International Journal of Otolaryngology and Head & Neck Surgery
Vol.1 No.2(2012), Article ID:21712,6 pages DOI:10.4236/ijohns.2012.12004
The Utility of Fine-Needle Aspiration in the Diagnosis and Management of Follicular Thyroid Neoplasms: One Institution’s 10-Year Experience
1Department of Otolaryngology-Head and Neck Surgery
2Henry Ford Health System, Detroit, Michigan
3Department of Pathology
4University of South Florida, Tampa, Florida
5Department of Public Health Sciences
Email: *tghanem1@hfhs.org
Received March 30, 2012; revised May 7, 2012; accepted May 31, 2012
Keywords: Fine Needle Aspiration; Follicular; Thyroid; Adenoma; Carcinoma
ABSTRACT
Background: Classical teaching dictates that follicular adenoma (FA) can be distinguished from follicular carcinoma (FC) based on histologic features only. We retrospectively reviewed our institution’s 10-year experience in the use of fine-needle aspiration (FNA) to diagnose follicular thyroid neoplasms. Methods: Patients who had FNA of a thyroid neoplasm from 2000 to 2010 were reviewed. Diagnoses of FA, FC, or follicular neoplasm-not otherwise specified (NOS) were included. Cytopathological results were correlated with surgical pathology. Results: Of 138 patients, 65% underwent surgery. FNA diagnosis for FA had a sensitivity of 50% and specificity of 71%. 25% of patients with an FNA diagnosis of FA were found to have cancer after surgical specimen examination. FNA diagnosis for FC had a sensitivity of 60% and specificity of 94%. Conclusions: FNA has a low sensitivity for diagnosing FA. Surgical pathology remains the gold standard for differentiating follicular carcinoma from adenoma.
1. Introduction
Fine-needle aspiration (FNA) has become a prominent diagnostic modality in evaluating many masses in the head and neck. For thyroid disease, FNA has become the initial step in the management of thyroid nodules. The primary purpose of FNA is to provide a rational guideline for the management of patients with thyroid nodules and to allow surgical planning for those requiring surgery. FNA is relatively easy to perform, cost effective, and a non-traumatic procedure to help evaluate any nodule larger than 1 cm in diameter or deemed suspicious on ultrasound [1,2]. Before the routine use of FNA in preoperative workup, only 14% of surgically resected thyroid nodules were found to be malignant [3,4].
FNA cytology has proven to be highly effective in diagnosing papillary thyroid cancer with a sensitivity and specificity approaching 98% [5]. Papillary thyroid cancer is the only thyroid malignancy that is diagnosed based on its nuclear morphology regardless of cytoplasmic features, growth pattern, special stains, and immunohistochemical markers.
The application of FNA to the diagnosis and management of follicular patterned lesions has been more controversial because distinguishing these lesions requires histological evidence of capsular or vascular invasion and metastasis [4]. Currently, no consensus exists on distinguishing follicular carcinoma (FC) from benign follicular adenoma (FA) using FNA alone. Thus all patients with large follicular epithelial cells on FNA are recommended to undergo a diagnostic lobectomy to further evaluate the thyroid nodule.
The spectrum of follicular patterned thyroid lesions is broad (Figure 1), [1] and a variety of classification schemes have been used in their analysis (Figure 2) [2, 6-8]. Various terms used in these schemes include “follicular lesion,” “atypical follicular lesion,” and “follicular neoplasm.” The American Thyroid Association (ATA) and the American Association of Clinical Endocrinologists (AACE) have proposed the term “indeterminate for malignancy.” The most widely accepted classification system is commonly referred to as the Bethesda system which was proposed by the National Cancer Institute in 2008 (Figure 2).
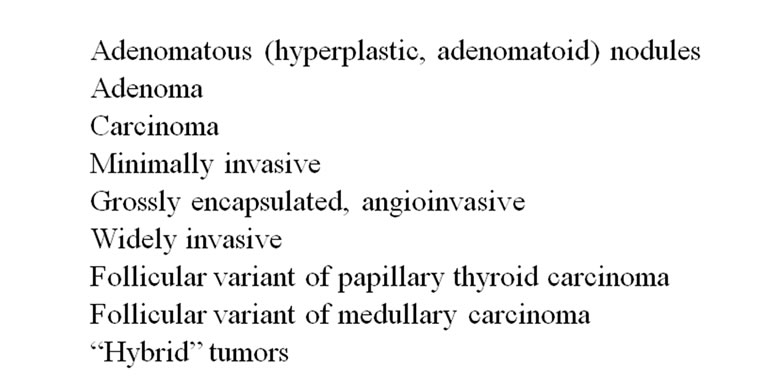
Figure 1. Follicular-patterned thyroid lesions.


Figure 2. Thyroid FNA classification schemes.
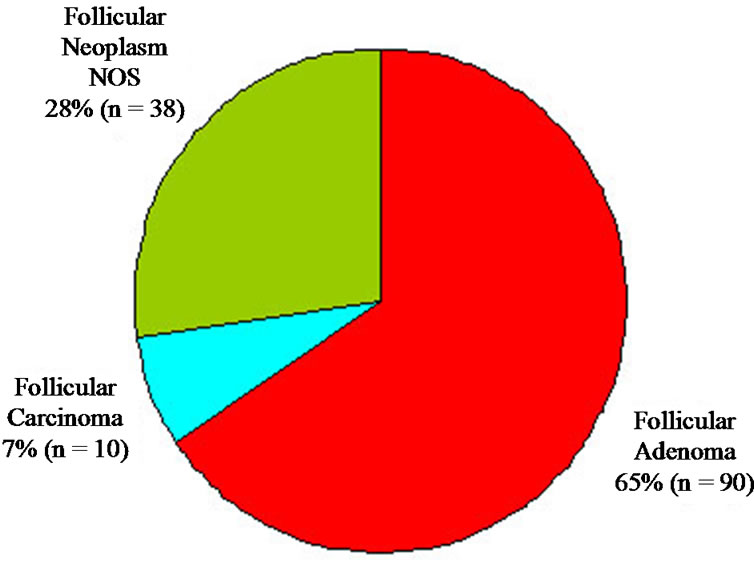
Figure 3. Distribution of all FNA reports.
The interpretation of follicular thyroid lesions is somewhat unique in our institution. Some pathologists believe a confident diagnosis of follicular thyroid cancer can be made based on cytological evaluation alone, supported by a study in which of 158 lesions cytologically interpreted as benign adenoma, 82% were confirmed to be benign after surgical excision [3]. This same study also showed that of 52 FCs diagnosed histologically, 36 (70%) were either suspected or diagnosed cytologically.
The gold standard for diagnosis of follicular carcinoma requires formal histological evaluation of the capsule to identify invasion. However, some institutions, including ours, utilize cytopathological analysis to differentiate follicular adenoma and carcinoma, with the primary purpose to decrease unnecessary thyroidectomies. We sought to evaluate this practice at our institution retrospectively by using an evidence-based approach to determine its validity.
2. Materials and Methods
A database search was performed of all patients who underwent FNA of the thyroid gland at our urban tertiary care hospital between 2000 and 2010. Institutional Review Board approval was obtained. Written informed consent was not required as unique patient identifiers were not used in this study. All patients who were diagnosed with papillary thyroid cancer or nodular goiter based on cytology alone were excluded. The charts of all patients whose diagnosis showed any type of follicular neoplasm were further reviewed. Data collected included age, gender, date of FNA, whether the patient had surgery, and, if so, date of surgery, and type of surgery performed.
Cytopathological diagnoses were grouped into three categories to simplify analysis: 1) definitive diagnosis of FA; 2) definitive diagnosis of FC; and 3) follicular neoplasm-not otherwise specified (NOS). For simplicity of data analysis, all hurthle cell tumors were classified as follicular tumors. Thus, a diagnosis of hurthle cell adenoma was included in category 1 while that of hurthle cell carcinoma was included in category 2. To maintain consistency, phrases in the cytopathology report such as “most consistent with” or “strongly suggestive of” were placed into one of the definitive diagnostic categories (category 1 or 2). Category 3 included cases where “possibility” of a diagnosis was noted in the report and the pathologist would not commit to benign versus malignant diagnosis. For patients who subsequently underwent lobectomy or total thyroidectomy, surgical pathology reports were reviewed to determine if the final histological diagnosis correlated with the aforementioned cytopathologic diagnostic categories.
Chi-square or Fisher Exact test was used to study the association between preoperative FNA diagnosis, the patients who underwent surgery, and FNA confirmation via pathological evaluation after surgery. Sensitivity and specificity were calculated between the FNA diagnosis and its confirmation.
3. Results
A total of 138 patients met inclusion criteria for the study. The mean age of patients was 54 years, with 72% being female. Of 91 patients (66%) who underwent surgery, final histological reports were available in 89. Of these 89 patients, 74 (83%) underwent a total thyroidectomy. Surgery was performed on average 3.8 months after FNA. The distribution of FNA results is shown in Figure 3.
Approximately two-thirds (90 patients) were diagnosed with FA. Of these, 48 patients ( 53%) went on to have surgery with a majority (80%) undergoing total thyroidectomy. Surgical pathology results are shown in Figure 4. The diagnosis of FA was confirmed in only 50% of patients, and a diagnosis of carcinoma, either follicular or papillary, occurred in only 25%. Overall, FNA diagnosis for FA had a sensitivity of 50% and a specificity of 71%.
53%) went on to have surgery with a majority (80%) undergoing total thyroidectomy. Surgical pathology results are shown in Figure 4. The diagnosis of FA was confirmed in only 50% of patients, and a diagnosis of carcinoma, either follicular or papillary, occurred in only 25%. Overall, FNA diagnosis for FA had a sensitivity of 50% and a specificity of 71%.
Among patients with FNA diagnosis of FC, all 10 patients (100%) went on to have total thyroidectomy. The surgical pathology results are summarized in Figure 5. A diagnosis of FC was confirmed in six patients (60%), with an additional two patients found to have papillary carcinoma. Overall, FNA diagnosis for FC had a sensitivity of 60% and specificity of 94%.
Among patients with an FNA diagnosis of follicular neoplasm-NOS, 31 patients (82%) went on to have surgery. The surgical pathology results are outlined in Figure 6. This group had an even distribution of diagnoses
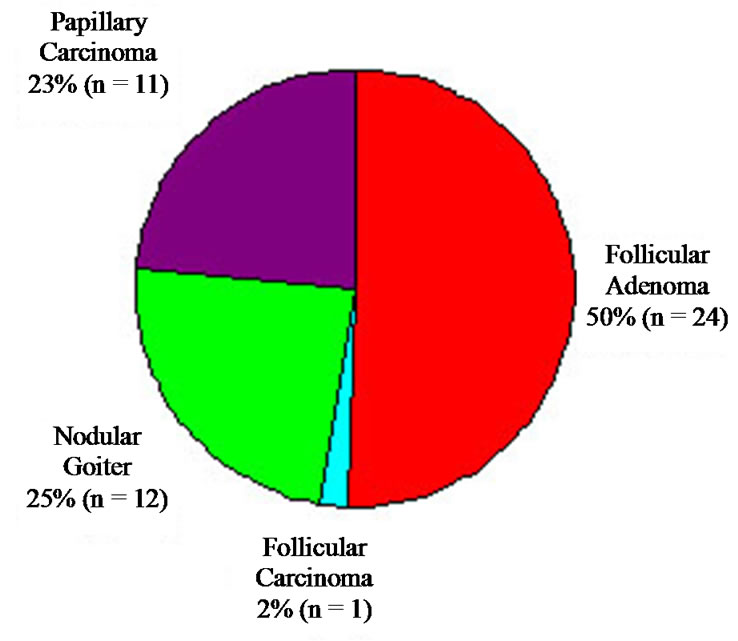
Figure 4. Final histologic diagnosis of all lesions diagnosed as FA on FNA.
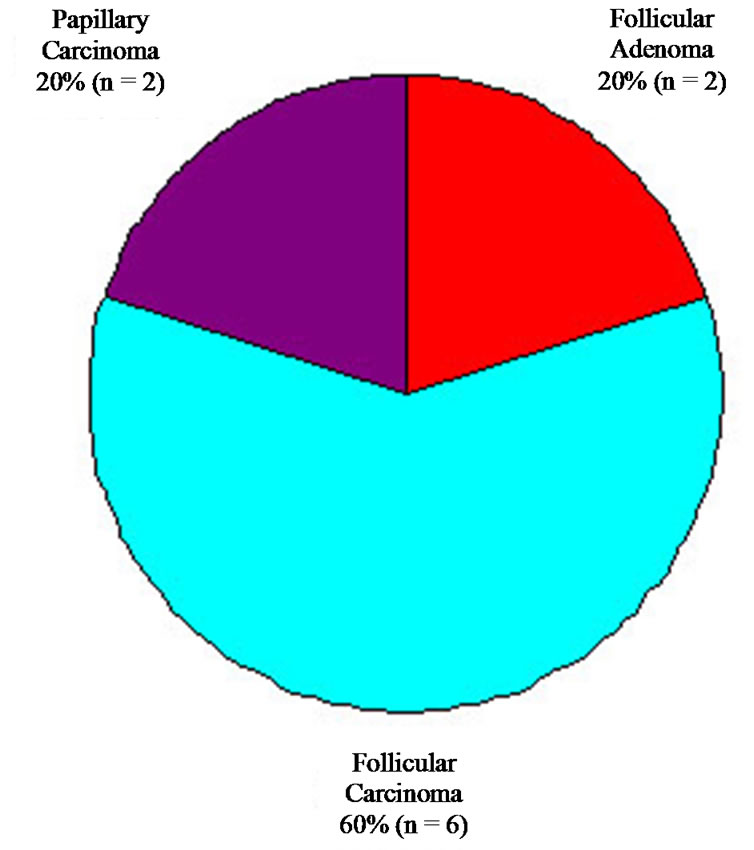
Figure 5. Final histologic diagnosis of all lesions diagnosed as follicular carcinoma on FNA.
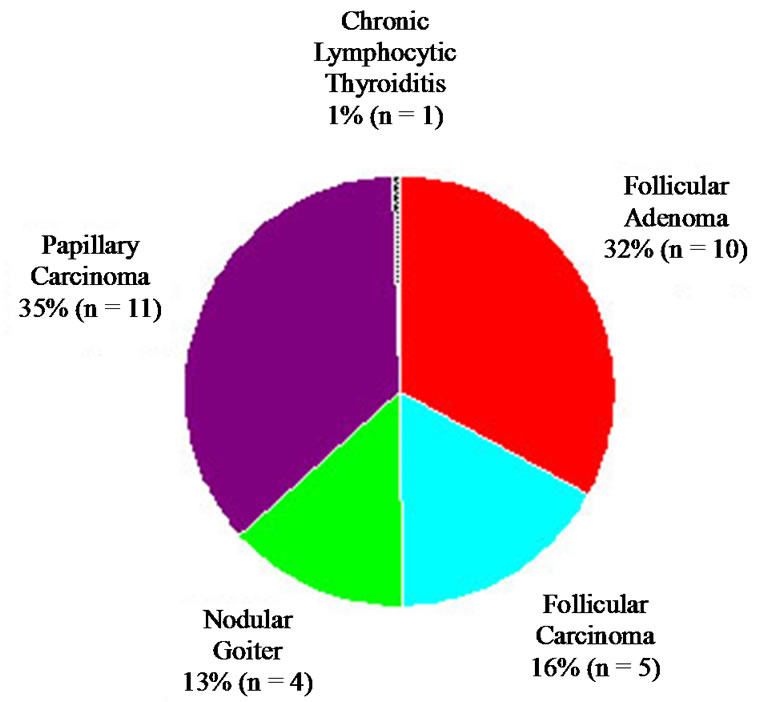
Figure 6. Final histologic diagnosis of all lesions diagnosed as follicular neoplasm-NOS on FNA.
of FA, FC, papillary carcinoma, and nodular goiter.
A total of 47 patients did not undergo any surgical intervention after their FNA diagnosis. A thorough chart review was performed on these patients. Twenty-four patients had no further follow-up within our health system, and we were not able to contact them. Surgery was subsequently recommended by the endocrinologists in eight patients; two of these patients refused surgery while the other six did not undergo surgery for unknown reasons. An additional seven patients were recommended to undergo observational management and no further thyroid work-up was performed. Two patients underwent repeat FNA’s; both patients were initially diagnosed with FA and repeat FNA showed chronic lymphocytic thyroiditis in one patient and was non-diagnostic in the other. Two patients are being followed with regular ultrasound while four patients died of unrelated causes.
4. Discussion
The gold standard of differentiating FA versus FC is surgical pathology. In an attempt to decrease unnecessary thyroid surgery for lesions that turn out to be benign FA, some cytopathologists utilize nuclear features of cells to make the distinction between FA and FC on FNA [3]. In our institutional review of all thyroid FNA diagnoses over 10 years, selecting only cases with cytologic diagnosis of the defined categories, a diagnosis of FC was uncommon (only 10 cases [7% overall]). This may reflect the decrease in overall incidence of FC in the past decade due to iodine supplementation and also may represent the selection preference of some pathologists at our institution to include this diagnosis in follicular neoplasm-NOS. This issue highlights the inherent difficulty with use of non-uniform reporting of follicular lesions. Of these 10 patients, six were proven to have FC, two to be a follicular variant of papillary carcinoma (FVPC), and two FA. Although the number of cases is small, 20% deemed FC on cytology were in fact benign.
In the group diagnosed cytologically as FA, 24 cases (50%) proved to be FA whereas 12 (25%) proved to be nodular goiter and 11 (24%) represented papillary carcinoma. One case was FC. A discrepancy occurred in half of the diagnoses initially thought to be adenoma. Based on this finding, if a clinician decides on conservative management in patients cytologically diagnosed with FA, there will be a 25% missed cancer rate.
Due to the inherent limitation in differentiating adenoma from carcinoma in cytologic preparation (FNA), some authors have suggested that follicular neoplasms can be stratified into two broad categories based on certain clinical parameters: those with high risk of malignnancy and those that can be managed by clinical observation [9-11]. Tyler et al. found that follicular neoplasms in patients older than 50 years had a higher risk of malignancy (40%) compared to younger patients [11]. In a study of 167 patients with a diagnosis of “follicular neoplasm,” Baloch et al. found a higher risk for malignnancy if the patient was male, older than 40 years, or the nodule was larger than 3.0 cm in size [12]. Schlinkert et al. studied 219 patients diagnosed as “suspicious for follicular neoplasm” and found that the characteristics of larger nodule size, fixation of the mass, and younger age were associated with a higher risk of malignancy [9].
It appears unlikely that the armamentarium of pathologists can serve to improve the specificity for diagnosing malignancy in non-papillary follicular lesions if morphologic criteria alone are used. Many investigators have attempted the use of ancillary techniques including immunohistochemistry and molecular markers to increase the accuracy of cytologic diagnosis in follicular neoplasms.
In addition to clinical parameters, immunohistochemical stains have been studied, including cytokeratin 19, Galectin-3, HBME-1, and Leu MI. Studies have shown significant overlap between benign and malignant lesions [13-18]. Overall, these studies are inconclusive and hindered by many limitations. Recently molecular markers have also been utilized to diagnose malignant thyroid lesions. Most of the studies were done on histological section while only a few have involved cytologic material. These markers include Ret-PTC translocation, BRAF mutation, K-ras and others [19-24]. In summary, unless larger studies are done specifically utilizing molecular markers in FNA material, clinical and cytological features remain the mainstay for diagnosis of follicular thyroid lesions.
Due to the known limitations of cytological diagnosis in follicular lesions and variability in terminology of diagnostic categories, the National Cancer Institute (NCI) hosted the “NCI thyroid FNA state of the science” conference in 2009. This led to the development of the Bethesda system for reporting thyroid cytopathology. In this system, a category of follicular neoplasm or “suspicious” for follicular neoplasm was created to describe a cellular aspirate showing a follicular patterned lesion that lacks the classical features of papillary carcinoma or any other frank malignant features. This category is intended to include cases with FA, FC, FVPC, and even hyperplastic nodules such as nodular goiter [25].
Our study has several drawbacks. Only 65% of the initial cohort went on to have surgery. Thus the diagnostic accuracy of 35% of our patients remains unknown. We believe this large percentage is due to an institutional bias. Though surgery was recommended in some of these patients, the majority did not have significant follow-up within our health system. The non-uniform reporting of follicular lesions by our institution’s pathologists, including many patients who were given a definitive diagnosis of follicular adenoma, may have led to these patients not being referred to surgery. Additionally, no clinical features, such as age or size of the nodule, were used in the statistical analysis. It is well known that larger sized nodules as well as older patient age are both risk factors for a diagnosis of carcinoma.
There were two primary purposes of this study. First was to assess our unique institutional preference to categorize benign versus malignant follicular lesions based on cytopathological diagnosis. The results of this study show that it is not yet possible to accurately differentiate FA from FC on cytology alone. The second purpose is to achieve institutional change in the way cytopathologists report follicular lesions and perhaps also in the clinical practice of endocrinologists and surgeons. As a result of this study, our institution has adopted the Bethesda system for reporting thyroid cytopathology. Clinicians have also become more aware that the distinction between benign and malignant follicular lesions is not possible based on cytopathology alone. Future studies regarding the effects of these changes are ongoing to assess their impact on the number and type of thyroid surgeries performed.
5. Conclusion
Our results reveal that FNA has a relatively low sensitiveity and specificity for diagnosing FA. We conclude that a definitive diagnosis beyond follicular neoplasmNOS is difficult based on FNA alone and histologic evaluation remains the gold standard. These lesions should be reported as follicular neoplasm-NOS which should prompt an appropriately planned surgical excision. Only then can a definitive histologic diagnosis be made which will help decide further management.
REFERENCES
- Z. W. Baloch and V. A. Livolsi, “Follicular-patterned lesions of the thyroid: the bane of the pathologist,” American journal of clinical pathology, Vol. 117, No. 1, 2002, pp. 143-150.
- Z. W. Baloch, V. A. LiVolsi and S. L. Asa, et al., “Diagnostic terminology and morphologic criteria for cytologic diagnosis of thyroid lesions: a synopsis of the National Cancer Institute Thyroid Fine-Needle Aspiration State of the Science Conference,” Diagnostic Cytopathology, Vol. 36, No. 6, 2008, pp. 425-437. doi:10.1002/dc.20830
- S. R. Kini, J. M. Miller, J. I. Hamburger and M. J. Smith-Purslow, “Cytopathology of follicular lesions of the thyroid gland,” Diagnostic Cytopathology, Vol. 1, No. 2, 1985, pp. 123-132. doi:10.1002/dc.2840010208
- J. Maruta, H. Hashimoto and Y. Suehisa, et al., “Improving the diagnostic accuracy of thyroid follicular neoplasms: cytological features in fine-needle aspiration cytology,” Diagnostic Cytopathology, Vol. 39, No. 1, 2011, pp. 28-34. doi:10.1002/dc.21321
- H. Gharib and J. R. Goellner, “Fine-needle aspiration biopsy of the thyroid: an appraisal,” Annals of Internal Medicine, Vol. 118, No. 4, 1993, pp. 282-289.
- K. Suen, “Guidelines of the Papanicolaou Society of Cytopathology for the examination of fine-needle aspiration specimens from thyroid nodules: The Papanicolaou Society of Cytopathology Task Force on Standards of Practice,” Diagnostic Cytopathology, Vol. 15, No. 1, 1996, pp. 84-89. doi:10.1002/(SICI)1097-0339(199607)15:1<84::AID-DC18>3.0.CO;2-8
- D. S. Cooper, G. M. Doherty and B. R. Haugen, et al., “Management guidelines for patients with thyroid nodules and differentiated thyroid cancer,” Thyroid, Vol. 16, No. 2, 2006, pp. 109-142. doi:10.1089/thy.2006.16.109
- H. Gharib, E. Papini and R. Valcavi, et al., “American Association of Clinical Endocrinologists and Associazione Medici Endocrinologi medical guidelines for clinical practice for the diagnosis and management of thyroid nodules,” Endocrine Practice, Vol. 12, No. 1, 2006, pp. 63-102.
- R. T. Schlinkert, J. A. van Heerden and J. R. Goellner, et al., “Factors that predict malignant thyroid lesions when fine-needle aspiration is ‘suspicious for follicular neoplasm’,” Mayo Clinic Proceedings, Vol. 72, No. 10, 1997, pp. 913-916.doi:10.1016/S0025-6196(11)63360-0
- R. M. Tuttle, H. Lemar and H. B. Burch, “Clinical features associated with an increased risk of thyroid malignancy in patients with follicular neoplasia by fine-needle Aspiration,” Thyroid, Vol. 8, No. 5, 1998, pp. 377-383. doi:10.1089/thy.1998.8.377
- D. S. Tyler, D. J. Winchester, N. P. Caraway, R. C. Hickey and D. B. Evans, “Indeterminate fine-needle aspiration biopsy of the thyroid: identification of subgroups at high risk for invasive carcinoma,” Surgery, Vol. 116, No. 6, 1994, pp. 1054-1060.
- Z. W. Baloch, S. Fleisher, V. A. LiVolsi and P. K. Gupta, “Diagnosis of ‘follicular neoplasm’: a gray zone in thyroid fine-needle aspiration cytology,” Diagnostic Cytopathology, Vol. 26, No. 1, 2002, pp. 41-44. doi:10.1002/dc.10043
- M. Alejo, G. Peiro, E. Oliva, X. Matias-Guiu and S. Schröder, “Leu-M 1 immunoreactivity in papillary carcinomas of the thyroid gland; microcarcinoma, encapsulated, conventional and diffuse sclerosing subtypes,” Virchows Archiv, Vol. 419, No. 5, 1991, pp. 447-448. doi:10.1007/BF01605080
- Z. W. Baloch, S. Abraham, S. Roberts and V. A. LiVolsi, “Differential expression of cytokeratins in follicular variant of papillary carcinoma: an immunohistochemical study and its diagnostic utility,” Human Pathology, Vol. 30, No. 10, 1999, pp. 1166-1171. doi:10.1016/S0046-8177(99)90033-3
- P. L. Fernandez, M. J. Merino and M. Gomez, et al., “Galectin-3 and laminin expression in neoplastic and non-neoplastic thyroid tissue,” The Journal of Pathology, Vol. 181, No. 1, 1997, pp. 80-86. doi:10.1002/(SICI)1096-9896(199701)181:1<80::AID-PATH699>3.0.CO;2-E
- K. T. Mai, J. C. Ford, H. M. Yazdi, D. G. Perkins and A. S. Commons, “Immunohistochemical study of papillary thyroid carcinoma and possible papillary thyroid carcinoma-related benign thyroid nodules,” PathologyResearch and Practice, Vol. 196, No. 8, 2000, pp. 533-540. doi:10.1016/S0344-0338(00)80025-4
- M. J. Sack, C. Astengo-Osuna, B. T. Lin, H. Battifora and V. A. LiVolsi, “HBME-1 immunostaining in thyroid fine-needle aspirations: a useful marker in the diagnosis of carcinoma,” Modern Pathology, Vol. 10, No. 7, 1997, pp. 668-674.
- K. H. van Hoeven, A. J. Kovatich and M. Miettinen, “Immunocytochemical evaluation of HBME-1, CA 19-9, and CD-15 (Leu-M1) in fine-needle aspirates of thyroid nodules,” Diagnostic Cytopathology, Vol. 18, No. 2, 1998, pp. 93-97.doi:10.1002/(SICI)1097-0339(199802)18:2<93::AID-DC3>3.0.CO;2-U
- C. C. Cheung, B. Carydis, S. Ezzat, Y. C. Bedard and S. L. Asa, “Analysis of ret/PTC gene rearrangements refines the fine needle aspiration diagnosis of thyroid cancer,” The Journal of Clinical Endocrinology & Metabolism, Vol. 86, No. 5, 2001, pp. 2187-2190. doi:10.1210/jc.86.5.2187
- Y. Cohen, E. Rosenbaum and D. P. Clark, et al., “Mutational analysis of BRAF in fine needle aspiration biopsies of the thyroid: a potential application for the preoperative assessment of thyroid nodules,” Clinical Cancer Research, Vol. 10, 2004, pp. 2761-2765. doi:10.1158/1078-0432.CCR-03-0273
- L. Jin, T. J. Sebo and N. Nakamura, et al., “BRAF mutation analysis in fine needle aspiration (FNA) cytology of the thyroid,” Diagnostic Molecular Pathology, Vol. 15, No. 3, 2006, pp. 136-143. doi:10.1097/01.pdm.0000213461.53021.84
- Z. Kucukodaci, E. Akar, A. Haholu and H. Baloglu, “A valuable adjunct to FNA diagnosis of papillary thyroid carcinoma: In-house PCR assay for BRAF T1799A (V600E),” Diagnostic Cytopathology, Vol. 39, No. 6, 2011, pp. 424-427. doi:10.1002/dc.21406
- Y. E. Nikiforov, D. L. Steward and T. M. RobinsonSmith, et al., “Molecular testing for mutations in improving the fine-needle aspiration diagnosis of thyroid nodules,” The Journal of Clinical Endocrinology & Metabolism, Vol. 94, No. 6, 2009, pp. 2092-2098.doi:10.1210/jc.2009-0247
- G. Tallini and G. Brandao, “Assessment of RET/PTC oncogene activation in thyroid nodules utilizing laser microdissection followed by nested RT-PCR,” Methods in Molecular Biology, Vol. 293, 2005, pp. 103-111.
- E. S. Cibas and S. Z. Ali, “The bethesda system for reporting thyroid cytopathology,” American Journal of Clinical Pathology, Vol. 132, 2009, pp. 658-665. doi:10.1309/AJCPPHLWMI3JV4LA
NOTES
*Corresponding author.

