International Journal of Otolaryngology and Head & Neck Surgery
Vol.2 No.1(2013), Article ID:26559,3 pages DOI:10.4236/ijohns.2013.21003
Myoepithelioma of Tongue
Depatment of Ear, Nose and Throat & Head & Neck Surgery, Grant Medical College, Mumbai, India
Email: dr.saurabhagarwal@yahoo.com
Received October 9, 2012; received November 11, 2012; accepted November 24, 2012
Keywords: Salivary Gland Tumour; Tongue; Myoepithelioma
ABSTRACT
Myoepithelioma is a rare neoplasm of the salivary glands, generally occurring in the parotid gland and less often in the minor accessory salivary gland of the oral cavity. The histological appearance includes solid, myxoid and reticular growth patterns. Vimentin and S-100 protein are very sensitive but non-specific immunohistochemical markers of neoplastic myoepithelium. Conservative surgery is the treatment of choice. A case of myoepithelioma of the minor salivary gland of tongue is described, focusing on clinical behaviour, histopathological and immunohistochemical features.
1. Introduction
Myoepithelioma is defined as a benign tumour composed of myoepithelial cells. Myoepithelioma are uncommon, representing approximately 1% of salivary gland tumors. The vast majority are benign, with only rare malignant examples reported in the literature. There is no sex predilection, and the peak age of occurrence is in the third decade of life (range, 9 - 85 yr). The most common locations of this neoplasm are the parotid gland and soft palate these cells, which are found in many organs (mainly the salivary glands), are major components of various salivary gland tumours. Morphologic presentation may vary considerably [1]. The growth patterns may be solid, myxoid or reticular, and the component cells may be spindle-shaped, plasmacytoid, hyaline, clear or epithelioid [7]. When benign, the tumour usually appears as an asymptomatic mass that slowly increases in size over a period of several months or years.
We hereby describe an unusual case of myoepithelioma of tongue. Only a few cases of myoepithelioma of tongue have been described in the literature.
2. Case Report
A 35 years old female presented to the department of otolaryngology with a 1 year history of gradually progressive swelling on the dorsum of posterior part of the tongue. The swelling was painless in nature with no other significant complaints. The lesion was soft and slightly tender to palpation, measuring around 2.5 × 2 × 1.3 cm (Figure 1). The remainder of the physical examination and laboratory studies were normal. An incisional biopsy of the lesion was performed and was suggestive of tumour of myoepithlial origin.
Subsequently, a complete excision of the tumour was planned. The patient underwent a wide local tumourresection via a transoral approach under general anaesthesia. The mass was excised with the help of diode laser. The excised mass measured 2.5 × 2.3 × 1.2 cm (Figures 2 and 3). Histological examination revealed a nodular tumour covered by mildly hyperplastic epithelium. It was composed of sheets, broad anastomosing trabeculae, and cords of cuboidal cells with moderate amount of pale staining cytoplasm (Figure 4). Intervening stroma showed hyalinisation and collections of lymphocytes and plasma cells. These features were suggestive of minor salivary gland tumour favouring myoepithelioma. Further Immunohistochemical studies revealed positivity to Calponin and P63. These results were consistent with the diagnosis of myoepithelioma. Patient had no recurrence during a follow-up period of 2 years.
3. Discussion
Myoepitheliomas are rare tumors that account for only about 1% of all salivary gland tumors. Most are benign,
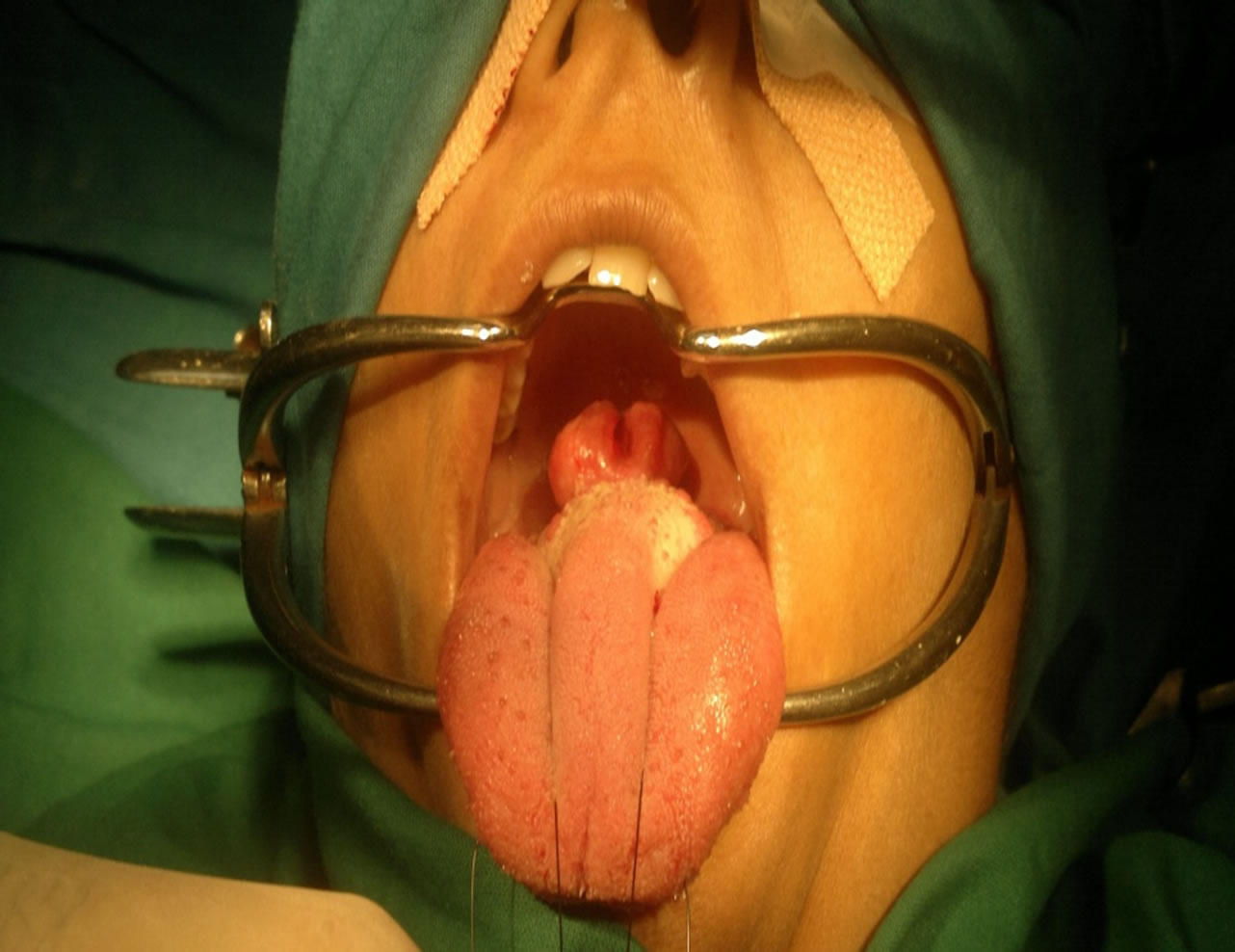
Figure 1. Myoepithelioma present at the tongue base.
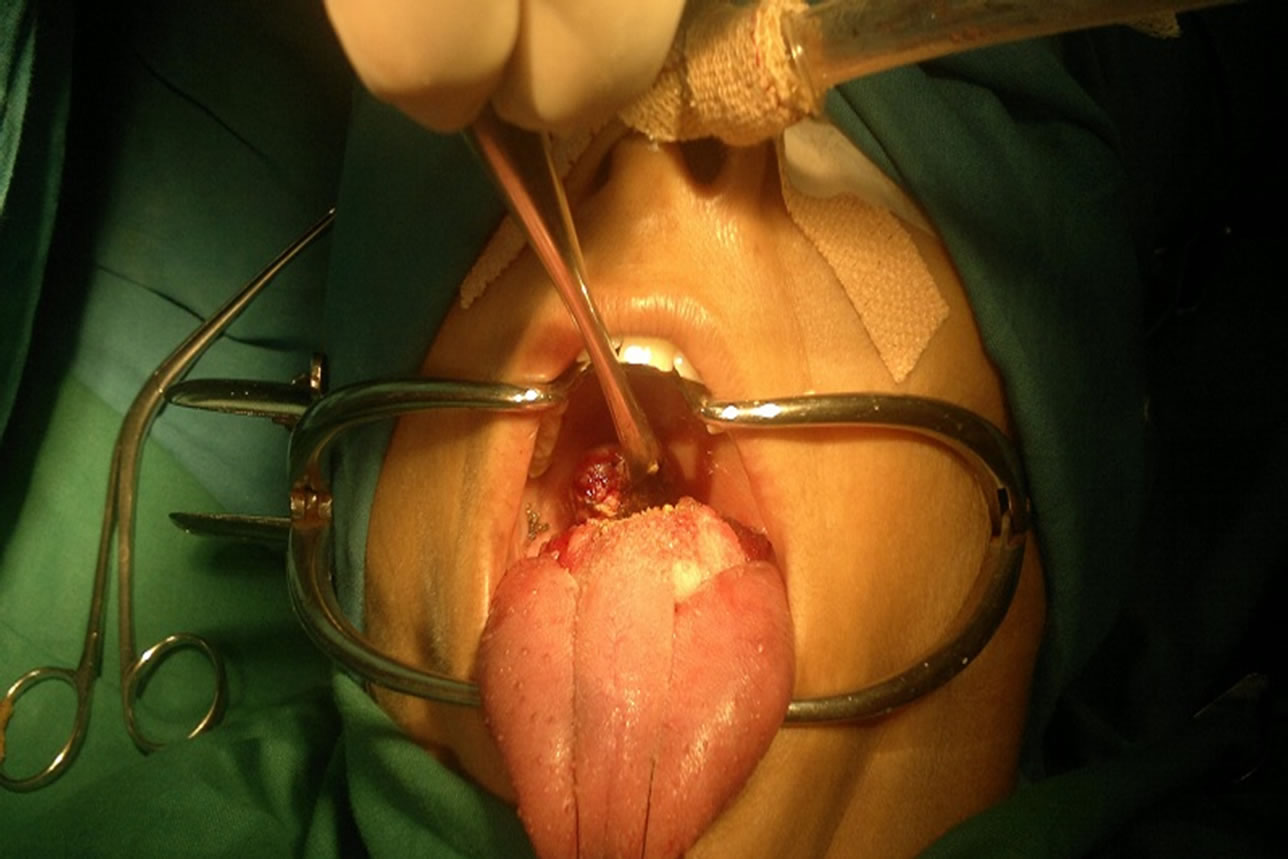
Figure 2. Myoepithelioma removed with laser.
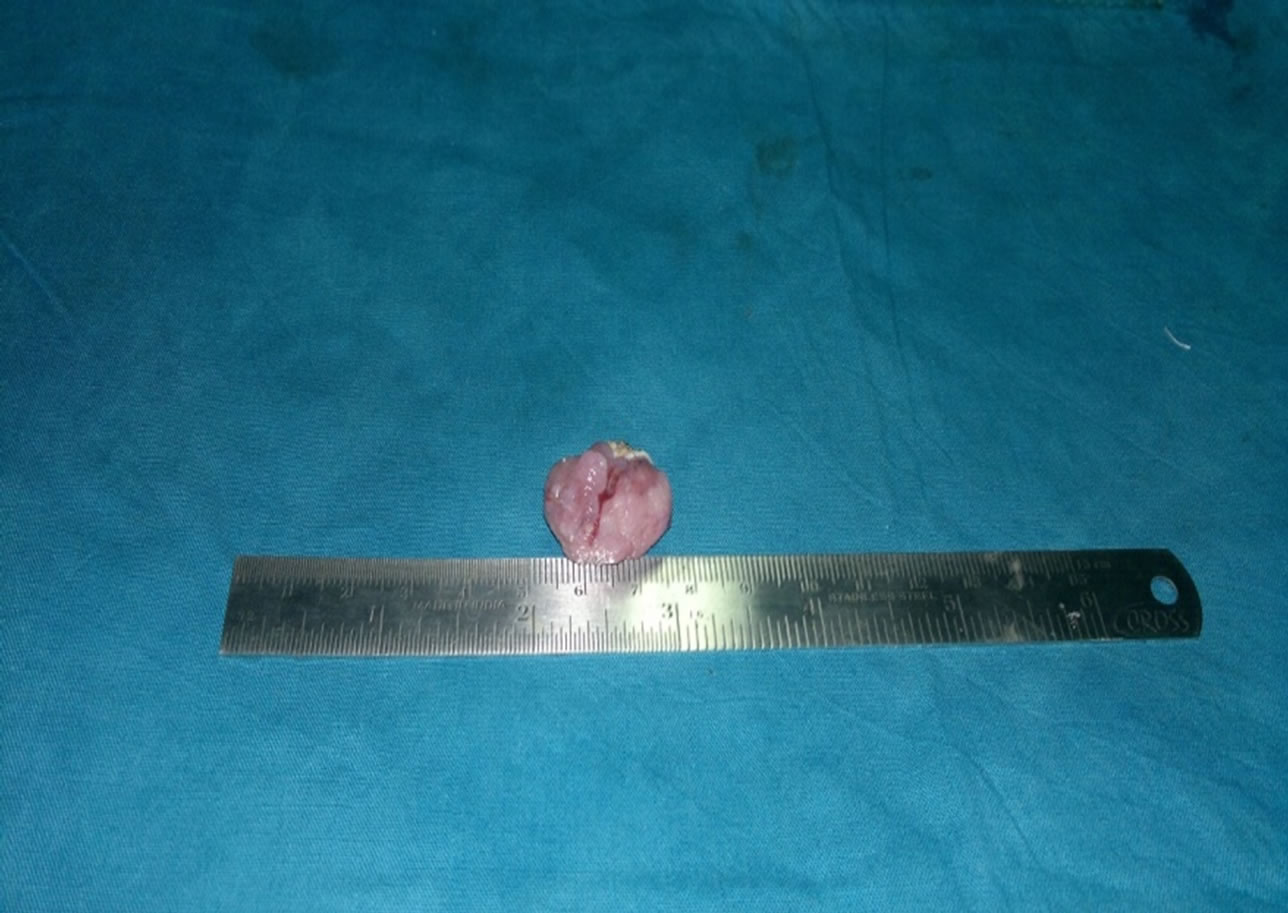
Figure 3. Myoepithelioma dissected out.
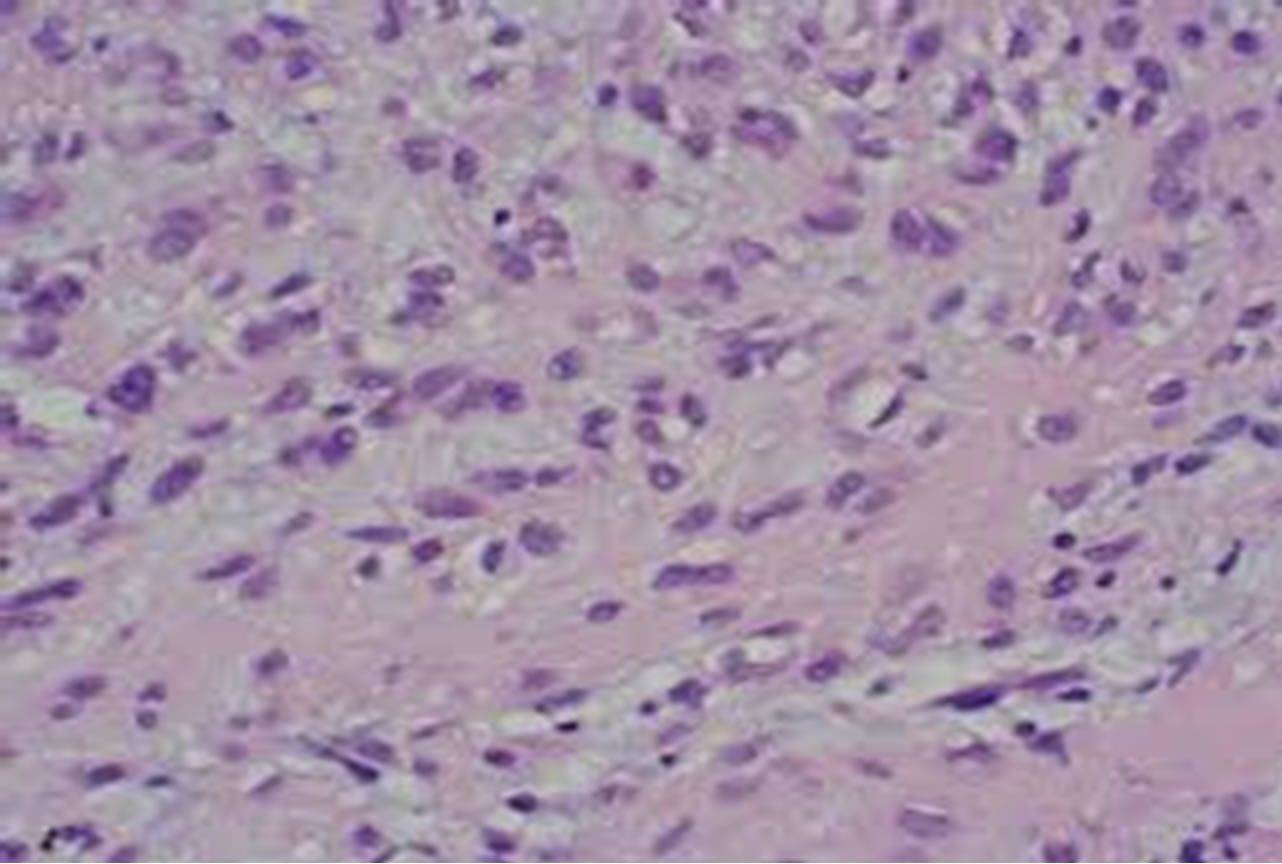
Figure 4. Histopathologic picture showing myoepithelioma.
but some can be malignant. In 1943 Sheldon first described myoepithelioma in the literature and then later in 1975 Stromeyer et al. reported the first documented case of a malignant myoepithelioma [2].
Myoepithelial cells are present in many secretory organs and have a dual epithelial and smooth muscle phenotype. Salivary glands have these cells in their acini and intercalated ducts. These cells form an important component in many salivary gland neoplasms. The tumor occurs over a wide age range with a median of 53 years and has no sex predilection. It usually presents as a painless, slow growing mass of benign nature, but sometimes it may be locally aggressive. The parotid gland is preferentially involved as compared to the other salivary glands [8].
Salivary-gland neoplasms that frequently contain myoepithelial cells are pleomorphic adenoma, adenoid-cystic carcinoma and epithelial-myoepithelial carcinoma of intercalated duct origin [11,12]. Neoplasms composed exclusively of myoepithelial cells are uncommon accounting for less than 1% of all salivary gland tumours. Most of these tumours are located in the parotid gland, while others occur in the submandibular gland or in the accessory glands of the oral cavity (hard and soft palate, lip, cheek, tongue, floor of the mouth, gingiva, retromolar area) [2-6,13]. They sometimes arise from the glands of the respiratory tract (nasal cavity, nasopharynx, larynx, lung).
The complex and varied morphologic and immunophenotypic expressions of neoplastic myoepithelium have always attracted numerous investigators, with valuable, but often contradictory, data being presented [9,10]. The traditional definition included only three types of myoepithelioma: plasmacytoid, spindle and mixed cells forms [4,8]. However, with the recent recognition of the different phenotypic and ultrastructural modifications displayed by the neoplastic myoepithelial cells of salivary gland tumours, the morphologic spectrum of myoepithelioma has been expanded [10,14]. By taking into account the variety of cytoarchitectural patterns displayed by the myoepitheliomatous regions of pleomorphic adenoma, Dardick et al. have proposed broader histopathologic guidelines for myoepitheliomas and have included a few previously unrecognized variants [14,15] .
Vimentin and S-100 protein are not usually present in normal myoepithelial cells and are very sensitive, but non-specific, markers of neoplastic myoepithelium. As with neoplastic transformation, the myoepithelium loses or modifies its smooth-muscle phenotype, immunohistochemical studies to demonstrate smooth-muscle differentiation in these cells have been less fruitful. In most tumours, alpha smooth muscle actin positivity has been observed, at least in a few cells, both of the spindleshaped and plasmacytoid types. Therefore, myoepithelial carcinomas invariably express keratins and their absence would not be in favour of this diagnosis [17].
The major differential diagnosis of myoepithelioma is from a pleomorphic adenoma. Myoepitheliomas are composed completely, or almost completely, of myoepithelial cells, whereas the amount is variable in the pleomorphic adenoma, but may reach levels comparable to those in myoepithelioma. Pleomorphic adenoma contains abundant ducts, whereas myoepithelioma have few, if any. The range of stromal components is identical, and myxoid and even chondroid areas can be seen in both. The only difference being that a much greater amount is likely to be found in the pleomorphic adenoma. Myoepithelioma probably constitutes one end of a biological spectrum which also includes pleomorphic adenoma and some (non-membranous) basal cell adenomas [7]. Other differential diagnosis includes soft tissues tumours such as leiomyoma, which is S-100 protein negative. Schwannomas are S-100 positive but most display characteristic histological features, although Verocay bodies have been reported in myoepitheliomas [18]. Polymorphous low grade adenocarcinoma may display areas of myoepithelial differentiation, both on Haematoxylin Eosin sections and on immunohistochemical stains. It is infiltrative, unlike the usually well-circumscribed myoepithelioma [19].
Biologically, myoepitheliomas are benign, in most cases, but occasionally infiltrate locally and metastasize. The malignancy is supported by infiltrative growth, necrotic areas, cytologic atypia, high mitotic rate and cellular pleomorphism. It has been suggested, in the literature, that assessment of cell proliferative activity may be helpful in the differential diagnosis between benign and malignant myoepitheliomas, and that a Ki-67 labelling index of more than 10% is diagnostic of myoepithelial carcinoma [20].
The prognosis of benign myoepithelioma would appear to be good, provided surgical excision is complete. Radiation therapy is used only when surgery is not considered feasible.
REFERENCES
- F. Martinez-Madrigal and C. Micheau, “Histology of the Major Salivary Glands,” The American Journal of Surgical Pathology, Vol. 13, No. 10, 1989, pp. 879-899. doi:10.1097/00000478-198910000-00008
- J. J. Sciubba and R. B. Brannon, “Myoepithelioma of Salivary Glands: Report of 23 Cases,” Cancer, Vol. 49, No. 3, 1982, pp. 52-72. doi:10.1002/1097-0142(19820201)49:3<562::AID-CNCR2820490328>3.0.CO;2-6
- J. G. Batsakis, “Myoepithelioma,” The Annals of Otology, Rhinology, and Laryngology, Vol. 94, No. 5, 1985, pp. 523-524.
- G. Seifert, “World Health Organization International Histological Classification of Tumours,” Histological Typing of Salivary Gland Tumours, 2nd Edition, Heidelberg, Berlin, 1991.
- E. Katsuyama, A. Kaneoka and K. Higuchi, “Myoepithelioma of the Soft Palate,” Acta Cytologica, Vol. 41, 1997, pp. 1856-1886.
- A. Piattelli, M. Fioroni and C. Rubini, “Myoepithelioma of Gengiva. Report of a Case,” Journal of Periodontology, Vol. 70, No. 6, 1999, pp. 683-687. doi:10.1902/jop.1999.70.6.683
- R. H. W. Simpson, H. Jones and P. Beasley, “Benign Myoepithelioma of the Salivary Glands: A True Entity?” Histopathology, Vol. 27, No. 1, 1995, pp. 1-9. doi:10.1111/j.1365-2559.1995.tb00284.x
- G. L. Ellis and P. L. Auclair, “Tumours of the Salivary Glands,” Armed Forces Institute of Pathology, Washington DC, 1996.
- J. G. Batsakis, B. Kraemer and J. J. Sciubba, “The Pathology of Head and Neck Tumours: The Myoepithelial Cell and Its Participation in Salivary Gland Neoplasia, Part 17,” Head and Neck Surgery, Vol. 5, No. 3, 1983, pp. 222-233. doi:10.1002/hed.2890050307
- I. Dardick and A. W. Van Nostrand, “Myoepithelial Cells in Salivary Gland Tumour,” Head and Neck Surgery, Vol. 7, No. 5, 1985, pp. 395-408. doi:10.1002/hed.2890070509
- R. L. Corio, J. J. Sciubba and R. B. Brannon, “Epithelial-Myoepithelial Carcinoma of Intercalated Duct Origin,” Oral Surgery, Vol. 53, No. 3, 1982, pp. 280-287. doi:10.1016/0030-4220(82)90304-8
- S. H. Thompson, S. Bender and A. Richards, “Plasmacytoid Myoepithelioma of a Minor Salivary Glands,” Journal of Oral and Maxillofacial Surgery, Vol. 43, No. 4, 1985, pp. 285-288. doi:10.1016/0278-2391(85)90289-7
- J. Taylor and J. V. Tighe, “A Minor Salivar Gland Tumour Presenting with Dysphagia,” The Journal of Laryngology & Otology, Vol. 113, No. 8, 1999, pp. 569-572. doi:10.1017/S0022215100144512
- I. Dardick, M. J. Thomas and A. W. Van Nostrand, “Myoepithelioma—New Concepts of Histology and Classification: A Light and Electron Microscopic Study,” Ultrastructural Pathology, Vol. 13, No. 2-3, 1989, pp. 187-224. doi:10.3109/01913128909057442
- I. Dardick, “Myoepitheliomas: Definitions and Diagnostic Criteria,” Ultrastructural Pathology, Vol. 19, No. 5, 1995, pp. 359-369. doi:10.3109/01913129509021906
- A. K. El-Naggar, M. Lovell, D. L. Callender, N. G. Ordonez and A. M. Killary, “Cytogenetic Analysis of a Primary Salivary Gland Myoepithelioma,” Cancer Genetics and Cytogenetics, Vol. 113, No. 1, 1999, pp. 49-53. doi:10.1016/S0165-4608(98)00280-5
- L. Alos, A. Cardesa, J. A. Bombi, C. Mallofre, A. Cuchi and J. Traserra, “Myoepithelial Tumours of Salivary glands: A Clinicopathologic, Immunohistochemical, Ultrastructural and Flow-Cytometric Study,” Seminars in Diagnostic Pathology, Vol. 13, No. 2, 1996, pp. 138-147.
- M. J. Merino and V. A. LiVolsi, “Pleomorphic Adenomas of the Parotid Gland Resembling Mesenchymal Tumours,” Oral Surgery, Vol. 44, No. 3, 1977, pp. 405-410. doi:10.1016/0030-4220(77)90410-8
- I. Dardick and A. P. W. Van Nostrand, “Polymorphus Low-Grade Adenocarcinoma: A Case Report with Ultrastructural Findings,” Oral Surgery, Oral Medicine, Oral Pathology, Vol. 66, No. 4, 1988, pp. 459-465. doi:10.1016/0030-4220(88)90269-1
- T. Nagao, I. Sugano and Y. Ishida, “Salivary Gland Malignant Myoepithelioma. A Clinicopathologic and Immunohistochemical Study of Ten Cases,” Cancer, Vol. 83, No. 7, 1988, pp. 1292-1299. doi:10.1002/(SICI)1097-0142(19981001)83:7<1292::AID-CNCR4>3.0.CO;2-L

