrane library, Scopus, CRD, Central Register of Controlled Trials to January 2015. Our searches will not be limited by language, publication status or setting. We also searched ClinicalTrials.gov, International depressive disorder Conference and the Anxiety Disorders and Depression Conference. For the reference lists of articles contact authors of included studies to acquire other data that may either be unpublished (Figure 1). Data collection, summary and analysis of the identification in this systematic review will be presented as a PRISMA [11] . Two review authors (Masoud. B, Meysam. B) will independently searched. First,
Figure 1. Flowchart of included studies.
screening the titles and abstracts of RCTs, Secondly, (H.D, A.AS) review author will independently full text of all trials. Compared the contents of each review author’s list, and Conflicts were resolved by discussion.
2.2. Inclusion Criteria
Clinical trials testing the efficacy of Vortioxetine 20 mg for the short-term treatment (8 wks.) of major depressive disorder were eligible for inclusion. Included studies had to be RCTs comparing Vortioxetine 20 mg with placebo. Patients needed to meet the criteria for major depressive disorder used in the individual trials. Studies were excluded if the main outcome was prevention of relapse or if treatment outcomes based on rating scales of major depressive disorder were not available.
2.3. Data Extraction
We collected data on participant characteristics, treatment details, study procedures, efficacy measures and Adverse Events (AEs). These data included, for example, group (treatment, placebo), size sample, age, sex, duration of treatment, baseline MADRS, doses and study location. Outcome data related to the characteristics of the individual trial and the reported results were extracted for each trial. For instance, the mean changes or reported numbers for Adverse Events were extracted from the individual study when appropriate. The efficacy measures were the mean change from baseline in on Montgomery-Åsberg Depression Rating Scale (MADRS), Clinical Global Impression Scale-Improvement (CGI-I), Sheehan Disability Scale (SDS) study [12] - [14] .
2.4. Quality Assessment
We will to assess the quality of studies, used Cochrane Collaboration “Risk of bias” assessment tool [15] , including Random sequence generation, Allocation concealment, Blinding of participants and personnel, Blinding of outcome assessment ,incomplete outcome data, Selective reporting and other bias (Figure 2).
2.5. Quality of RCTs Included
The study quality was assessed with Jadad scores [16] . The Jadad score is an instrument used to assess the quality of randomized clinical trials (RCTs). It includes three items as follows: randomization, blindness and dropouts. The score standards and the results of our included studies are shown in Table 1, respectively. Were rated as providing good methodological quality based on a Jadad score of 1 - 5. So the total scores for all included articles indicated a high study quality.
2.6. Statistical Analysis
In the review, we assessed values, Montgomery-Åsberg Depression Rating Scale (MADRS), Clinical Global Impression Scale-Improvement (CGI-I), Sheehan Disability Scale (SDS) and adverse effects randomized into the Vortioxetine 20 mg/day and placebo groups for each trial were statistically combined using the Mantel- Haenszel random effects model. The effects were expressed as Standard mean different ratios (SMD) with 95% confidence intervals (CIs) and P values. The incidence of adverse effects between the Vortioxetine 20 mg/day and placebo groups was also determined using the Mantel-Haenszel model, and the results were expressed as the Odds Ratio (ORs) with the 95% CI and P values. The heterogeneity across each effect size was evaluated with using the I2 and Chi-squared tests statistic. This measure evaluates how much of the variance among studies can be attributed to the actual differences among the studies rather than to chance. A magnitude of considerable heterogeneity is usually I2 = 75% - 100% [16] . A sensitivity analysis was performed to rule out the possibility that any single study strongly influenced the pooled effect. Publication bias was assessed by a funnel plot, Egger’s test [17] , and Begg’s rank correlation test [18] . All the statistical analyses were performed using Review Manager (Rev Man 5.3) software and Stata 12 software.
3. Results and Discussion
3.1. Efficacy
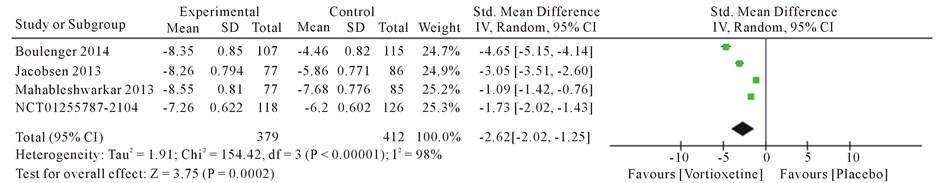
Overall, we found 4 articles met the inclusion criteria and were finally used for this meta-analysis (Table 2). This article consist Boulenger JP [19] , Mahableshwarkar AR [20] , Jacobsen PL [21] and trial no NCT01255787 [22] . A total of five studies with 1337 patients, 609 in the 20 mg/day Vortioxetine group and 728 patients in the placebo group. The standard mean different ratios (SMD) for Montgomery-Åsberg Depression Rating Scale (MADRS) with Vortioxetine 20 mg compared to placebo was −4.75 with 95% CI [−6.84, −2.65] and P value < 0.00001 and heterogeneity for MADRS scale was I2 = 99%, The Standard mean different ratios (SMD) for Clinical Global Impression Scale-Improvement (CGI-I) was −4.34 with 95% CI [−6.41, −2.27] and P value < 0.00001 and heterogeneity for SMD was I2 = 99% and Standard mean different ratios (SMD) for Sheehan Disability Scale (SDS) was −2.62 with 95% CI [−3.99, −1.25] and P value < 0.00001 and heterogeneity for SDS was I2 = 98% (Figure 3).
Table 1. Jadad score quality assessment of the included studies.
Figure 2. Risk of bias graph in the included studies.
Table 2. Summary of the included studies in the meta-analysis.
aThe Montgomery-Åsberg Depression Rating Scale (MADRS) is a depression rating scale consisting of 10 items, each rated 0 to 6. The 10 items represent the core symptoms of depressive illness. The overall score ranges from 0 (symptoms absent) to 60 (severe depression); bVortioxetine.
MADRS:
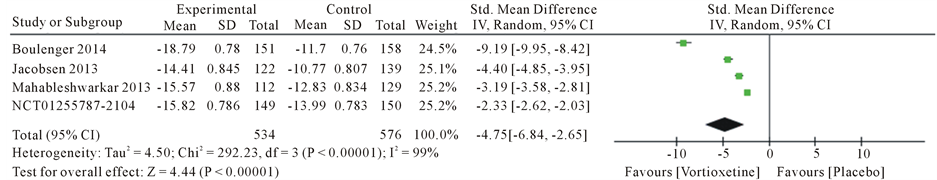
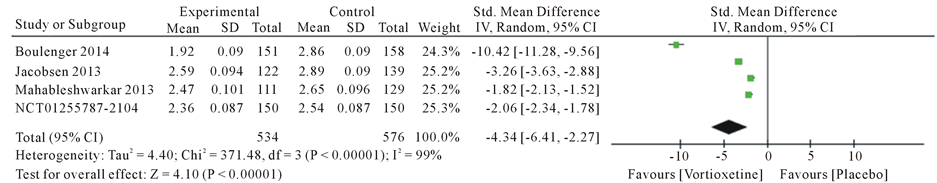
Figure 3. Forest plot of standard different mean ratios (SMD) and 95% confidence intervals (CIs) of change from baseline in the Montgomery-Åsberg Depression Rating Scale (MADRS), Clinical Global Impression Scale-Improvement (CGI-I) and Sheehan Disability Scale (SDS) total score at week 8 in the included studies.
3.2. Safety
Drug safety evaluation for symptoms that have been observed in studies for was meta-analysis. The most common side effects were diarrhea, dry mouth, dizziness, fatigue, headache and nausea.
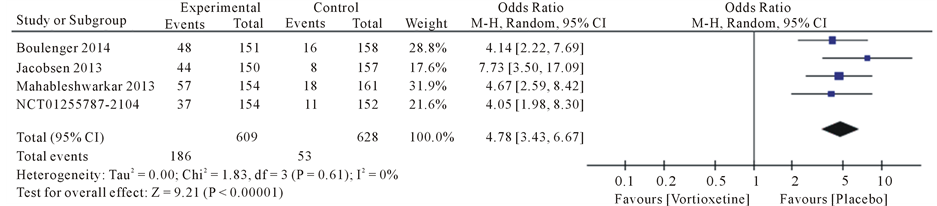
Results the 20 mg/day Vortioxetine compared to placebo showed for diarrhea OR = 0.92 with 95% CI [0.46, 1.83], P value = 0.09, for dry mouth OR = 1.74 with 95% CI [1.07, 2.83], P value = 0.80, for dizziness OR = 1.62 with 95% CI [0.72, 3.66], P value = 0.05, for fatigue OR = 1.17 with 95% CI [0.34, 4.08], P value = 0.07, for headache OR = 1.28 with 95% CI [0.91, 1.79], P value = 0.60 and for nausea OR = 4.78 with 95% CI [3.43, 6.67], P value = 0.61 (Figure 4).
3.3. Sensitivity Analysis
Sensitivity analysis not found that the pooled remission rate was significantly influenced when we excluded the study from .trial no NCT01255787.
3.4. Analysis for Publication Bias
Analysis for publication bias showed, no publication bias was observed for MADRS, CGI-I and SDS (Egger’s test: P = 0.006, P = 0.010, P = 0.105 respectively, and Begg’s test: P = 0.042, P = 0.174, P = 0.174, respectively). Results showed no publication bias was observed for Adverse Events contain Diarrhea, Dry mouth, Dizziness, Fatigue, Headache and Nausea in the included studies (Egger’s test: P = 0.823, P = 0.257, P =0.617, P = 0.149, P = 0.227, P = 0.205, P = 0.197 respectively and Begg’s test: P = 1.000, P = 0.174, P = 0.497, P = 0.174, P = 0.174, P = 0.174, respectively).
This study evaluated the efficacy, safety of Vortioxetine 20 mg/d compared with placebo treatment in patients with major depressive disorder. We identified four RCTs examining the efficacy of Vortioxetine 20 mg/d versus placebo for treatment major depressive disorder. The present meta-analysis demonstrated the superior efficacy of Vortioxetine compared with placebo for the treatment of major depressive disorder in terms of mean change MADRS scale (SMD = −4.75). Our results showed that the treatment of the Vortioxetine 20 mg/day group based on both depression rating SDS (SMD = −2.62) and CGI-H (SMD = −4.34) was greater than the placebo group. The decrease in depression symptoms seems too associated with 20 mg/d of Vortioxetine versus placebo. Vortioxetine 20 mg/d were statistically significantly superior to placebo in three scales. Efficacy has been replicated at the 20 m/g doses in adults, demonstrated efficacy in study Boulenger et al., Mahableshwarker et al., Jacobsen et al. and trial NCT01255787. In the studies Alvarez et al. [23] , Mahableshwarkar et al. [24] , Jain et al. [25] , Katona et al. [26] , and Henigsberg et al. [27] showed Vortioxetine efficacy. Improve symptoms in patient by major depressive disorder Obtained in these studies. Results of Adverse Events (AEs) showed a significant for dry mouth OR = 1.74 [1.07 - 2.83] and nausea OR = 4.78 [3.43 - 6.67]. But no significant differences were observed for the other four adverse effects. AEs discontinuation rates were generally low. It suggested that the negative results in previous double-blind, random-controlled studies may have been due to an inadequate sample size, which can be overcome by the meta-analytic method. These findings indicate that compared to placebo, 20 mg/d mg/day Vortioxetine significantly improved depressive symptoms in patients with major depressive disorder. In the randomized clinical analyzed, the common adverse effects of Vortioxetine include diarrhea, dizziness, dry mouth, nausea, headache and fatigue.
The limitations of this meta-analysis include the following: the inclusion of patients only during the acute phase, which did not enable us to analyze the long-term efficacy and safety of Vortioxetine in treating major depressive disorder. The included studies did not include data on the onset time of Vortioxetine’s efficacy, and thus, we did not compare the onset time between 20 mg/d Vortioxetine and placebo. All included trials were supported by the Takeda company, Ltd. All included studies did not include the efficacy and adverse effects based on sex; thus, we could not evaluate gender differences. Due to the limited number of the published articles, we did not analyze the efficacy and safety of different doses of Vortioxetine in the treatment of major depressive disorder. The small number of included studies and the relatively small sample size, which may influence the reliability of the results. However, depression is frequently associated with coronary heart diseases [28] , diabetes mellitus [29] , stroke [30] , pregnancy, and the postpartum period [31] . Thus, the use of Vortioxetine should also benefit the physical state of these patients. Due to the small number of trials in our meta-analysis, our results warrant additional studies to verify these findings. In the future, additional large-scale and well-designed Studies are needed to determine the optimal dose, the most appropriate treatment group, and the efficacy and safety of Vortioxetine combined with other antidepressants in treating depression [32] .
4. Conclusion
We found that Vortioxetine may be another treatment option for major depressive disorder. However, our results
Diarrhea:
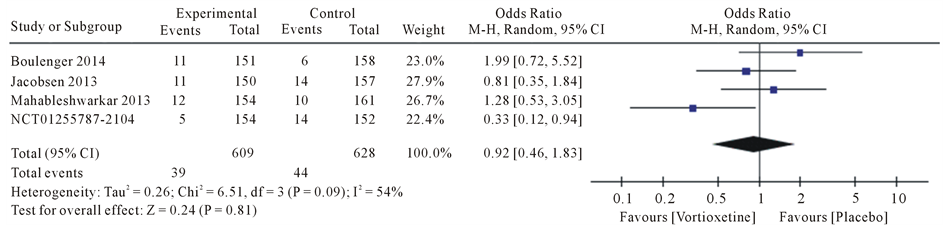
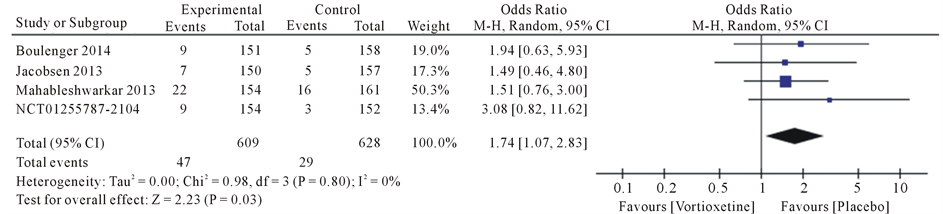
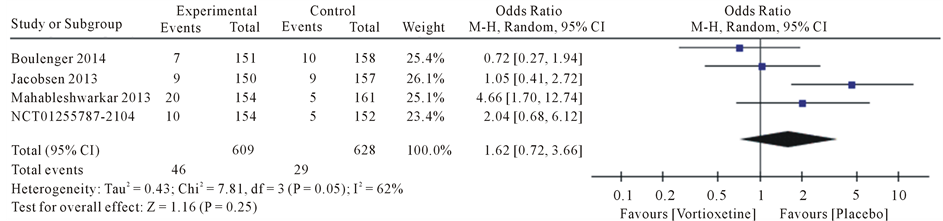
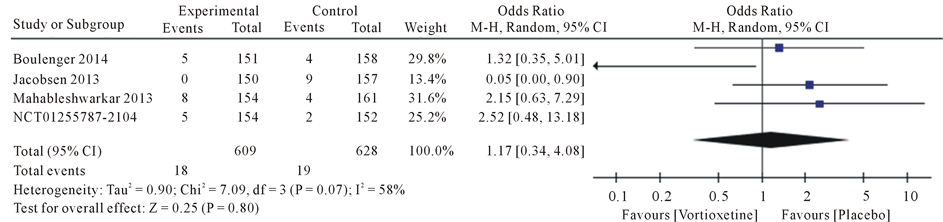
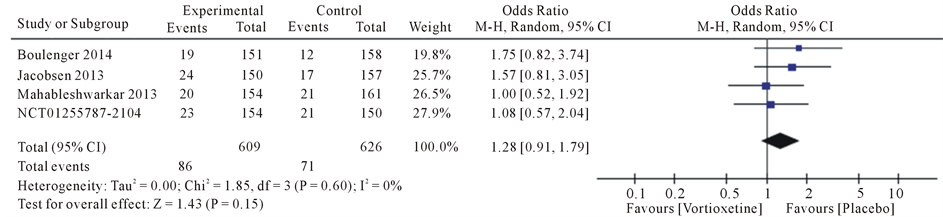
Figure 4. Forest plot of Odds ratio (OR) and 95% confidence intervals (CIs) of Diarrhea, Dry mouth, Dizziness, Fatigue, Headache and Nausea AEs in the included studies.
should be interpreted and translated into clinical practice with caution, small effect sizes of the clinical trials included in present the meta-analysis. Adequately powered, well-designed, and direct-comparison clinical trials should also more clearly address the comparative efficacy of Vortioxetine and different antidepressants. The current meta-analysis of published RCTs has shed light on the benefits of 20 mg/d Vortioxetine for the treatment of major depression disorder. As a novel antidepressant, there is increasingly greater interest in Vortioxetine. In several studies of the drug Vortioxetine 20 mg/d, the treatment of major depressive illness has been more effective for evaluating the effectiveness of this drug, which must be more clinical studies of sound.
Acknowledgements
This study was part of the MSc (Health Technology Assessment (HTA)) thesis of first author (Masoud Behzadifar). The authors would like to thank all participants who made this study possible.
References
- Ferrari, A.J., Charlson, F.J., Norman, R.E., et al. (2013) Burden of Depressive Disorders by Country, Sex, Age, and Year: Findings from the Global Burden of Disease Study 2010. PLoS Medicine, 10, e1001547.
- World Health Organization Mental Health Depression. http://www.who.int/mediacentre/factsheets/fs369/en/
- Murray, C.J. and Lopez, A.D. (1997) Alternative Projections of Mortality and Disability by Cause 1990-2020: Global Burden of Disease Study. The Lancet, 349, 1498-1504. http://dx.doi.org/10.1016/S0140-6736(96)07492-2
- Souery, D., Papakostas, G.I. and Trivedi, M.H. (2006) Treatment Resistant Depression. Journal of Clinical Psychiatry, 67, 16-22.
- Liu, M.T., Maroney, M.E. and Hermes-DeSantis, E.R. (2015) Levomilnacipran and Vortioxetine: Review of New Pharmacotherapies for Major Depressive Disorder. World Journal of Pharmacology, 4, 17-30. http://dx.doi.org/10.5497/wjp.v4.i1.17
- Halperin, D. and Reber, G. (2007) Influence of Antidepressants on Hemostasis. Dialogues in Clinical NeuroSciences, 9, 47-59.
- National Institute of Mental Health What Is Depression? http://www.nimh.nih.gov/health/topics/depression/index.shtml
- Centers for Disease Control and Prevention Depression. http://www.cdc.gov/mentalhealth/basics/mental-illness/depression.htm
- American Psychiatric Association (2013) DSM-5 Task Force. Diagnostic and Statistical Manual of Mental Disorders. 5th Edition, American Psychiatric Association, Washington DC.
- Wesolowska, A., Tatarczynska, E., Nikiforuk, A. and Chojnacka-Wojcik, E. (2007) Enhancement of the Anti Immobility Action of Anti-Depressants by a Selective 5-HT7 Receptor Antagonist in the Forced Swimming Test in Mice. European Journal of Pharmacology, 555, 43-47. http://dx.doi.org/10.1016/j.ejphar.2006.10.001
- Liberati, A., Altman, D.G., Tetzlaff, J., Mulrow, C., Gøtzsche, P.C., Ioannidis, J.P.A., et al. (2009) The PRISMA Statement for Reporting Systematic Reviews and Meta-Analyses of Studies That Evaluate Health Care Interventions: Explanation and Elaboration. PLoS Medicine, 6, e1000100. http://dx.doi.org/10.1371/journal.pmed.1000100
- Montgomery, S.A. and Asberg, M. (1979) A New Depression Scale Designed to Be Sensitive to Change. The British Journal of Psychiatry, 134, 382-389. http://dx.doi.org/10.1192/bjp.134.4.382
- Forkmann, T., Scherer, A., Boecker, M., Pawelzik, M., Jostes, R. and Gauggel, S. (2011) The Clinical Global Impression Scale and the Influence of Patient or Staff Perspective on Outcome. BMC Psychiatry, 11, 83. http://dx.doi.org/10.1186/1471-244X-11-83
- Leon, A.C., Olfson, M., Portera, L., Farber, L. and Sheehan, D.V. (1997) Assessing Psychiatric Impairment in Primary Care with the Sheehan Disability Scale. The International Journal of Psychiatry in Medicine, 27, 93-105. http://dx.doi.org/10.2190/T8EM-C8YH-373N-1UWD
- Higgins, J.P., Altman, D.G., Gøtzsche, P.C., Jüni, P., Moher, D., Oxman, A.D., Savovic, J., Schulz, K.F., Weeks, L. and Sterne, J.A. (2011) The Cochrane Collaboration’s Tool for Assessing Risk of Bias in Randomized Trials. BMJ, 343, Article ID: d5928.
- Jadad, A.R., Moore, R.A., Carroll, D., Jenkinson, C., Reynolds, D.J., Gavaghan, D.J. and Mc Quay, H.J. (1996) Assessing the Quality of Reports of Randomized Clinical Trials: Is Blinding Necessary? Controlled Clinical Trials, 17, 1- 12. http://dx.doi.org/10.1016/0197-2456(95)00134-4
- Egger, M., Davey Smith, G., Schneider, M. and Minder, C. (1997) Bias in Meta-Analysis Detected by a Simple, Graphical Test. BMJ, 315, 629-634. http://dx.doi.org/10.1136/bmj.315.7109.629
- Begg, C.B. and Mazumdar, M. (1994) Operating Characteristics of a Rank Correlation Test for Publication Bias. Biometrics, 50, 1088-1101. http://dx.doi.org/10.2307/2533446
- Boulanger, J.P., Loft, H. and Olsen, C.K. (2014) Efficacy and Safety of Vortioxetine (Lu AA21004), 15 and 20 mg/day: A Randomized, Double-Blind, Placebo-Controlled, Duloxetine-Referenced Study in the Acute Treatment of Adult Patients with Major Depressive Disorder. International Clinical Psychopharmacology, 29, 138-149. http://dx.doi.org/10.1097/YIC.0000000000000018
- Mahableshwarkar, A.R., Jacobsen, P.L., Serenko, M., et al. (2013) A Duloxetine-Referenced, Fixed-Dose Study Comparing Efficacy and Safety of 2 Vortioxetine Doses in the Acute Treatment of Adult MDD Patients (NCT01153009) (Poster). American Psychiatric Association 166th Annual Meeting, San Francisco, 18-22 May 2013.
- Jacobsen, P.L., Mahableshwarkar, A.R., Serenko, M., et al. (2013) A Randomized, Double-Blind, Placebo-Controlled Study of the Efficacy and Safety of Vortioxetine 10 mg and 20 mg in Adults with Major Depressive Disorder (NCT01163266) (Poster). American Psychiatric Association 166th Annual Meeting, San Francisco, 18-22 May 2013.
- Efficacy and Safety Study of Vortioxetine (Lu AA21004) for Treatment of Major Depressive Disorder. NCT01255787. http://clinicaltrials.gov/show/NCT01255787
- Alvarez, E., Perez, V., Dragheim, M., Loft, H. and Artigas, F. (2012) A Double-Blind, Randomized, Placebo-Controlled, Active Reference Study of Lu AA21004 in Patients with Major Depressive Disorder. International Journal of Neuropsychopharmacology, 15, 589-600. http://dx.doi.org/10.1017/S1461145711001027
- Mahableshwarkar, A.R., Jacobsen, P.L. and Chen, Y. (2013) A Randomized, Double-Blind Trial of 2.5 mg and 5 mg Vortioxetine (Lu AA21004) versus Placebo for 8 Weeks in Adults with Major Depressive Disorder. Current Medical Research & Opinion, 29, 217-226. http://dx.doi.org/10.1185/03007995.2012.761600
- Jain, R., Mahableshwarkar, A.R., Jacobsen, P.L., Chen, Y. and Thase, M.E. (2013) A Randomized, Double-Blind, Placebo-Controlled 6-wk Trial of the Efficacy and Tolerability of 5 mg Vortioxetine in Adults with Major Depressive Disorder. International Journal of Neuropsychopharmacology, 16, 313-321. http://dx.doi.org/10.1017/S1461145712000727
- Katona, C., Hansen, T. and Olsen, C.K. (2012) A Randomized, Double-Blind, Placebo-Controlled, Duloxetine-Referenced, Fixed-Dose Study Comparing the Efficacy and Safety of Lu AA21004 in Elderly Patients with Major Depressive Disorder. International Clinical Psychopharmacology, 27, 215-223. http://dx.doi.org/10.1097/YIC.0b013e3283542457
- Henigsberg, N., Mahableshwarkar, A.R., Jacobsen, P., Chen, Y. and Thase, M.E. (2012) A Randomized, Double-Blind, Placebo-Controlled 8-Week Trial of the Efficacy and Tolerability of Multiple Doses of Lu AA21004 in Adults with Major Depressive Disorder. The Journal of Clinical Psychiatry, 73, 953-959. http://dx.doi.org/10.4088/JCP.11m07470
- Penninx, B.W., Beekman, A.T., Honig, A., Deeg, D.J., Schoevers, R.A., van Eijk, J.T. and van Tilburg, W. (2001) Depression and Cardiac Mortality: Results from a Community-Based Longitudinal Study. Archives of General Psychiatry, 58, 221-227. http://dx.doi.org/10.1001/archpsyc.58.3.221
- Schlienger, J.L. (2013) Type 2 Diabetes Complications. La Presse Médicale, 42, 839-848. http://dx.doi.org/10.1016/j.lpm.2013.02.313
- Robinson, R.G. and Spalletta, G. (2010) Post Stroke Depression: A Review. Canadian Journal of Psychiatry, 55, 341- 349.
- Di Florio, A., Forty, L., Gordon-Smith, K., Heron, J., Jones, L., Craddock, N. and Jones, I. (2013) Perinatal Episodes across the Mood Disorder Spectrum. JAMA Psychiatry, 70, 168-175. http://dx.doi.org/10.1001/jamapsychiatry.2013.279
- Fu, J. and Chen, Y. (2015) The Efficacy and Safety of 5 mg/d Vortioxetine Compared to Placebo for Major Depressive Disorder: A Meta-Analysis. Psychopharmacology, 232, 7-16. http://dx.doi.org/10.1007/s00213-014-3633-z
NOTES

*Corresponding author.