Surgical Science
Vol. 3 No. 4 (2012) , Article ID: 18642 , 4 pages DOI:10.4236/ss.2012.34035
Pre-Application Evaporation of Surgical Preparation Solutions: Does It Matter?
1Department of Surgery, Southern Health, Melbourne, Australia
2Faculty of Medicine, Nursing and Health Sciences, Monash University, Melbourne, Australia
3Department of Orthopaedic Surgery, Southern Health, Melbourne, Australia
Email: *osama.elsewaisy@southernhealth.org.au
Received January 18, 2012; revised February 20, 2012; accepted March 2, 2012
Keywords: Preoperative Antisepsis; Surgical Preparation Solution; Alcohol; Evaporation
ABSTRACT
Introduction: Surgical site infections (SSIs) remain the most common health care associated infections in the surgical population. Preoperative surgical preparation solutions containing alcohol are believed to be best at eradicating skin microorganisms. The efficacy of alcohol is concentration dependant, with a concentration of greater than 60% most bactericidal. Surgical antisepsis guidelines do not stipulate how long alcoholic preparation solutions can be left out prior to use. Method: 30 ml of Alcoholic Iodine (Iodine 1% in Alcohol 70% (v/v)) and Alcoholic Chlorhexidine (Chlorhexidine 2% in Alcohol 70% (v/v)) were left to stand in gallipots in an operating theatre equipped with laminar flow. Samples were taken at 0, 30, 60 and 120 minutes and the alcohol content was analyzed. The experiment was repeated 3 times for each time interval. Results: The Alcoholic Iodine group demonstrated significant decrease in alcohol concentration, from a mean of 76% (SD 3.6) to a mean of 37.7% (SD 2.9) in only 30 minutes. This effect was sustained, reaching a mean concentration of 26% (SD 2.9) alcohol at 120 minutes. The Alcoholic Chlorhexidine group did not exhibit the same degree of concentration drop, the concentration dropped marginally to 71.5% (SD 2.7) at 120 minutes from 83.4% (SD 0.4). Conclusion: Alcoholic Iodine exhibits significant evaporation under operating room conditions after 30 minutes. Alcoholic Chlorhexidine does not appear to undergo similar losses in concentration. We recommend that alcoholic surgical preparation solutions must be poured immediately prior to use, and must be discarded if left uncovered for more than a few minutes.
1. Introduction
Despite multiple clinical interventions throughout the perioperative process, surgical site infections (SSIs) remain the most common health care associated infections in the surgical population. SSIs impose a significant financial burden on health care systems and adversely effect morbidity and mortality of patients undergoing surgical procedures [1-3].
Current measures to reduce the risk of SSIs include face masks, hand washing, gowning and gloving, air filtration techniques, antibiotic prophylaxis and patient skin decontamination [1,3]. While there is insufficient evidence in the literature to support the use of one skin antiseptic over another, there is reasonable consensus that alcohol based solutions are most effective in the prevention of SSIs [2,4-7]. The U.S. Centers for Disease Control (CDC) has labeled it “the most effective and rapidacting skin antiseptic” for preventing SSIs [2]. Alcohol provides the most rapid and greatest reduction in initial microbial counts on skin, but has no persistent activity, and is therefore most effective when combined with agents that exhibit residual antimicrobial effect [1,4,7].
Alcohol concentrations of 70% (v/v) are recommended, as the bactericidal effect of alcohol decreases below this concentration [4]. Morton investigated the relationship of alcohol concentration on bactericidal activity [8], and found that a concentration of greater than 60% (v/v) eradicated Streptococcus pyogenes and Staphylococcus aureus species in less than 10 seconds, while a concentration of less than 40% (v/v) required 45 and 60 minutes for each organism respectively. The concentration related efficacy of alcohol on bactericidal activity was demonstrated more recently by Kampf, who reported superior bactericidal activity in higher concentration hand antiseptics [9].
Alcohol is known to be volatile, and while most guidelines warn against flammability, and recommend sealing of alcohol containers [4,7], we could not find any guidelines that stipulate how long alcoholic surgical antiseptic solutions can be left in the preparation bowl (gallipot) prior to use. This included the manufacturer guidelines, our local hospital guidelines and state/international guidelines for surgical site antisepsis [4,7].
There are many reasons why surgical cases can be delayed including surgical, anaesthetic, nursing and patient factors. These delays can be lengthy, and sometimes occur after surgical sets have been prepared—and surgical preparation solution has been poured. We hypothesized that there may be significant, time-dependant evaporation of the alcohol in surgical preparation solutions prior to use. If the concentration decreases significantly, it could markedly affect the bactericidal activity of the solution.
The purpose of this study was to measure the concentration of alcohol in Alcoholic Iodine (Iodine 1% in Alcohol 70% (v/v): ORION Laboratories Pty Ltd) and Alcoholic Chlorhexidine (Chlorhexidine 2% in Alcohol 70% (v/v): ORION Laboratories Pty Ltd) solutions after evaporation for half hour increments up to two hours. We chose to investigate these solutions as they are the two most commonly used at our organization for preoperative skin antisepsis, and are two of the most widely recommended in the literature [1,4,5,10-14] .
2. Method
We set up three 120 ml gallipots, 50 cm apart, on a standard 92 cm high surgical trolley in an empty operating room with laminar flow activated, with the temperature set at 20 degrees Celsius (68 degrees Fahrenheit).
30 ml of Alcoholic Iodine (Iodine 1% in Alcohol 70% (v/v): ORION Laboratories Pty Ltd) was poured into each of the gallipots (from 3 separate sealed bottles) and left to stand for 30 minutes, after which a 2.5 ml sample was drawn up from each gallipot and transferred into separate 2.5 ml test-tubes with sealable lids. The remainder of the solutions was discarded.
Three new Alcoholic Iodine solutions were opened and 30 ml was poured into a new set of three gallipots, which were left to stand for 60 minutes, after which a 2.5 ml sample was collected from each and sealed as described above. This was repeated again at 120 minutes.
The same procedure was undertaken with Alcoholic Chlorhexidine (Chlorhexidine 2% in Alcohol 70% (v/v): ORION Laboratories Pty Ltd), at 30, 60 and 120 minutes.
Three sets of 2.5 ml were taken from three new bottles of Alcoholic Iodine and Alcoholic Chlorhexidine, and immediately sealed in three separate 2.5 ml test-tubes— in order to validate our results at time “0”.
New syringes and gallipots were used for each phase of the experiment, to ensure that there was no mixing of different concentrations of solutions. New bottles of Alcoholic Chlorhexidine and Alcoholic Iodine were used for each gallipot to ensure that no alcohol evaporated from opened bottles between experiments, and that a different bottle was used for each individual experiment.
The 24 sealed 2.5 ml test-tubes were labeled and transported to our organization’s pathology research laboratory, where the alcohol concentration of each was measured using a Beckman Coulter SYNCHRON DXC800 (Beckman Coulter Diagnostics: Sydney, Australia).
Institutional review board (IRB) approval was not required as no human participants were involved in the study.
3. Results
The Alcoholic Iodine group demonstrated significant decrease in alcohol concentration, from a mean of 76% (SD 3.6) at time “0” to a mean of 37.7% (SD 2.9) in only 30 minutes. This decrease was sustained reaching a mean of 26% (SD 2.9) at 120 minutes. The average standard deviation for the Alcoholic Iodine groups was 2.6. This data is summarized in Table 1 and Figure 1.
The Alcoholic Chlorhexidine group did not exhibit the same degree of concentration drop, maintaining a mean concentration of 84.3% (SD 1.3) at 30 minutes, from 83.4% (SD 0.4) at time “0”. The concentration dropped marginally to 71.5% (SD 2.7) at 120 minutes. The average standard deviation for the Alcoholic Chlorhexidine groups was 2.2. These results are summarized in Table 2 and Figure 2.
4. Discussion
Findings from our study indicate that Alcoholic Iodine
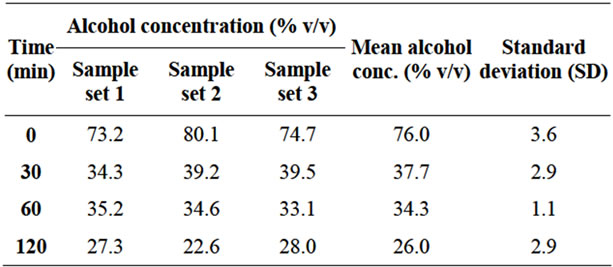
Table 1. Alcohol concentration (iodine 1% in alcohol 70% v/v) vs. time.
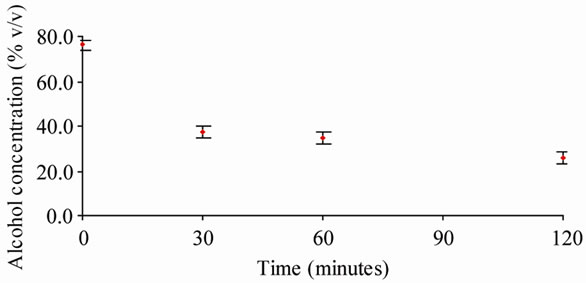
Figure 1. Alcohol concentration (iodine 1% in alcohol 70% v/v) vs. time.
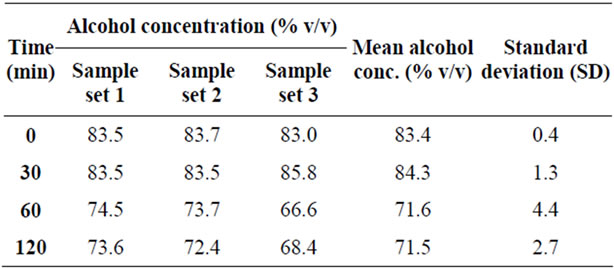
Table 2. Alcohol concentration (chlorhexidine 2% in alcohol 70% v/v) vs. time.
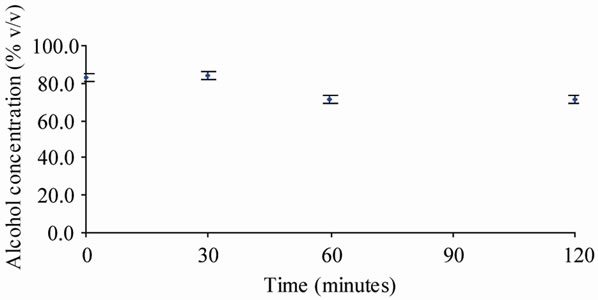
Figure 2. Alcohol concentration (chlorhexidine 2% in 70% alcohol 70% v/v) vs. time.
solution (Iodine 1%, Alcohol 70% v/v) exhibits significant evaporation of the alcohol component to less than 40% alcohol when left in a gallipot for only 30 minutes. This concentration of alcohol could take up to 45 and 60 minutes to eradicate Streptococcus pyogenes and Staphylococcus aureus species respectively [8].
The same concentration drop was not seen in the Alcoholic Chlorhexidine solutions, which maintained a mean alcohol concentration of 71.5% (SD 2.5) after being exposed to the same conditions for 2 hours.
It is not clear why the Alcoholic Chlorhexidine solutions did not demonstrate the same trend, possibilities include:
1) The Alcoholic Chlorhexidine solutions we sampled may have contained a higher alcohol concentration to begin with.
Our mean concentrations at time “0” were 83.4% (SD 0.4) for Alcoholic Chlorhexidine versus 76.0% (SD 3.6) for Alcoholic Iodine. In addition, the composition information sheets from the supplier (ORION Laboratories Pty Ltd) list a proportion of “70% Alcohol (v/v)” for Alcoholic Iodine and “>60% Alcohol (v/v)” for Alcoholic Chlorhexidine, indicating that the concentration of alcohol may be slightly variable in these solutions.
The scientific literature indicates that in an ethanolwater mixture, the rate of each component’s evaporation is different, and that the ethanol component’s evaporation rate increases as the concentration of alcohol decreases [15]. This means that the evaporation of the alcohol component would be faster in solutions with lower alcohol concentrations, and this effect would be compounded as time progressed.
2) The Alcoholic Chlorhexidine solution may have decreased volatility compared with the Alcoholic Iodine due to differing chemical structure and bonds.
3) The apparent difference may be due to observational error, however this is unlikely given the reproducibility of each result three times and consistent narrow standard deviations.
5. Conclusions
1) Alcoholic Iodine (Iodine 1%, Alcohol 70%) solutions exhibit significant evaporation to less than 40% alcohol when left in a gallipot under operating room conditions for 30 minutes.
This effect is sustained, reaching a mean concentration of 26% (SD 2.9) alcohol at 120 minutes.
2) The time after which Alcoholic Iodine begins to significantly lose alcohol concentration is unknown.
3) Alcoholic Chlorhexidine (Chlorhexidine 2%, Alcohol 70%) does not appear to undergo significant losses of alcohol concentration secondary to evaporation after 120 minutes under operating room conditions.
Based on these observations, we recommend that alcoholic surgical preparation solutions must be poured immediately prior to use, and surgical antisepsis guidelines should explicitly state this. We also believe that alcoholic preparation solutions left in a gallipot for more than a few minutes prior to use must be discarded, and a new solution opened immediately prior to use.
5.1. Limitations
The main limitation of our study is the relatively small sample size, which precludes statistical significance— meaning that we cannot prove that our results did not occur by chance. However, based on our rigorous experimental design, and narrow standard deviations throughout the results, we feel that the results are valid.
5.2. Recommendations for Future Research
Further studies on this topic should incorporate a larger sample size, in order to achieve statistical significance. They could also investigate alcohol concentration after shorter periods of evaporation (e.g. 10, 20 minutes), in order to delineate a “safe” period, after which the solution must be discarded. There is also scope to further investigate the evaporation of Alcoholic Chlorhexidine solutions, to confirm whether these solutions exhibit less alcohol concentration loss due to evaporation.
REFERENCES
- N. Fletcher, D. Sofianos, M. Berkes and W. Obremskey, “Prevention of Perioperative Infection,” The Journal of Bone & Joint Surgery, Vol. 89, No. 7, 2007, pp. 1605-1618. doi:10.2106/JBJS.F.00901
- A. Mangram, T. Horan, M. Pearson, L. Silver and W. Jarvis, “U.S. Centers for Disease Control and Prevention. Guideline for Prevention of Surgical Site Infection,” Infection Control and Hospital Epidemiology, Vol. 20, No. 4, 1999, pp. 250-278. doi:10.1086/501620
- J. Zinn, J. Jenkins, V. Swofford, B. Harrelson and S. McCarter, “Intraoperative Patient Skin Prep Agents: Is There a Difference?” AORN Journal, Vol. 92, No. 6, 2010, pp. 662-671. doi:10.1016/j.aorn.2010.07.016
- “Surgical Skin Antisepsis in Operating Theatres. Version 1.0,” Queensland Health, 2011.
- R. Darouiche, M. Wall, K. Itani, M. Otterson, A. Webb, M. Carrick, H. Miller, S. Awad, C. Crosby, M. Mosier, A. AlSharif and D. Berger, “Chlorhexidine-Alcohol versus Povidone-Iodine for Surgical-Site Antisepsis,” New England Journal of Medicine, Vol. 362, No. 1, 2010, pp. 18-26. doi:10.1056/NEJMoa0810988
- D. Keblish, D. Zurakowski, M. Wilson and C. Chiodo, “Preoperative Skin Preparation of the Foot and Ankle: Bristles and Alcohol Are Better,” Journal of Bone and Joint Surgery, Vol. 87, No. 5, 2005, pp. 986-992. doi:10.2106/JBJS.D.02695
- “Recommended Practices for Preoperative Patient Skin Antisepsis,” Perioperative Standards and Recommended Practices, Association of Perioperative Registered Nurses (AORN), Inc., Denver, 2010.
- H. Morton, “The Relationship of Concentration and Germicidal Efficiency of Ethyl Alcohol,” Annals of the New York Academy of Sciences, Vol. 53, No. 1, 1950, pp. 191-196. doi:10.1111/j.1749-6632.1950.tb31944.x
- G. Kampf, “How Effective Are Hand Antiseptics for the Postcontamination Treatment of Hands When Used as Recommended?” American Journal of Infection Control, Vol. 36, No. 5, 2008, pp. 356-360. doi:10.1016/j.ajic.2007.07.017
- S. Sistla, G. Prabhu, S. Sistla and J. Sadasivan, “Minimizing Wound Contamination in a ‘Clean’ Surgery: Comparison of Chlorhexidine-Ethanol and Povidone-Iodine,” Chemotherapy, Vol. 56, No. 4, 2010, pp. 261-267. doi:10.1159/000319901
- M. Saltzman, G. Nuber, S. Gryzlo, G. Marecek and J. Koh, “Efficacy of Surgical Preparation Solutions in Shoulder Surgery,” Journal of Bone and Joint Surgery, Vol. 91, No. 8, 2009, pp. 1949-1953. doi:10.2106/JBJS.H.00768
- R. Ostrander, M. Botte and M. Brage, “Efficacy of Surgical Preparation Solutions in Foot and Ankle Surgery,” Journal of Bone and Joint Surgery, Vol. 57, 2005, pp. 980-985. doi:10.2106/JBJS.D.01977
- C. Bibbo, D. Patel, R. Gehrmann and S. S. Lin, “Chlorhexidine Provides Superior Skin Decontamination in Foot and Ankle Surgery: A Prospective Randomized Study,” Clinical Orthopaedics & Related Research, Vol. 438, 2005, pp. 204-208. doi:10.1097/01.blo.0000167832.47464.22
- J. Hibbard, G. Mulberry and A. Brady, “A Clinical Study Comparing the Skin Antisepsis and Safety of ChloraPrep, 70% Isopropyl Alcohol, and 2% Aqueous Chlorhexidine,” Journal of Infusion Nursing, Vol. 25, No. 4, 2002, pp. 244-249. doi:10.1097/00129804-200207000-00007
- K. O’Hare, P. Spedding and J. Grimshaw, “Evaporation of the Ethanol and Water Components Comprising a Binary Liquid Mixture,” Asia-Pacific Journal of Chemical Engineering, Vol. 1, No. 2, 1993, pp. 118-128.
NOTES
*Corresponding author.

