Open Journal of Veterinary Medicine
Vol. 2 No. 2 (2012) , Article ID: 19643 , 8 pages DOI:10.4236/ojvm.2012.22009
Gut Peculiarities of Feed Deprived White Sturgeons (Acipenser transmontanus, Richardson 1836)*
1Department of Veterinary Sciences and Technologies for Food Safety, Università degli Studi di Milano, Milan, Italy
2Department of Animal Pathology, Hygiene and Public Health, Faculty of Veterinary Medicine, Università degli Studi di Milano, Milan, Italy
Email: #alessia.digiancamillo@unimi.it, alessiadg73@hotmail.com
Received February 8, 2012; revised March 1, 2012; accepted March 22, 2012
Keywords: Acipenser Transmontanus; Runt Sturgeons; Reared Sturgeons; Gut, Diatom Identification
ABSTRACT
In the White sturgeon fish farms, some individuals have difficulty in getting access to food: such sturgeons are called “runts”, and they result in a slower growth rate than normally feeding fish. In this paper, we have studied the gut peculiarities of runt sturgeons. Utilizing in paralleling an analysis of diatom populations in both the fish gut tissues and the rearing tank waters, we hypothesized a causative relation between the occurrence of runt sturgeons and periodic diatom blooms. In fact, we have observed that the diatom species identified in the aquatic environment were also detected in organs (Fragilaria spp and Rhoicosfenia spp for both glandular body, mid-intestine) of the runt sturgeon’s gut, but not in tissues of normally feeding individuals. Owing to their siliceous wall, diatoms can be responsible for areas of epithetlial detachment in the mucosal surfaces of the alimentary canal and a catharral inflammation in both the gastric pits and intestinal folds which may be the cause of secondary bacterial diseases. We suggest that diatom blooms may contribute to the occurrence of runt sturgeons in the studied Italian fish farm.
1. Introduction
When farming white sturgeons (Acipenser transmontanus Richardson, 1836), is unusual within the same stock, some sturgeons have a prolonged difficulty in getting access to food, and become emaciated (feed-deprived sturgeons are called “runts”, Figure 1(a)). We previously described in runt sturgeons that some gut structures demonstrate clear alterations [1]. The runt condition results in a slower growth rate than that of normally feeding fish, as well as slower swimming [2,3], giving rise to the occurrence of growth depensation phenomena in fish tanks. Growth depensation phenomena are not infrequent in fish farms, and may depend on individual sizeand food-related factors [4,5]. These factors possibly depend on hierarchical relationships among fish, including competition for food, stocking density, and differences in swimming activity [6,7], as well as on the presence of stressful conditions, surely not infrequent in culturing conditions. In addition to changes affecting the skin and gills, which are directly in contact with the aquatic environment, environmental stressors may also influence the structure of the gastrointestinal tract, because its surfaces are constantly exposed to the forces of the external environment via the food intake [8]. Hypothesizing that a possible cause in the emerging of runt condition in a sturgeon farm may reside on a not yet described relationship of fishes with the farm aquatic micro-habitat, we have in this paper thrown our attention to the presence of Bacillariophyceae (diatoms) in the water. Diatoms are microscopic unicellular algae, in which the structure of the siliceous skeleton (“frustule”) shows a geometrical shape. Diatoms are extremely numerous in both marine and fresh waters, where they periodically release domoic acid [9] and other toxins. Biotoxins, which in most cases are neurotoxic [10] are released in large quantities during the periodic diatom blooms, which are sometimes linked to changes in the colour of the water, and may be related to the occurrence of poisoning in animals and in people who eat them [11-13]. We have recently shown that diatoms are identifiable and microscopically recognizable in mammalian tissues, applying appropriate digesting methodologies [14,15].With the aim of elucidating a possible relationship between the occurrence of runt sturgeons in an Italian fish farm and diatoms, we have examined in this multi-disciplinary study both runt sturgeons and waters of the rearing tanks during algal blooms. Normally feeding sturgeons were studied as well in parallel. Either runt and normal sturgeons alimentary canals were in addition studied by a microbiological point of view. Additionally, the runt and normal sturgeons alimentary canals were studied microbiologically. In parallel with intensifying production, fish diseases have in recent years acquired important practical and economic importance, and some of them may cause emaciation, among other signs, in cultured fish species [16]. Bacterial diseases, from both primary and secondary occurrences [17-19], do not spare sturgeon fish farms [20,21].
2. Materials and Methods Employed
The study was conducted at the Agroittica Lombarda fish hatchery (Calvisano, Italy). In spring, when a characteristic green colour was evident in some tank waters, the occurrence of runt individuals in them among the numerically predominant normally feeding sturgeons was observed. Even if variable, the percentage of the occurrence of runt individuals was around 3% - 5%. In winter, when the colour of tank water was as usual colourless, only normally feeding sturgeons were seen.
2.1. Micro-Anatomical Analyses
A total number of twenty-two adult A. transmontanus was used for this study, and their alimentary canal pieces were sampled immediately after slaughtering at the fish farm. The slaughtering was preceded by a blow to the head. Both runt or normally feeding sturgeons were sampled (see below) coming from the same tanks, which were characterized by the presence of runt individuals.
The normally feeding sturgeons (total number = 12) had body weights ranging between 8.5 and 10 kg, and their body length was 95 - 110 cm. The total number of studied runt sturgeons was 10. The body weights were variably diminished in runt sturgeons (from 1.9 to 7.8 kg) in comparison with the normally feeding ones, whereas their length was similar to that of normal individuals (from 90 to 107 cm). Immediately after the sacrifice, the gastrointestinal tract were excised from all the studied animals, and small samples of oesophagus (proximal, mid, and distal), stomach (in its different glandular zones: cardiac, gastric proper, pyloric) and intestine (pyloric caeca constituting the glandular body, proximal intestine, mid-intestine with spiral valve, distal intestine or rectum) were collected. Small tissues from the liver and spleen were also sampled from all the fish. All the tissue samples were promptly fixed in 4% paraformaldehyde in phosphate-buffered saline (PBS), and then paraffin-embedded, after processing through a graded ethanol series. De-waxed microtome sections (4 - 6 µm thick) were stained for histology with haematoxylin-eosin (HE) sequential staining to ascertain structural details. The observation was made with a light microscope (Olympus BX51, Olympus Italy), equipped with a digital camera (Camedia C3030, Olympus) and an image analysis software (DP Software, Olympus). The observer was not aware of the provenience of each studied gut section.
2.2. Collecting and Studying Diatoms in Sturgeon Tissues
Other pieces of the guts (from the same organs and from the same fish that were collected and used for the micro-anatomical analyses) from both normally feeding (N = 12) and runt sturgeons (N = 10) were used to demonstrate the possible presence of diatoms [14,15]. Briefly, the tissue specimens were treated with strong acids combined with high temperature to dissolve the organic components of the tissues examined while preserving the siliceous frustules of diatoms. One gram of each organ specimen was placed in 20 ml of 12 N HCl and boiled at 100˚C for 15 minutes until the tissues were completely dissolved. This procedure was repeated two times for each organ. The fluid obtained was collected and spread on slides (1 ml per slide, two repetitions). Owing to the chemical digestion, the fluid spread on the slides contained inorganic remnants only, in particular the siliceous frustules of diatoms. The siliceous frustules were identified by light microscopy based on their species-specific shapes [22-25]. Diatom counts were performed with the light microscope, by calculating the number of diatoms in each millilitre of material with the DP software. All reagents used for chemical digestion were tested to exclude the presence of exogenous diatoms.
2.3. Collecting and Studying Diatoms in Tanks
Diatom analysis was performed on the mud sediment and on the open water (planktonic sediment) from the water tanks in which runt sturgeons were present. We investigated the mud in addition to open water because sturgeons are benthophagous. Three repetitions of the mud sediment were sampled by submerging a glass into the sand at the centre of each tank (1.5 m deep × 50 m long × 20 m wide). The planktonic sediment was collected in the open water at the centre of the tank (three repetitions). Mud sediments were placed in H2O2 (30%, in a quantity corresponding to 1/3 of the total volume of the mud) for at least 72 h, according with Round et al. [26] to dissolve the organic matter present in the mud. After sedimentation, a quantity of 5 ml per sample was collected and spread on five slides (1 ml of fluid material per slide). Open water was treated with 3:1 acetone: water, and after sediments fell to the bottom of the glass (3 days), 5 ml of the material was collected as described for the mud (1 ml per slide). All reagents used for extraction were periodically tested to exclude the presence of exogenous diatoms. Slides from both mud and open waters were analysed with the light microscope. The observer was not aware of the origin of the samples. The diatoms were identified according Krammer and Lange-Bertalot [22-25]. Diatom counts were performed with the light microscope, by calculating the number of diatoms in each millilitre of material with the DP software.
2.4. Microbiological Analysis
The microbiological examination was performed on 10 animals, five normally feeding sturgeons and five runt sturgeons. For each animal, a small piece, 1 cm long, was sampled from the glandular body, proximal intestine, mid-intestine with spiral valve, and distal intestine. The samples were sterile taken. The content of each sample was diluted into 10 ml sterile physiological solution (NaCl, 9 g in distilled H2O, 1000 ml) at 4˚C until it reached the laboratory. After homogenizing, 100 ml from each sample was directly inoculated on the following agar plates, using standard protocols: Tryptic Soy Agar (TSA, Oxoid, Italy) for total bacterial count, MRS (Biolife, Italy) for Lactobacillus spp, MacConkey medium (Oxoid, Italy) for Enterobacteriaceae, and Mannitol Salt Agar medium (MSA, Oxoid, Italy) for Staphylococcus spp. [27]. The plates were incubated in an aerobic atmosphere at 20˚C for 24 h (for TSA, MSA, and MacConkey medium), and at 20˚C for 48 - 72 h under 5% CO2 atmosphere (for MRS medium). After incubation, the colonies grown on the bacteriological media were identified by both macroscopic criteria (colony size and morphology) and microscopic observations following Gram’s stain. Biochemical tests were performed to evaluate urease, catalase and oxidase activities. The API System (BioMerièux, France), a miniaturized biochemical panel, has been used for the identification of Micrococci (API Staph®), Enterobacteriaceae (API 20E®), and non-Enterobacteriaceae (API 20NE®). For each sample, examined in a “blind” way, we counted the Colony Forming Units (CFUs) using the dilution methods, and processed the results as CFU/ml of homogenate.
3. Results
3.1. Micro-Anatomical Analysis
Using HE sequential staining, the alimentary tract of the examined normally feeding sturgeons showed the expected structure [28,29], and pathological changes were never detected in any of the examined tissues, including liver and spleen. On the other hand, in runt sturgeons areas of epithelial detachment were frequently encountered within the mucosal surfaces of the oesophagus (Figure 1(b)), stomach (Figure 1(c)), and intestine (Figure 1(d)).
A catarrhal inflammation with oedema and hyperaemia of the tunica propria-submucosa was especially evident in gastric pits and intestinal folds. The gut associated lymphoid tissue (GALT) showed hyperplasia, which was especially prominent in the oesophagus (Figure 2(a)), fundic stomach (Figure 2(b)) and mid-intestine. In the oesophagus, particularly in the distal region, an excess of mucous-secreting activity was observed (Figure 2(c)). The liver (Figure 2(d)) and spleen contained a large number of melano-macrophagic centres (MMCs), which were also detected in similar large quantities in the
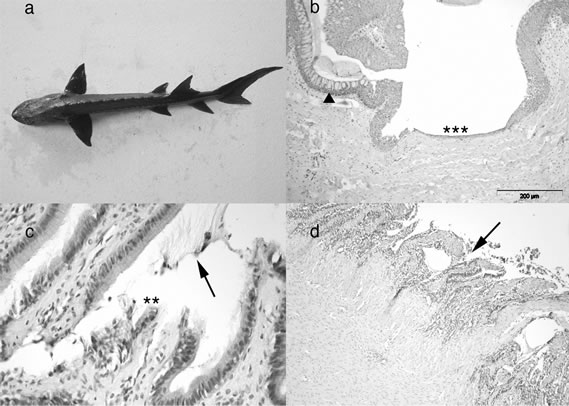
Figure 1. Runt sturgeon. (a) A runt sturgeon is clearly identified by its emaciated profile; (b) Distal oesophagus, HE. A wide area of epithelial hypoplasia (asterisks) is seen at the mucosal surface, and epithelial goblet cell hyperplasia is also evident (arrowhead). Scale bar 200 µm; (c) Pyloric stomach, HE. The epithelial layer of the tunica mucosa shows signs of detachment (asterisks), and a moderate excess of secretion (arrow). Scale bar, 50 µm; (d) Mid-intestine, HE. The tunica mucosa (arrowhead) is almost entirely devoid of the epithelial layer. Scale bar, 200 µm.
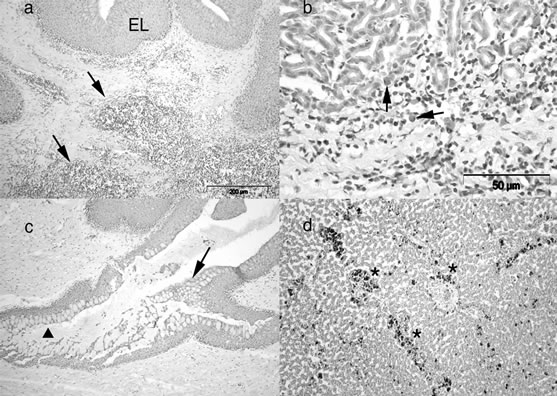
Figure 2. Runt sturgeon. (a) Distal oesophagus, HE. A noticeable inflammation is present in the superficial zone of the tunica propria-submucosa (arrows). EL = epithelial layer. Scale bar, 200 µm; (b) Fundic stomach, HE. Several inflammatory cells (arrows) are observed within the tunica propria-submucosa. Scale bar, 50 µm; (c) Distal oesophagus, HE. An important catarrhal oesophagitis (arrow) is detected, and epithelial goblet cell hyperplasia is also evident (arrowhead). Scale bar, 200 µm; (d) Liver, HE. Several melano-macrophagic centres (asterisks) are observed within the parenchyma. Scale bar, 100 µm.
pancreatic parenchyma embedded in the sub-serosal connective tissue of the glandular body and proximal intestine. The presence of MMCs was negligible in the same organs of the normally feeding sturgeons.
3.2. Diatoms in Sturgeon Tissues
The results of tissue digestion with strong acids combined with the high temperature showed that the glandular body and mid-intestine of runt sturgeons contained frustules of diatoms (Figure 3).
Based on the encountered frustules, the genera of the diatoms in runt sturgeon glandular body and mid-intestine were identified and their relative frequencies determined. The results are summarized in Table 1.
In the glandular body, Fragilaria spp. (Ehrenberg) (82.76% of total identified diatoms) was the most prominent taxon. Rhoicosphenia spp (Kützing) (15.37% of the total identified diatoms) was the second commonest taxon present in the digested tissues. In the mid-intestine, Fragilaria spp. was also the most frequent taxon (91.62% of total identified diatoms), and Rhoicosphenia spp. was the second commonest taxon (4.99% of the total identified diatoms) present in the digested tissues.
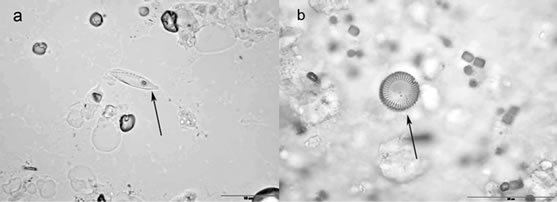
Figure 3. Tissue digestion. (a) Rhoicosphenia curvata in glandular body of runt sturgeon (arrow). Scale bar, 10 µm; (b) Fragilaria spp. in mid-intestine of runt (arrow). Scale bar, 10 µm.
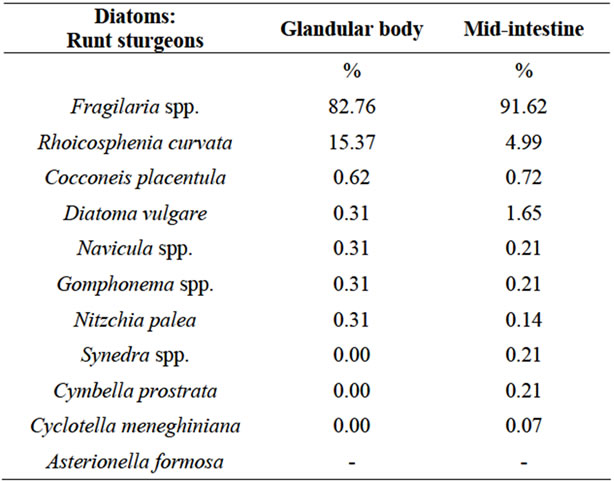
Table 1. Diatoms identified by light microscopy and counted (as %) in digested tissues (1 g) from both the glandular body and mid-intestine of runt sturgeons.
3.3. Diatoms in Open Water and Mud
Diatom counts and distribution in water are presented in Table 2. Fragilaria spp was the most prominent taxon present in the open water (50.81% of the total identified diatoms). Cyclotella meneghiniana (Kützing) was the second commonest taxon present in the water (42.21% of the total identified diatoms). Diatom counts and distribution in mud are also shown in Table 2. C. meneghiniana was the most frequent taxon present in the sediment, and Fragilaria the next commonest (51.70% and 43.07% of the total diatoms, respectively).
3.4. Microbiological Examination
All the five normally feeding sturgeons showed an increase in the total intestinal bacterial counts from the glandular body (>102 CFU/ml intestinal content) to the distal intestine (>104 CFU/ml). A high concentration of Lactobacillus spp (102 - 104 CFU/ml) was identified all along the intestine, whereas Escherichia coli was moderately present (103 CFU/ml). We also observed the presence of Micrococcus luteus (~102 CFU/ml), a Grampositive bacterium known to come from the environment, and the absence of Staphylococcus spp.
The total microbial counts performed on the intestinal content of the five runt sturgeons were uniformly high (>104 CFU/ml) from the glandular body to the distal intestine. Staphylococcus spp. populations were not identified. The presence of lactobacilli was variable, because the count was high in some fish (>104 CFU/ml) and low in others (<102 CFU/ml). In all the runt sturgeons, E. coli and other Enterobacteriaceae were absent, but other potential fish pathogens, such as Klebsiella pneumoniae, Citrobacter freundii, Serratia spp. and Aeromonas hydrophila, were not identified in normally feeding sturgeon intestine.
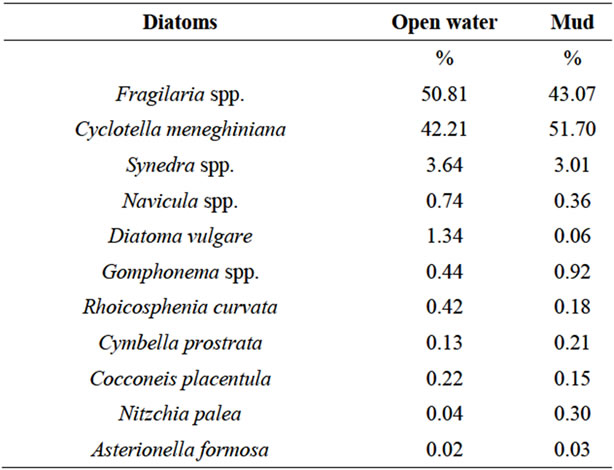
Table 2. Diatoms identified and counted (%) in open water and mud (5 ml).
4. Discussion and Conclusion
The aim of this study was to describe the gut changes/ lesions in either normally feeding or “runt” sturgeons, as well as examine with a multidisciplinary approach the possible relationship between the occurrence of runt sturgeons (A. transmontanus, white sturgeon) and variations in the aquatic diatom communities in an Italian (Northern Italy) fish farm.
Micro-anatomical findings revealed that the digestive tract of runt sturgeons showed variable degrees of pathologic changes, from catarrhal to desquamating oesophago-gastro-enteritis, not present in normally feeding sturgeons. Functional damage related to epithelial loss may be of great physiological relevance in runt sturgeons, and may contribute to the assessment of these emaciated fish, because epithelial cells disruption and sloughing are present in the runt condition impairing processes of digesting and absorbing. The runt sturgeon’s liver, pancreas and spleen contained several melanomacrophage centres (MMCs).
MMCs (also called macrophage aggregates, MAs) are frequently encountered in the parenchyma of teleost fish organs, such as liver, spleen and cephalic kidney, in which lympho-hematopoietic functions occur [30-32]. The increasing numbers of MMCs are associated with either an inflammatory status or cachectic diseases [30,33]. The sturgeon runt condition certainly resembles a cachectic disease, and the food deprivation is considered per se able of inducing inflammatory responses [34]. We suggest that the pathological aspects we have observed in the gut of runt sturgeons may depend on the fact that their alimentary canals contain unwelcome guests, the diatoms, which were by us discovered in them by tissues chemical digestion. In sharp contrast, we never found diatoms in the alimentary canals of the normally feeding sturgeons examined in parallel. Accordingly, the guts of these latter were fully normal in their structure, which we have al0 ready described [30,31].
Interestingly, the diatoms were not detected in all the examined organs from the runt sturgeons, but only in those ones (the glandular body and the mid-intestine with spiral valve) in which, due to their complex structure with many complicated folds [29,30], the luminal contents move slowly and the diatoms within the alimentary canal could have prolonged contact with the intestinal mucosa. The prolonged contact of diatoms with the runt sturgeon intestinal mucosa may be detrimental for the host animal due to : 1) the siliceous frustule, which can only be digested with difficulty, if not at all, and may mechanically damage the intestinal cells; and 2) the possible synthesis and periodical release of biotoxins (e.g., domoic acid) by the diatoms. We hypothesize that diatoms enter the alimentary canal of runt sturgeons, when hierarchical relationships or social stressors among the fishes of a stock prevent some sturgeons from feeding properly, and this for such a long time to permanently impair the access to feed for the runt individuals. So, mixed with the normally feeding sturgeons that continue to feed properly and quickly, the feeding status of the “runt” sturgeons gradually worsens. Because sturgeons are benthophagous, they latter graze the only feed which is utilizable, that is bottom mud, and ingest diatoms, maybe during their periodical blooms. Consequently, a variable number of diatoms enter the alimentary canals of runt sturgeons, and gradually worsen the emaciated status of fish as a result of the poor (or incorrect) food ingested.
The presence of diatoms in the fish alimentary canal, as well as in tissues different from the gastrointestinal tract, is not a frequent report. Xie [34] has shown that the alimentary canal of the bighead carp [Aristichthys nobilis (Richardson, 1845)] can partially disrupt the algal (Cyclotella spp.) cell walls, above all by the action of pharyngeal teeth. Sanchez Rueda [35] observed that benthic diatoms were the basic food of Mugil spp. Casatti et al. [36] have described the presence of diatoms in Brazilian algivorous fish species, without mentioning lesions. Speare et al. [37] described a severe gill pathology in Coho salmon linked to the presence of diatom and microsporidian infections.
In the present paper, the most frequent taxon identified in the glandular body was Fragilaria spp (82.76%), followed by Rhoicosphenia curvata (Kützing) (15.37%), and the same genera with similar percentages were observed in the mid-intestine (91.62% and 4.99% respecttively). Additionally, other taxa such as Diatoma vulgare, Synedra spp., Navicula spp., Nitzschia palea, and others were observed in smaller numbers.
Chemical treatments of both waters and mud sediments revealed the presence of species, which are frequently described in artificial systems, and the most of them live on either aquatic plants or walls of tanks.
Comparing the results from sturgeon tissues and those ones coming from tanks, it was observed that Fragilaria spp and R. curvata were present in both tissues and tanks, whereas Cyclotella was not (glandular body) or only minimally (mid-intestine) detected in the organs of runt sturgeons, even if it was always present in the tanks. These data suggest that shape and size of diatoms can be considered as limiting factors for the possible tissue penetration into the alimentary canal of sturgeons that ingest sediments. In fact, Cyclotella (belonging to Centrales diatoms) is cylindrical in shape and has a round profile, and for this reason it is presumed that diatoms belonging to this taxon, even if they enter the alimentary canal, move smoothly along the mucosal surfaces and do not come into intimate contact with them or do not cause important epithelial cell losses. On the contrary, Fragilaria spp and R. curvata (belonging to Pennales diatoms) have a long axis with acuminate extremities, and can be 10 μm long. These latter two taxa may cause the changes in the intestinal mucosa, above all when the movements of the luminal contents are delayed by the absence of proper feed and by the complex structure, so rich of folds, of both the glandular body and mid-intestine.
The detrimental structural effects linked to the diatoms, above all Pennales diatoms, may be the cause of seconddary bacterial pathologies in runt sturgeons. Even if this hypothesis is not confirmed or substantiated by other studies, the results of the preliminary microbiological analyses conducted in parallel upon runt and normally feeding sturgeon intestines are interesting and worthy of further investigation. The total bacterial count was uniformly high from the glandular body to the distal intestine in runt sturgeons. Therefore it could be hypothesized that the total bacterial count has been influenced in runt sturgeon intestines by the mucosal lesions and structural changes observed in the tunica propria-submucosa, possibly linked to the presence of diatoms. The evaluation of the presence of Enterobacteriaceae appeared interesting, because the intestines of normal animals were colonized by E. coli and other typical Enterobacteriaceae, whereas in runt sturgeons the intestinal microbial flora consisted of potentially pathogenic bacteria (Aeromonas hydrophila, Klebsiella pneumoniae, Serratia spp, Citrobacter freundii), which perhaps are responsible, together with diatoms, for the reason described in the gut of runt sturgeons. Interestingly, Grossart et al., [38] have recently reported that marine diatoms, especially during algal blooms, harbour bacterial species, including potential fish pathogens, such as those belonging to Flavobacteria and Sphingobacteria.
In conclusion, this report describes, for the first time to our knowledge, a possible relationship between the presence of runt sturgeons and the periodic algal blooms caused by the diatom populations in some rearing tanks of an Italian fish farm, suggesting a coherent hypothesis for runt sturgeon etiopathogenesis, at least valid for the observed fish farm.
We can thus suggest a coherent hypothesis for runt sturgeon ethio-pathogenesis, at least valid for the observed fish farm. The variable percentages of the runt occurrence in the studied fish farm is coherent with the hypothesized causative mechanism, because the diatom blooms may vary in number and taxa during either one year or in successive years, depending on the environmental variables. The fact that recovering runt sturgeons in tanks different from those ones, in which the cachectic syndrome was observed, was without effect (unpublished observations), also appears congruent with our hypothesis concerning a durative, possible detrimental relationship between diatoms and mucosal surfaces of the runt gut.
5. Acknowledgements
Authors wish to thank Dr. Mario Pazzaglia (Agroittica Lombarda) for helping in collecting sturgeon samples. This work was supported by grants of the University of Milan, Italy (F.I.R.S.T. 2004, 2005) and of CARIPLO Foundation (2004-2005) to Cinzia Domeneghini.
REFERENCES
- C. Domeneghini, G. Radaelli, G. Bosi, S. Arrighi, A. Di Giancamillo, M. Pazzaglia and F. Mascarello, “Morphological and Histochemical Differences in the Structure of the Alimentary Canal in Feeding and Runt (Feed Deprived) White Sturgeons (Acipenser transmontanus),” Journal of Applied Ichthyology, Vol. 18, No. 4-6, 2002, pp. 341-346. doi:10.1046/j.1439-0426.2002.00384.x
- M. P. Georgiadis, R. P. Hedrick, W. O. Johnson and I. A. Gardner, “Mortality and Recovery of Runt White Sturgeons (Acipenser transmontanus) in a Commercial Farm in California, USA,” Preventive Veterinary Medicine, Vol. 43, No. 4, 2000, pp. 269-281. doi:10.1016/S0167-5877(99)00105-1
- M. P. Georgiadis, R. P. Hedrick, W. O. Johnson and I. A. Gardner, “Growth of White Sturgeon (Acipenser transmontanus) Following Recovery from the Stunted Stage in a Commercial Farm in California, USA,” Preventive Veterinary Medicine, Vol. 29, 2000, pp. 283-291. doi:10.1016/S0167-5877(99)00106-3
- D. J. Martell, J. D. Kieffer and E. A. Trippel, “Effects of Temperature during Early Life History on Embryonic and Larval Development and Growth in Haddock,” Journal of Fish Biology, Vol. 66, No. 6, 2005, pp. 1558-1575. doi:10.1111/j.0022-1112.2005.00699.x
- J. T. Silverstein, M. Hostuttler and K. P. Bleming, “Strain Differences in Feed Efficiency Measured as Residual Feed Intake in Individually Reared Rainbow Trout, Oncorhynchus mykiss (Walbaum),” Aquaculture Research, Vol. 36, No. 7, 2005, pp. 704-711. doi:10.1111/j.1365-2109.2005.01278.x
- S. A. Correa, M. O. Fernandes, K. K. Iseki and J. A. Negrao, “Effect of Establishment of Dominance Relationship on Cortisol and Other Metabolic Parameters in Nile Tilapia (Oreochromis niloticus),” Brazilian Journal of Medical and Biological Research, Vol. 36, No. 12, 2003, pp. 1725-1731. doi:10.1590/S0100-879X2003001200015
- S. Rafatnezhad, B. Falahatkar and M. H. T. Gilani, “Effects of Stocking Density on Haematological Parameters, Growth and Fin Erosion of Great Sturgeon (Huso huso) Juveniles,” Aquaculture Research, Vol. 39, No. 14, 2008, pp. 1506-1513. doi:10.1111/j.1365-2109.2008.02020.x
- S. E. Wendelaar Bonga, “The Stress Response in Fish,” Physiology Review, Vol. 77, No. 3, 1997, pp. 591-625.
- J. L. C. Wright, R. K. Boyd, A. S. W. de Freitas, M. Falk, R. A. Foxall and W. D. Jamieson, “Identification of Domoic Acid, a Neuroexcitatory Amino Acid, in Toxic Mussels from Eastern Prince Edward Island,” Canadian Journal of Chemistry, Vol. 67, No. 3, 1989, pp. 481-490. doi:10.1139/v89-075
- Z. Amzil, J. Fresnel, D. Le Gal and C. Billard, “Domoic Acid Accumulation in French Shellfish in Relation to Toxic Species of Pseudo-Nitschia multiseries and P. Pseudodelicatissima,” Toxicon, Vol. 39, No. 8, 2001, pp. 1245-1251. doi:10.1016/S0041-0101(01)00096-4
- A. Sierra Beltran, M. Palafox-Uribe, J. Grajales-Montiel, A. Cruz-Villacorta and J. L. Ochoa, “Sea Bird Mortality at Cabo San Lucas, Mexico: Evidence That Toxic Diatom Blooms Are Spreading,” Toxicon, Vol. 35, No. 3, 1997, pp. 447-453. doi:10.1016/S0041-0101(96)00140-7
- K. A. Lefebvre, S. Bargu, T. Kieckhefer and M. W. Silver, “From Sand Dabs to Blue Whales: The Pervasiveness of Domoic Acid,” Toxicon, Vol. 40, No. 7, 2002, pp. 971-977. doi:10.1016/S0041-0101(02)00093-4
- P. R. Costa, R. Rosa, R., A. Duarte-Silva, V. Brotas and M. A. Sampayo, “Accumulation, Transformation and Tissue Distribution of Domoic Acid, the Amnesic Shellfish Poisoning Toxin, in the Common Cuttlefish, Sepia officinalis,” Aquatic Toxicology, Vol. 74, No. 1, 2005, pp. 82-91. doi:10.1016/j.aquatox.2005.01.011
- A. Di Giancamillo, E. Giudici, S. Andreola, D. Porta, D. Gibelli, C. Domeneghini, M. Grandi and C. Cattaneo, “Immersion of Piglet Carcasses in Water—The Applicability of Microscopic Analysis and Limits of Diatom Testing on an Animal Model,” Legal Medicine, Vol. 12, No. 1, 2010, pp. 13-18. doi:10.1016/j.legalmed.2009.09.007
- A. Di Giancamillo, C. Domeneghini, D. Gibelli and C. Cattaneo, “Diatom Extraction with HCl from Animal Tissues: A Technical Note,” Legal Medicine, Vol. 13, No. 5, 2011, pp. 268-271. doi:10.1016/j.legalmed.2011.05.005
- A. C. Camus, P. L. Shewmaker, M. J. Mauel and D. J. Wise, “Streptococcus ictaluri Arthritis, Osteolysis, Myositis and Spinal Cord Meningitis in Channel Catfish Broodstock,” Journal of Aquatic Animal Health, Vol. 20, No. 1, 2008, pp. 54-62.
- S. Jeremic, V. Radosavljevic and D. Jakic-Dimic, “Current Bacterial Diseases of Fresh Water Fishes,” Biotechnology in Animal Husbandry, Vol. 21, No. 3-4, 2005, pp. 141-151. doi:10.2298/BAH0504141J
- O. Bergh, “The Dual Myths of the Healthy Wild Fish and the Unhealthy Farmed Fish,” Disease of Aquatic Organisms, Vol. 75, No. 2, 2007, pp. 159-164. doi:10.3354/dao075159
- K. Pulkkinen, L. R. Suomalainen, A. F. Read, D. Ebert, P. Rintamäki and E. T. Valtonen, “Intensive Fish Farming and the Evolution of Pathogen Virulence: The Case of Columnaris Disease in Finland,” Proceedings of Biological Science, Vol. 277, No. 1681, 2010, 593-600. doi:10.1098/rspb.2009.1659
- O. N. Bauer, O. N. Pugachev and V. N. Voronin, “Study of Parasites and Diseases of Sturgeons in Russia: A Review,” Journal of Applied Ichthyology, Vol. 18, No. 4-6, 2002, pp. 420-429.
- Z. Ma, H. Yang, T. Li, L. Luo and J. Gao, “Isolation and Identification of Pathogenic Aeromonas veronii Isolated from Infected Siberian Sturgeon (Acipenser baerii),” Wei Shen Wu Xue Bao, Vol. 49, 2009, pp. 1289-1294.
- K. Krammer and H. Lange-Bertalot (Nachdr. 1997) “Bacillariophyceae 1. Teil Naviculaceae,” In: H. Ettl, J. Gerloff, H. Heynig and D. Mollenhauer, Eds., Süsswasserflora von Mitteleuropa, Band 2/1, Gustav Fisher, Jena, 1986.
- K. Krammer and H. Lange-Bertalot (Nachdr. 1997) “Bacillariophyceae 2. Teil Bacillariaceae, Epithemiaceae, Surirellaceae,” In: H. Ettl, J. Gerloff, H. Heynig and D. Mollenhauer, Eds., Band 2/2, Gustav Fisher, Jena, 1988.
- K. Krammer and H. Lange-Bertalot, “Bacillariophyceae 3. Teil Centrales, Fragilariaceae, Eunotiaceae,” In: H. Ettl, J. Gerloff, H. Heynig and D. Mollenhauer, Eds., Süsswasserflora von Mitteleuropa, Gustav Fisher, Jena, 1991.
- K. Krammer and H. Lange-Bertalot, “Bacillariophyceae 4. Teil Achnantaceae,” In: J. Gerloff, H. Heynig and D. Mollenhauer, Eds., Kritische Ergänzungen zu Navicula (Lineolatae) und Gomphonema, Gustav Fisher, Jena, 1991.
- F. E. Round, R. M. Crawford and D. G. Mann, “The Diatoms. Biology and Morphology of the Genera,” Cambridge University Press, Cambridge, 1990.
- I. Dalsgaar, “Selection of Media for Antimicrobial Susceptibility Testing of Fish Pathogenic Bacteria,” Aquaculture, Vol. 196, No. 3-4, 2001, pp. 267-275. doi:10.1016/S0044-8486(01)00538-5
- C. Domeneghini, C. Straini, R. Pannelli and A. Veggetti, “Gut Glycoconjugates in Sparus aurata L. (Pisces, Teleostei). A Comparative Histochemical Study in Larval and Adult Ages,” Histology and Histopathology, Vol. 13, 1998, pp. 359-372.
- C. Domeneghini, S. Arrighi, G. Radaelli, G. Bosi and F. Mascarello, “Morphological and Histochemical Peculiarities of the Gut in the White Sturgeon, Acipenser transmontanus,” European Journal of Histochemistry, Vol. 43, 1999, pp. 135-145.
- C. Agius and R. J. Roberts, “Melano-Macrophage Centres and Their Role in Fish Pathology,” Journal of Fish Disease, Vol. 26, No. 9, 2003, pp. 499-509. doi:10.1046/j.1365-2761.2003.00485.x
- I. L. Leknes, “Melano-Macrophage Centres in the Liver of Platyfish, Xiphophorous maculatus, Poeciliidae: Teleostei,” Zoology, Vol. 107, No. 3, 2004, pp. 201-204. doi:10.1016/j.zool.2004.07.002
- P. N. Rodrigues and F. A. Pereira, “A Model for Acute Iron Overload in Sea Bass (Dicentrarchus labrax L.),” Laboratory Animals, Vol. 38, No. 4, 2004, 418-424. doi:10.1258/0023677041958909
- D. J. Wise, T. Greenway, M. H. Li, A. C. Camus and E. H. Robinson, “Effects of Variable Periods of Food Deprivation on the Development of Enteric Septicaemia in Channel Catfish,” Journal of Aquatic Animal Health, Vol. 20, No. 1, 2008, pp. 39-44. doi:10.1577/H07-008.1
- P. Xie, “Gut Contents of Bighead Carp (Aristichthyis nobilis) and the Processing and Digestion of Algal Cells in the Alimentary Canal,” Aquaculture, Vol. 195, No. 1-2, 2001, pp. 149-161. doi:10.1016/S0044-8486(00)00549-4
- P. Sanchez Rueda, “Stomach Content of Mugil cephalus and Mugil curema (Mugiliformes: Mugilidae) with Emphasis on Diatoms in the Tamiahua Lagoon,” Revista de Biologia Tropical, Vol. 50, No. 1, 2002, pp. 245-252.
- L. Casatti, H. F. Mendes and K. M. Ferreira, “Aquatic Macrophytes as Feeding Site for Small Fishes in the Rosana Reservoir, Paranapanema River, South-Eastern,” Brazilian Journal of Biology, Vol. 63, No. 2, 2003, pp. 213-222. doi:10.1590/S1519-69842003000200006
- D. J. Speare, J. Brackett and H. W. Ferguson, “Sequential Pathology of the Gills of Coho Salmon with a Combined Diatom and Microsporidian Gill Infection,” Canadian Veterinary Journal, Vol. 30, 1989, pp. 571-575.
- H. P. Grossart, F. Levold, M. Allgaier, M. Simon and T. Brinkhoff, “Marine Diatoms Species Harbour Distinct Bacterial Communities,” Environmental Microbiology, Vol. 7, No. 6, 2005, pp. 860-873. doi:10.1111/j.1462-2920.2005.00759.x
NOTES
*Conflict of interest: The Authors have no personal relationships with the fish farm, so that no bias influenced their work.
#Corresponding author.

