International Journal of Otolaryngology and Head & Neck Surgery
Vol.1 No.3(2012), Article ID:24780,8 pages DOI:10.4236/ijohns.2012.13014
Localization of Active Caspase-3 and Caspase-8 in Nasal Polyps and Nasal Hyperplasia in Consideration of Mast Cell Function: A Semiquantitatively Analysis
1Department of Dermatology, University Hamburg-Eppendorf, Hamburg, Germany
2Department of Histology, University of Goettingen, Goettingen, Germany
3Department of Otorhinolaryngology, University of Goettingen, Goettingen, Germany
4Institute of Pathology, Klinikum Kassel, Kassel, Germany
5Dulbecco Telethon Institute, Department of Biology, University of Rome Tor Vergata, Rome, Italy
6IRCCS Fondazione Santa Lucia, Rome, Italy
7Anatomy, National University of Ireland, Galway, Ireland
8Department of Audiology and Phoniatrics, Charite, Medical University of Berlin, Berlin, Germany
Email: *saskia.rohrbach@charite.de
Received September 28, 2012; revised October 26, 2012; accepted November 5, 2012
Keywords: Nasal Polyposis; Caspase-3; Caspase-8; Allergic Rhinitis; Non-Allergic Rhinitis; Mast Cells; Nasal Pathology; Chronic Rhinosinusitis; Apoptosis; Nasal Hyperplasia
ABSTRACT
Introduction: The pathogenesis of nasal polyposis and nasal hyperplasia is still unknown. The localization of caspases in nasal polyps and nasal hyperplasia of patients with and without allergic rhinitis was studied. Methods: Sections of human nasal polyps (n = 5) and hyperplastic nasal turbinates (5 with, 5 without allergy) were stained for active caspase- 3 and caspase-8. Double immunofluorescence was used to evaluate colocalization of the caspases with Ki-M1P and tryptase. TUNEL was performed. Results: Active caspase-3 and caspase-8 were seen in nearly all nasal polyps and hyperplastic nasal turbinates. Active caspase-3 was predominantly localized in stromal cells, identified as mast cells. Caspase-8 was localized in mast cells with the pattern similar to active caspase-3 and additionally found in epithelial cells at the nasal and polyp surface and in epithelial cells of glands. Conclusion: Our results suggest that mast cell apoptosis may be involved in the pathological mechanisms which characterize and sustain chronic inflammatory disorders of the nasal mucosa with and without allergy.
1. Introduction
Over the last decade chronic inflammatory nasal diseases have become more and more frequent and the rate of their occurrence continues to grow. This trend is not solely attributable to the incidence of allergic rhinitis. Nasal polyposis is the ultimate manifestation of chronic inflammation of the upper airway mucosa [1]. Though not fatal, nasal polyposis is a common nasal disease with a high rate of recurrence [2]. The pathogenesis of nasal polyposis is still enigmatic. Several investigations give evidence that the accumulation of inflammatory cells, especially mast cells and eosinophils and their increased amount of inflammatory mediators have enormous influence on polyp development [3-5]. Apoptosis, often defined as programmed cell death, is an essential mechanism including several important physiological and pathological processes to eliminate unwanted cells during the development, to initiate remodelling of tissues and to regulate homeostasis of multicellular organisms [6,7]. An imbalance between apoptosis and survival is responsible for various diseases, like ischemic damage, neurodegenerative and autoimmune diseases or cancer [8-10]. A deregulation of this system also seems to play a central role in the pathogenesis of nasal polyposis [2,11,12]. Several investigations give evidence that the accumulation of infiltrating inflammatory cells in nasal polyposis is caused by a decrease in apoptosis [13-15]. Kowalski et al. (2002) found a strong positive correlation between the duration of nasal polyposis and the density of apoptotic cells in atopic patients [13]. In their investigation, TUNEL, a nick end-labelling technique to detect DNA strand breaks, and apoptotic morphology was used to identify apoptosis. Caspases are key players in programmed cell death. Depending on the nature of stimuli and cell type, two major caspase activation pathways have been described: The death receptor-mediated caspase-8-dependent extrinsic pathway and the mitochondrial-initiated intrinsic pathway [16-18]. Activated initiator caspases (caspase-8 or -9) start a downstream cascade of effector caspases, such as caspase-3, which cleaves various substrates and leads to the execution of cell death [19,20]. Nevertheless, this pathway is not a one-way mechanism, but to some point can be reversible.Results of the localization of caspases in healthy nasal mucosa are contradictory. Whereas some exclude the presence of caspases in healthy nasal tissue [21], recent studies describe caspase activity in normal mucosa as well as in nasal pathologies [22,23]. In order to understand better the role of caspases during the pathogenesis of chronic nasal diseases, we investigated the localization of caspase-3 and -8 in nasal polyps and in nasal mucosal hyperplasia of patients with and without nasal allergy.
2. Material and Methods
15 patients (7 females, 8 males, mean age 39.14 ± 17.99, range 21 - 70 years) were included in the study. Nasal polyps were obtained from 5 patients (all males, mean age 54 ± 10.37, range 41 - 68) undergoing elective polypectomy or conchotomy at the department for ENT of the Georg-August-University Hospital, Goettingen, Germany. The second group consisted of 10 patients (7 females, 3 males, mean age 41 ± 18.67, range 21 - 70) with hyperplastic middle nasal turbinates (5 with and 5 without nasal allergy). Nasal allergy was defined by a positive personal history in allergic rhinitis symptoms and a positive skin prick test. None of the patients had an ongoing drug treatment. Nasal surgery was unrelated to the goal of this study. The study was approved by the local committee on human experimentation and patients gave their consent to participate. Tissue fixation, HE-staining and immunohistochemistry were done as described elsewhere [24] by using caspase-3-antibody (polyclonal rabbit-anti-human/ mouse-caspase-3-active-antibody, R & D Systems, Wiesbaden, Germany), diluted 1:500 in TBS-BSA 1% for 2 hours and bridge-antibody (goat-anti-rabbit-immunoglobulin-antibody, DAKO A/S, Glostrup, Denmark), diluted 1:50 with TBS-BSA.
For localization of caspase-8 the indirect immunoperoxidase-method was applied using monoclonal mouseanti-human-FLICE/caspase-8-antibody as first antibody (Beckman Coulter, Krefeld, Germany) over night in a dilution of 1:100 in TBS-BSA and a peroxidase conjugated rabbit-anti-mouse-immunoglobulin-antibody as second antibody (DAKO A/S, Glostrup, Denmark), incubated for 30 minutes, diluted 1:50 in TBS-BSA. DABTris-HCl-solution served as chromogen. Rinsing between all steps was performed in TBS-buffer for 10 minutes. Incubation of antibodies was done in a humid chamber at room temperature. The final steps included counterstaining with hemalaun (according to Mayer) and mounting with DPX (Fluka, Steinheim, Germany). Controls included omission of the primary antibodies and replacement by TBS/BSA 1% in every charge.
For double immunofluorescence staining paraffin sections were put on silanized glass slides, deparaffinised, treated with boiling citrat buffer 3 times for 5 minutes in a microwave, cooled down and rinsed with water and TBS. Endogenous peroxidase activity was blocked with 10% BSA. Anti-caspase-3-antibody- (diluted 1:100 in TBS) versus anti-caspase-8-antibody (diluted 1:50 in TBS) served as first antibody, incubated over night in a humid chamber. Sections were then incubated with LINK (goat-antimouse/rabbit, DAKO, A/S, Glostrup, Denmark) for 30 minutes. From this point all steps were carried out in darkness: Incubation with streptavidin FITC (DAKO A/S, Glostrup, Denmark, diluted 1:40 in TBS, marked with green fluorescence) for 60 minutes and incubation with an unmarked F(ab)-fragment (goatanti-mouse, Dianova, Hamburg, Germany, diluted 1:50 in TBS) for further 30 minutes followed. To identify the origin of the caspase-positive free connective tissue cells, a primary antibody against mast cell tryptase (mouseanti-human, DAKO, A/S, Glostrup, Denmark, diluted 1:100 in TBS) versus Ki-M1P (monoclonal, mouse-antihuman, Radzun et al. [25], diluted 1:500 in TBS) was then added. A Cy3-marked F(ab)-fragment (goat-antimouse, Dianova, Hamburg, Germany, diluted 1:25 in TBS, marked with red fluorescence) served for staining. Rinsing between all steps was done in TBS-buffer for 5 minutes. Fluorescent-mounting medium (DAKO, Carpinteria, CA, USA) was used. Qualitative assessment: The extent of caspase-positive free connective tissue cells was graded semiquantitatively with a light microscope “KF 2” (Zeiss, Oberkochen, Germany) as (++) if there was a high amount of caspase-3/-8-positive cells, (+) if there was only a small extent of caspase-3/-8-positive cells, and (-) if no caspase-3/-8-positive cells could be detected. In the epithelial cells at the nasal and polyp surface and in the epithelial cells of the glands the staining for caspase-3 and -8 was graded as (+) if a clear staining was detected, and as (-) if there was no clear staining for caspase-3/-8. In these epithelial cells the clear staining was separated over again in a clear granular staining (1), a fine, sporadic granular staining (2) and a homogeneous staining (3). TUNEL-staining: The TUNEL assay was conducted with the ApopTag Fluorescin In Situ Apoptosis detection kit by Intergen (cat. S7111, Billerica, MA, USA) according to manufacturer’s instructions. Images of the labelled cells were taken with a fluorescence inverted-microscope (Leica DFC 350 Fx, Bensheim, Germany, Software Qwin Image 4.0).
3. Results
Localization and quantitative evaluation of active caspase-3 and caspase-8 in nasal polyps and in hyperplastic nasal mucosa of non-allergic and allergic patients are summarized in Tables 1-6. In nasal polyps, active caspase-3 was detected in 4 of the 5 nasal polyps (see Table 1). It was mainly localized in free connective tissue cells (Figure 1), whereas a few free intraepithelial cells, positive for active caspase-3, presumably inflammatory cells, were present at the polyp surface. A colocalization for active caspase-3 and mast cell tryptase in free connective tissue cells (Figures 2-4) was seen. In contrast, no colocalization for active caspase-3 and Ki-M1P was detected. Caspase-8 was found in all 5 nasal polyps (see Table 2). Similar to caspase-3, caspase-8 was localized in free connective tissue cells (Figure 5) and in one case also in free cells within the epithelium at the polyp surface. It was additionally found in epithelial cells at the polyp surface itself and in epithelial cells of glands. In most of this tissue, a clear staining for caspase-8 was noted within epithelial cells at the polyp surface, whereas epithelial cells of glands showed a clear staining for caspase-8 only in one nasal polyp, basically in excretory ducts. A colocalization for caspase-8 and mast cell tryptase in free connective tissue cells of the nasal polyps (Figures 6-8) was seen. Similar to caspase-3, no colocalization for caspase-8 and Ki-M1P was apparent. In hyperplastic nasal mucosa of patients without nasal allergy, active caspase-3 was localized in free connective tissue cells in 4 of 5 hyperplastic nasal turbinates (see Table 3, Figure 9). By double immunofluorescence, a colocalization for active caspase-3 and mast cell tryptase in the free connective tissue cells was found, whereas no colocalization for active caspase-3 and Ki-M1P was detected. Caspase-8 was found in all specimens (see Table 4), and in free connective tissue cells (Figure 10). Four of them showed clear staining of epithelial cells at the nasal surface. 4 of 5 hyperplastic nasal turbinates showed caspase- 8-positive free cells within the epithelium at the nasal surface. Epithelial cells of glands were stained irregularly (Figure 10, inlay down to the left). Double immunofluorescence staining for caspase-8 and mast cell tryptase showed a colocalization in the free connective tissue cells, but no colocalization for caspase-8 and Ki-M1P. In hyperplastic nasal turbinates of patients with nasal allergy, localization of active caspase-3 and caspase-8 corresponded to that of the tissue of patients without nasal allergy (see Tables 5 and 6, Figures 11 and 12); in one case this staining was predominantly localized in the excretory ducts (patient 12). TUNEL-staining revealed a clear and specific signal for a few positive cells per section, recognizable in all samples scattered in the stroma, from both the hyperplastic nasal turbinates of patients
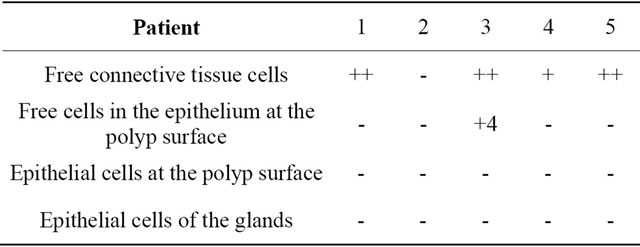
Table 1. Localization of caspase-3 active in nasal polyps.
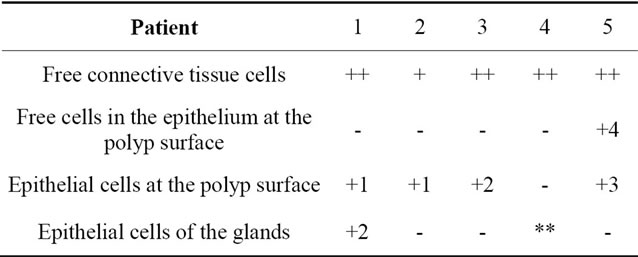
Table 2. Localization of caspase-8 in nasal polyps.
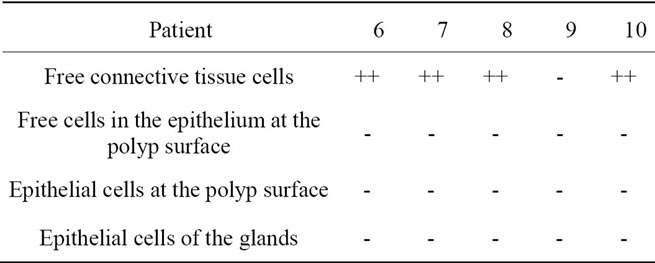
Table 3. Localization of caspase-3 active in hyperplastic nasal mucosa without nasal allergy.
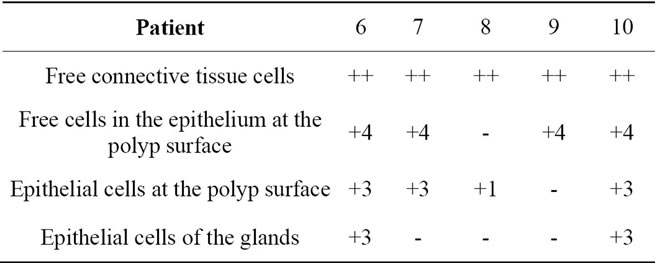
Table 4. Localization of caspase-8 in hyperplastic nasal mucosa without nasal allergy.
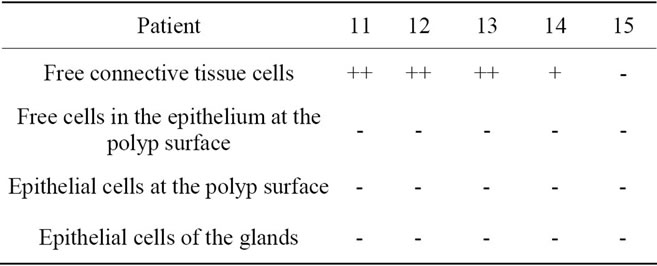
Table 5. Localization of caspase-3 active in hyperplastic nasal mucosa with nasal allergy.
with or without nasal allergy and the nasal polyps, as revealed by fluorescence microscopy (Figures 13(A)-(E)). Additionally, isolated positivecells for the TUNEL staining were also found within the epithelium in some sections (Figure 13(C)). Interestingly, TUNEL-positive signal was also revealed in the cytoplasm of some cells in the stroma (Figure 13(E)).
4. Discussion
The accumulation of inflammatory cells, especially mast cells, eosinophils and their inflammatory mediators seem to have a key role in the development of nasal polyps [26,27]. Caspase activation takes up an important posi-
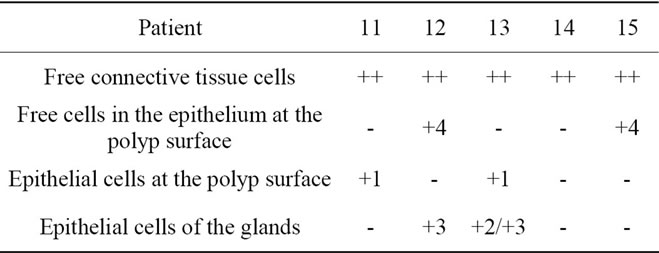
Table 6. Localization of caspase-8 in hyperplastic nasal mucosa with nasal allergy.
Legend for free connective tissue cells: (++) high extent/amount of caspase-3/-8-positive cells; (+) small extent of caspase-3/-8-positive cells; (-) no clear staining for caspase-3/-8. Legend for free cells within the epithelium at the polyp and nasal surface, epithelial cells at the polyp and nasal surface and epithelial cells of the glands: (+) clear staining for caspase-3/-8; (-) no clear staining for caspase-3/-8; (**) no glandular structures in the tissue; 1) clear granular staining; 2) fine, sporadic granular staining; 3) homogeneous staining clearly visible; 4) few caspase-3/-8-positive free cells in the epithelium at the polyp and nasal surface.
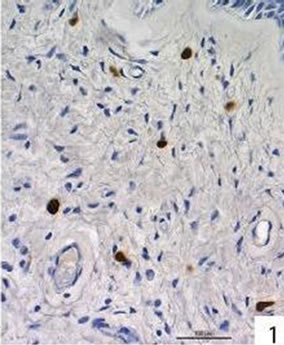
Figure 1. Active caspase-3 was seen in 4 of the 5 nasal polyps. Immunohistochemical localization of active caspase-3 in free cells in the connective tissue of a nasal polyp.
Figure 2. Double immunofluorescence staining for active caspase-3 in a nasal polyp.
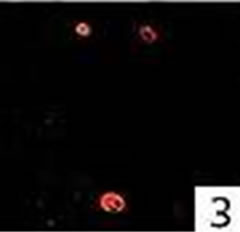
Figure 3. Double immunofluorescence staining for mast cell tryptase in a nasal polyp.
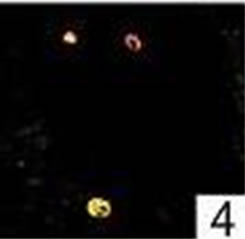
Figure 4. Double immunofluorescence staining: colocalization for active caspase-3 and mast cell tryptase in a nasal polyp. No colocalization for active caspase-3 and Ki-M1P was detected.
Figure 5. Caspases-8 was detected in all 5 polyps. Immunohistochemical localization of caspase-8 in free cells in the connective tissue of a nasal polyp. Caspase-8 was also found in free cells within the epithelium at the surface of one polyp, in epithelial cells at the polyp surface and in epithelial cells of glands (the latter 3 localizations are not shown).
Figure 6. Double immunofluorescence staining for caspase-8 in a nasal polyp.

Figure 7. Double immunofluorescence staining for mast cell tryptase in a nasal polyp.

Figure 8. Double immunofluorescence staining: colocalization for caspase-8 and mast cell tryptase in a nasal polyp. No colocalization for caspase-8 and Ki-M1P was detected.
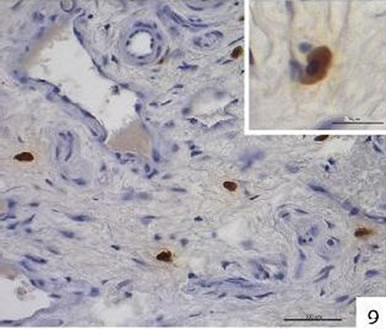
Figure 9. Immunohistochemical localization of active caspase-3 in free cells in the connective tissue of hyperplastic nasal mucosa (without allergic rhinitis). Inlay up to the right: active caspase-3-positive cell.
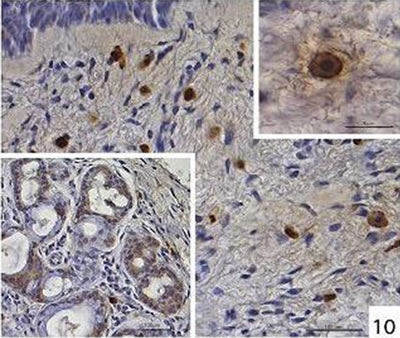
Figure 10. Immunohistochemical localization of caspase-8 in free cells in the connective tissue of hyperplastic nasal mucosa (without allergic rhinitis). Inlay up to the right: caspase-8-positive cell. Inlay down to the left: localization of caspase-8 in the glands in hyperplastic nasal mucosa (without allergic rhinitis), diffuse irregular staining.
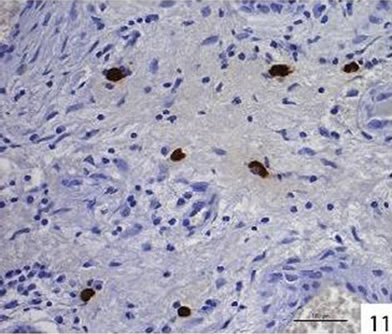
Figure 11. Immunohistochemical localization of active caspase-3 in free cells in the connective tissue of hyperplastic nasal mucosa (with allergic rhinitis).
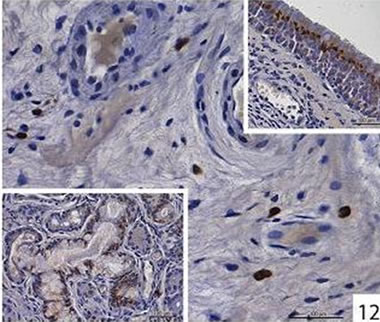
Figure 12. Immunohistochemical localization of caspase-8 in free cells in the connective tissue of hyperplastic nasal mucosa (with allergic rhinitis). Inlay up to the right: localization of caspase-8 in the surface epithelium in hyperplastic nasal mucosa (with allergic rhinitis), granular staining. Inlay down to the left: Localization of caspase-8 in the glands in hyperplastic nasal mucosa (with allergic rhinitis), granular staining.
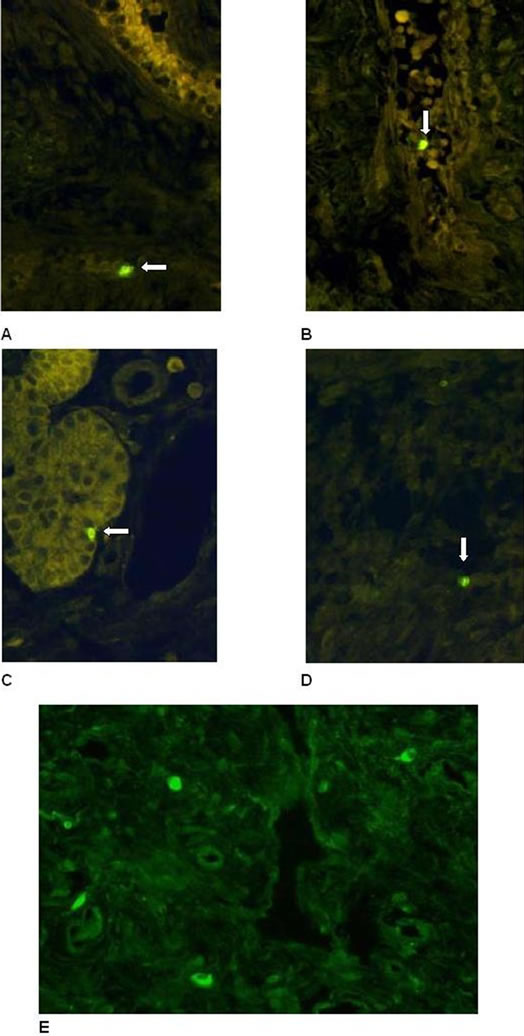
Figure 13. (A) + (B): TUNEL-staining. Hyperplastic nasal mucosa (without allergic rhinitis, (A)) and nasal polyp (B) with scattered positive cells in the stroma (arrow). (C) + (D): TUNEL-staining. Hyperplastic nasal mucosa (with allergic rhinitis) with scattered positive cells in the stroma (D). Note the isolated positive cells localized additionally within the epithelium ((C), arrow). (E) TUNEL-staining. Nasal polyp. Positive signals were detected in the cytoplasm of some cells in the stroma (arrow).
tion in the execution of apoptosis [7]. Nevertheless, little is known about the cellular localization of caspases and their involvement in chronic inflammatory nasal diseases, entities in which apoptosis could be reduced or even increased. Our study reveals the localization of caspase-3 and caspase-8 in human nasal polyps and hyperplastic nasal mucosa in patients with and without nasal allergy. Lately, rare caspase-3 activity has been shown in nasal mucosa of the lower nasal turbinates in healthy controls, whereas no or isolated caspase-3 activity in allergic rhinitis and defined activity in the endothelium and in the lamina muscularis of subepithelial vessels in polyposis nasi was detected [22]. Cho et al. (2008) found no significant differences in the level of expression of caspase-3 between normal mucosa and nasal polyps [23]. We found presence of both active caspase-3 and caspase-8 within nearly all nasal polyps and hyperplastic nasal turbinates primarily localized in scattered cells in the stroma without substantial differences in all groups. These results implicate similar involvement of active caspase-3 and of caspase-8 in the cellular pathological mechanisms of nasal polyposis and hyperplasia of nasal mucosa, regardless of the coincidence of nasal allergy. Double immunofluorescence labelling revealed localization of both caspases in mast cells, whereas localization in macrophages could be excluded. Caspase-3 is classically activated as one of the common effectors in the apoptotic cascade [19,20]. Its presence in mast cells of nasal polyps and hyperplastic nasal mucosa points out its participation in chronic nasal disorders. This is corroborated by the fact that scattered TUNEL-positive cells were found in connective tissue, indicating occurrence of apoptotic cell death in the stroma of the hyperplastic nasal turbinates. The fact that caspase-positive staining was widespread in the cytoplasm of mast cells points out a slow and long lasting activation of caspase-3 before DNA degradation is finally triggered [28]. Mast cells are known to undergo apoptosis, both in a caspase-dependent and a caspase-independent manner [29]. However, they are exceptionally long-living cells (up to months). Their number is, at least under normal in vivo conditions, relatively constant, suggesting that they are normally not programmed for spontaneous apoptosis [30]. Therefore, it is likely that elevated activation of caspases-3 in mast cells may be part of the mechanisms which underline pathogenesis of nasal polyps and mucosal hyperplasia. The presence of TUNEL-positive cells in the stroma underlines that cell death occurs in this cellular population. Apoptosis has been described as an essential host defence mechanism in order to prevent spreading of infections and to eliminate unwanted cells during tumour growth [31,32]. Some groups have reported caspase-3 and -8-mediated apoptosis in human mast cells in vitro or in mastocytoma and in various inflammatory and fibrotic diseases as liver fibrosis, Crohn’s and chronic graft-versus-host-disease [31,33,34]. Based on their in-vitro investigations of human mast cell lines, Berent-Moaz et al. (2006) proposed that the extrinsic apoptotic pathway, mediated by the death receptor TRAIL-R (tumor necrosis factor-related apoptosis-inducing ligand) and resulting in caspase-3 activation, might be a mechanism of regulating mast cell survival in vivo and, potentially, for downregulating or resolving mast cell hyperplasia in diseases [35]. According to this, caspase detection in mast cells of nasal polyps and hyperplastic nasal mucosa, in vivo as shown in this work, could reduce and resolve or even prevent mast cell hyperplasia. Another reason for the presence of active caspase-3 and -8 in mast cells in chronic nasal diseases may not primarily be related to ongoing cell death, but may indicate the involvement in maturation and processing of pro inflammatory cytokines, as has been reported for other caspases [36]. Mast cells are a cellular source of IL-16, a potential chemotaxin for CD+ T-cells and CD4+ eosinophils [37]. Eosinophils in turn are known to be important effectors in polyposis nasi and allergic rhinitis, so that caspase-3 may play a mature role in the inflammatory process of chronic nasal disorders. Whereas active caspase-3 was nearly exclusively stromal, caspase-8 was additionally found almost consistently in the cytoplasm of epithelial cells at the nasal and polyp surface and in glands. The fact that just some TUNEL-positivity was seen in epithelia, and that staining of active caspase-3 in these compartments was limited to intraepithelial inflammatory cells, suggests that no massive death is occurring in epithelial cells. The caspase-8 antibody used in our study cannot distinguish between the active and inactive form of caspase-8. We propose that caspase-8 might be constitutionally expressed in the nasal epithelium and just waits for a proper signal to be activated. Whether or not this is normally the case in all epithelia or if it is a hallmark of chronically damaged tissues, remains unclear to this point and requires further investigation. Interestingly, in some cells, TUNEL signal was clearly evident in the cytoplasm.
As it has been described for olfactory sensory neurons [38], phagocytic cells might engulf dying cells which contain fragmented DNA. Phagocytic cells are negative for caspase-3 and -8, and therefore are not expected to be TUNEL-positive. The presence of phagocyted DNAfragments in their cytoplasm might explain the cytoplasm positivity to TUNEL. It might indicate that for example cell death in the nasal stroma contributes to these pathologies.
5. Conclusion
We showed expression of active caspase-3 and caspase-8 in stroma cells, which have been identified as mast cells. This together with the presence of TUNEL suggests that ongoing cell death of mast cells characterize and sustain chronic inflammatory disorders of the nasal mucosa.
6. Acknowledgements
We would like to thank Rod Dungan, Elke Heyder, Berti Manshausen, Sonja Schwoch and Christina Zelent for advisory help in preparing histology, immunohistology and photografic documentation.
REFERENCES
- J. S. Lacroix, C. G. Zheng, S. H. Goytom, B. Landis, I. Szalay-Quinodoz and D. D. Malis, “Histological Comparison of Nasal Polyposis in Black African, Chinese and Caucasian Patients,” Rhinology, Vol. 40, No. 3, 2002, pp. 118-121.
- S.-Y. Fang and B.-C. Yang, “Overexpression of FasLigand in Human Nasal Polyps,” Annals of Otology, Rhinology and Laryngology, Vol. 109, No. 3, 2000, pp. 267- 270.
- G. Di Lorenzo, A. Drago, M. Esposito Pellitteri, G. Candore, A. Colombo, F. Gervasi, M. L. Pacor, F. Purello D’Ambrosio and C. Caruso, “Measurement of Inflammatory Mediators of Mast Cells and Eosinophils in Native Nasal Lavage Fluid in Nasal Polyposis,” International Archives of Allergy and Immunology, Vol. 125, No. 2, 2001, pp. 164-175. doi:10.1159/000053811
- G. Di Lorenzo, P. Mansueto, M. Melluso, G. Candore, A. Colombo, M. E. Pellitteri, et al., “Allergic Rhinitis to Grass Pollen: Measurement of Inflammatory Mediators of Mast-Cell and Eosinophils in Native Nasal Fluid Lavage and in Serum out of and during Pollen Season,” Journal of Allergy and Clinical Immunology, Vol. 100, No. 6, 1997, pp. 832-837.
- A. Davidsson, T. Andersson and H. B. Hellquist, “Apoptosis and Phagocytosis of Tissue-Dwelling Eosinophils in Sinunasal Polyps,” The Laryngoscope, Vol. 110, No. 1, 2000, pp. 111-116. doi:10.1097/00005537-200001000-00020
- M. Raff, “Cell Suicide for Beginners,” Nature, Vol. 396, No. 6707, 1998, pp. 119-122. doi:10.1038/24055
- M. D. Jacobson, M. Weil and M. C. Raff, “Programmed Cell Death in Animal Development,” Cell, Vol. 88, No. 3, 1998, pp. 347-354. doi:10.1016/S0092-8674(00)81873-5
- I. Jeremias, D. Reinhardt and K. M. Debatin, “Impaired Apoptosis Regulation as Cause for Illness,” HNO, Vol. 49, No. 8, 2001, pp. 673-683. doi:10.1007/s001060170070
- J. B. Schulz, M. Weller and M. A. Moskowitz, “Caspases as Treatment Targets in Stroke and Neurodegenerative Diseases,” Annals of Neurology, Vol. 45, No. 4, 1999, pp. 421-429. doi:10.1002/1531-8249(199904)45:4<421::AID-ANA2>3.0.CO;2-Q
- U. Törmänen-Näpänkangas, Y. Soini, V. Kinulla and P. Pääkkö, “Expression of Caspases-3, -6, and -8 and Their Relation to Apoptosis in Non-Small Cell Lung Carcinoma,” International Journal of Cancer, Vol. 93, No. 2, 2001, pp. 192-198. doi:10.1002/ijc.1315
- C. Delbrouck, H.-J. Gabius, H. Kaltner, C. Decaestecker, R. Kiss and S. Hassid, “Expression Patterns of Galectin-1 and Galectin-3 in Nasal Polyps and Middle and Inferior Turbinates in Relation to Growth Regulation and Immunosuppression,” Archives of Otolaryngology—Head & Neck Surgery, Vol. 129, No. 6, 2003, pp. 665-669. doi:10.1001/archotol.129.6.665
- H. Pacova, J. Astl and J. Martinek, “The Pathogenesis of Chronic Inflammation and Malignant Transformation in the Human Upper Airways: The Role of Beta-Defensins, eNOS, Cell Proliferation and Apoptosis,” Histology and Histopathology, Vol. 24, No. 7, 2009, pp. 815-820.
- M. L. Kowalski, J. Grzegorczyk, R. Pawliczak, T. Kornatowski, M. Wagrowska-Danilewicz and M. Danilewicz, “Decreased Apoptosis and Distinct Profile of Infiltrating Cells in Nasal Polyps of Patients with Aspirin Hypersensitivity,” Allergy, Vol. 57, No. 6, 2002, pp. 493-500. doi:10.1034/j.1398-9995.2002.13508.x
- H. U. Simon, S. Yousefi, C. Schranz, A. Schapowal, C. Bachert and K. Blaser, “Direct Demonstration of Delayed Eosinophil Apoptosis as a Mechanism Causing Tissue Eosinophilia,” Journal of Immunology, Vol. 158, No. 8, 1997, pp. 3902-3908.
- H. U. Simon, “Molecular Mechanisms of Defective Eosinophil Apoptosis in Diseases Associated with Eosinophilia,” International Archives of Allergy and Immunology, Vol. 113, No. 1-3, 1997, pp. 206-208. doi:10.1159/000237548
- B. M. Polster and G. Fiskum, “Mitochondrial Mechanisms of Neural Cell Apoptosis,” Journal of Neurochemistry, Vol. 90, No. 6, 2004, pp. 1281-1289.
- U. Sartorius, I. Schmitz and P. H. Krammer, “Molecular Mechanisms of Death-Receptor-Mediated Apoptosis,” Chembiochem, Vol. 2, No. 1, 2001, pp. 20-29.
- M. O. Hengartner, “The Biochemistry of Apoptosis,” Nature, Vol. 407, No. 6805, 2000, pp. 770-776. doi:10.1038/35037710
- P. E. Mirkes, “2001 Warkany Lecture: To Die or Not to Die, the Role of Apoptosis in Normal and Abnormal Development,” Teratology, Vol. 65, No. 5, 2002, pp. 228- 239. doi:10.1002/tera.10049
- M. G. Grütter, “Caspases: Key Players in Programmed Cell Death,” Current Opinion in Structuarl Biology, Vol. 10, No. 6, 2000, pp. 649-655. doi:10.1016/S0959-440X(00)00146-9
- M. Trimarchi, A. Miluzio, P. Nicolai, M. L. Morassi, M. Bussi and P. C. Marchisio, “Massive Apoptosis Erodes Nasal Mucosa of Cocaine Abusers,” American Journal of Rhinology, Vol. 20, No. 2, 2006, pp. 160-164.
- R. Hirt, F. Paulsen, K. Neumann and S. Knipping, “Immunocytochemical Detection of Caspase 3 in Various Diseases of Human Nasal Mucosa,” HNO, Vol. 57, No. 5, 2009, pp. 466-472. doi:10.1007/s00106-009-1905-4
- S. H. Cho, S. H. Lee, K. R. Kim, H. M. Lee, S. H. Lee and T. H. Kim, “Expression and Distribution Patterns of the Inhibitor of Apoptosis Protein Family and Caspase 3 in Nasal Polyps,” Archives of Otolaryngology—Head & Neck Surgery, Vol. 134, No. 3, 2008, pp. 316-321. doi:10.1001/archotol.134.3.316
- S. Rohrbach, A. Olthoff, R. Laskawi and W. Goetz, “Neuronal Nitric Oxide Synthase-Immunoreactivity. A Neuromodulating System Independent of Peripheral Nasal Gland Denervation in Guinea Pig Nasal Mucosal Tissue after Treatment with Botulinum Toxin Type A,” ORL, Vol. 64, No. 5, 2002, pp. 330-334.
- H. J. Radzun, M. L. Hansmann, H. J. Heidebrecht, S. Bödewadt-Radzun, H. H. Wacker, H. Kreipe, et al., “Detection of Monocyte/Macrophage Differentiation Antigen in Routinely Processed Paraffin-Embedded Tissue by Monoclonal Antibody Ki-M1P,” Laboratory Investigation, Vol. 65, No. 3, 1991, pp. 306-315.
- S. B. Haudek, G. E. Taffet, M. D. Schneider and D. L. Mann, “TNF Provokes Cardiomyocyte Apoptosis and Cardiac Remodelling through Activation of Multiple Cell Death Pathways,” Journal of Clinical Investigation, Vol. 117, No. 9, 2007, pp. 2692-2701. doi:10.1172/JCI29134
- R. Pawankar, “Nasal Polyposis: An Update,” Current Opinion in Allergy Clinical Immunology, Vol. 3, No. 1, 2003, pp. 1-6. doi:10.1097/00130832-200302000-00001
- S. Ohsawa, S. Hamada, H. Yoshida and M. Miura, “Caspase-Mediated Changes in Histone H1 in Early Apoptosis: Prolonged Caspase Activation in Developing Olfactory Sensory Neurons,” Cell Death and Differentiation, Vol. 15, No. 9, 2008, pp. 1429-1439. doi:10.1038/cdd.2008.71
- H. Yoshikawa and K. Tasaka, “Caspase-Dependent and -Independent Apoptosis of Mast Cells Induced by Withdrawal of IL-3 Is Prevented by Toll-Like Receptor 4-Mediated Lipopolysaccharide Stimulation,” European Journal of Immunology, Vol. 33, No. 8, 2003, pp. 2149- 2159. doi:10.1002/eji.200323270
- J. Padawer, “Mast Cells: Extended Lifespan and Lack of Granule Turnover under Normal in Vivo Conditions,” Experimental and Molecular Pathology, Vol. 20, No. 2, 1974, pp. 269-280. doi:10.1016/0014-4800(74)90059-8
- C. E. Jenkins, A. Swiatoniowski, A. C. Issekutz and T.-J. Lin, “Pseudomonas Aeruginosa Exotoxin A Induces Human Mast Cell Apoptosis by a Caspase-8 and -3-Dependent Mechanism,” Journal of Biological Chemistry, Vol. 279, No. 35, 2004, pp. 37201-37207. doi:10.1074/jbc.M405594200
- A. Nakagawara, “Molecular Basis of Spontaneous Regression of Neuroblastoma: Role of Neurotrophic Signals and Genetic Abnormalities,” Human Cell, Vol. 11, No. 3, 1998, pp. 115-124.
- T. Inoue, K. Yoneda, M. Kakurai, S. Fujita, M. Manabe and T. Demitsu, “Alteration of Mast Cell Proliferation/ Apoptosis and Expression of Stem Cell Factor in the Regression of Mastocytoma—Report of a Case and a Serial Immunhistochemical Study,” Journal of Cutaneous Pathology, Vol. 29, No. 5, 2002, pp. 305-312. doi:10.1034/j.1600-0560.2002.290509.x
- A. M. Piliponsky and F. Levi-Schaffer, “Regulation of Apoptosis in Mast Cells,” Apoptosis, Vol. 5, No. 5, 2000, pp. 435-441. doi:10.1023/A:1009680500988
- B. Berent-Moaz, A. M. Piliponsky, I. Daigle, H. U. Simon and F. Levi-Schaffer, “Human Mast Cell Undergo TRAIL-Induced Apoptosis,” Journal of Immunology, Vol. 176, No. 4, 2006, pp. 2272-2278.
- H. Y. Chang and X. Yang, “Proteases for Cell Suicide: Functions and Regulation of Caspases,” Microbiology and Molecular Biology Reviews, Vol. 64, No. 4, 2000, pp. 821-846. doi:10.1128/MMBR.64.4.821-846.2000
- Y. Zhang, D. Huang and G. Yu, “Survivin Expression and Its Relationship with Apoptosis and Prognosis in Nasal and Paranasal Sinus Carcinomas,” Acta Otolaryngologica, Vol. 125, No. 12, 2005, pp. 1345-1350. doi:10.1080/00016480510043963
- Y. Suzuki, J. Schafer and A. I. Farbmann, “Phagocytic Cells in the Rat Olfactory Epithelium after Bulbectomy,” Experimental Neurology, Vol. 136, No. 12, 1995, pp. 225- 233. doi:10.1006/exnr.1995.1099
NOTES
*Corresponding author.

