Advances in Microbiology
Vol.3 No.8A(2013), Article ID:41268,8 pages DOI:10.4236/aim.2013.38A003
Mycelial Growth of Pleurotus Spp in Se-Enriched Culture Media
Departamento de Microbiologia, Federal University of Viçosa, Campus UFV, Viçosa, Brazil
Email: *mkasuya@ufv.br
Copyright © 2013 Marliane de Cássia Soares da Silva et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received October 25, 2013; revised November 25, 2013; accepted December 2, 2013
Keywords: Mushrooms; Colony Morphology; Food; Sodium Selenite; Hyphae Diameter
ABSTRACT
Selenium (Se) is an essential element to human. However, this element can be in low content in soil of some regions. Se deficiency may cause Keshan disease, thyroid dysfunction and osteoarthritis. The Se-enriched cereals are an interesting way to prevent these diseases. But, recent studies have shown that Se-enriched mushrooms are a better Se source. This occurs due to the high capacity of the fungi to absorb and transform the inorganic Se to organic forms, which are more bioavailable. Pleurotus ostreatus and Pleurotus eryngii are mushrooms species worldwide consumed and able to Se bioaccumulate. However, depending on the level of this element, it can be toxic for the fungus. Here we showed that the presence of the Se in culture medium decreases fungal growth rate, hyphae diameter and septum distance and causes alteration in color of colony. A garlic strong smell was directly proportional to Se level. P. eryngii was more tolerant to Se than P. ostreatus. So, it is important to screen this element level for Se-enriched mushroom production.
1. Introduction
Selenium (Se) is an essential element to human, and the biological activity is attributed to Se-aminoacids and the cofactor of enzymes [1,2]. Some diseases, such as Keshan disease, thyroid dysfunction and osteoarthritis have been attributed to Se deficiency [3,4]. Some studies have also shown that the increase in the daily Se intake by patients with cancer decreases the collateral effects caused by the chemotherapy [5].
Wheat and other cereals have been fortified with Se [6], but when compared to mushrooms, the Se absorption is lower. Da Silva et al. [7] were able to produce Pleurotus ostreatus mushroom with 857.80 µg∙g−1 of Se. The P. ostreatus has been able to absorb Se in higher concentration than Agaricus bisporus [8], Boletus edulis [9], Pleurotus eryngii [10], Ganoderma lucidum [11] and Lentinula edodes [12].
Pleurotus sp. is a food with high nutritional value [13,14] and is worldwide consumed. It is suggested that 1.0 g of P. ostreatus [7] or 85 g of P. eryngii [10] fresh mushrooms is enough to supply the Se recommended for adults [15]. Se-enriched mushrooms produce antioxidants compounds [16,17], and decrease both the proliferation of carcinogenic cells [11] and the activation of a substance that induce tumor development [8]. Therefore, Se-enriched mushrooms could be a good source of this element which has important biological functions.
Although it has shown that the enrichment with sodium selenite does not affect the P. eryngii mushroom yield [10] or the mycelial growth of P. ostreatus [18] depending on the concentration, Se can be toxic to Pleurotus spp [19]. Indeed, da Silva et al. [7] showed that Seenriched substrate with more than 25.4 mg∙kg−1 of Se decreases the mushroom production of P. ostreatus. Therefore, depending on the Se level used for enrichment, the growth rate of the fungus can be decreased or inhibited.
The assessment of the highest Se concentration in the culture media that do not affect mycelial growth of Pleurotus spp is important to determine the Se concentration that can be used in the substrate for production of Se-enriched mushrooms. So, we selected isolates of P. ostreatus and P. eryngii to evaluate the mycelial growth and biomass production, besides the fungal macroscopic and microscopic morphological features, when growing in culture media with different concentrations of Se.
2. Material and Method
2.1. Microorganisms
We used three isolates of three of P. eryngii (PLE 01, PLE 02 and PLE 03) and P. ostreatus (PLO 02, PLO 06 and PLO 09) belong to collection of the Department of Microbiology of Federal University of Viçosa/BIOAGRO, MG, Brazil. The isolates were grown in a Petri dish containing potato dextrose agar (PDA) culture medium, pH 5.8, and incubated at 25˚C ± 2˚C during seven days. After this period, an agar disc of eight mm, cut from the border of the colony, was transferred to PDA media containing 0, 25.4, 50.9, 76.4 or 101.8 mg∙L−1 of Se in form of sodium selenite, and incubated at 25˚C ± 2˚C during seven days. These Se levels were choose based on previous studies [7,12].
2.2. Growth Rate and Biomass
The growth rate was determined by the colony diameter, measured by taken two measurements, perpendicular to each other, at 7th day, and divided by seven.
To biomass evaluation, the culture medium with mycelium was transferred to a flask with 200 mL of distilled water and boiled, in microwave oven, to liquefy agar. Then, the solution was filtered, rinsed in distilled water and the mycelium was taken and put in an oven at 60˚C, until constant weight.
2.3. Diameter and Septum of Hyphae
The diameter and septum of hyphae were measured after stained with calcofluor and observed under epifluorescence microscopy. The images were capture by digital camera FUJIX HC-300Z and processed with the software Image Pro Plus.
2.4. Colonies Morphology
Colonies morphology was evaluated by the following qualitative criterions: alteration in medium color, alteration in colony color, alteration in colony thickness.
2.5. Statistics
The experiment was a completely randomized design, with five repetitions. The assay was repeated twice. The data were subjected to analysis of variance and mean values were compared by Tukey’s test (p < 0.05).
3. Results
All isolates tested decreased the growth rate (Figure 1(a)) and the mycelial dry mass (Figure 1(b)) in presence of Se. These reductions were higher in P. ostreatus than P. enrygii and more accentuated up to 50.9 mg∙L−1 (Figure 1).
The reduction of 2.5 - 8 folds in dry mass shows that both fungi were sensitive to Se. But, the reductions of growth rate and dry mass were not proportional to the increases Se levels in culture medium (Figure 1). This indicates that the fungi could have some mechanism of tolerance to the toxic effect of this mineral. Note that for PLE 03 the twice of the Se concentration not caused alterations in growth rate and dry mass (Figure 1).
The dry mass methodology showed to be a method more sensitive than growth rate. Indeed, the highest decreases in dry mass of P. ostreatus demonstrate that this fungal specie was less tolerant to Se than P. eryngii. This fact is important for selection of appropriate isolates for production of mushrooms in substrate with highest Se levels.
The Se addition decreased the septum distance and the hyphae diameter of all isolates, except for PLO 09 and PLE 02, at 25.4 mg∙L−1 of Se which increased the septum distance, and PLO 06 which increased the hyphae di-

Figure 1. Growth rate (a) and dry mass (b) of Pleurotus eryngiii (PLE 01, PLE 02 and PLE 03) and Pleurotus ostreatus (PLO 02, PLO 06 and PLO 09) cultivated in different selenium concentrations.
ameter (Figures 2 and 3). Furthermore, we not observed significant difference (p < 0.05) in these parameters in Se levels higher than 50.9 mg∙L−1 (Figures 2 and 3). However, in the 101.8 mg∙L−1 of Se, for most strains tested, we observed a decrease in septa distance and hyphae diameter when compared with medium without Se (Figures 2-4). These observations affirm that fungi has mechanisms of tolerance to Se.
The colony morphology of all isolates was also affected by Se addition (Figure 5). The isolates of P. ostreatus had a lower mycelial density than P. eryngii (Figure 5). The colony color changed from white to strong orange, mainly in the center of the colony, suggesting that the contact time with Se influences the colony color. Halos formation and a strong smell were intensified with increasing the Se concentration. This suggested that some volatile compound are produced due to the Se presence.
4. Discussion
In this study, addition of Se was harmful to fungal growth and development (Figures 1 and 5), showing that Se in high concentrations is toxic [20]. A high Se concentration also inhibited mushroom formation [7]. Other metal can inhibit or reduce basidiomycetes growth, as Hg, Cu and Ni [21]. This is interesting, because theses metals have been used for development of antifungal wood preservatives, which allow us to conclude that concentrations higher than 101.8 mg∙L−1 of Se also can be used for the development of antifungal preservatives. Fungal growth in wood structures is a problem, because during the fungus growth, lignin, cellulose and other compounds are degraded [22,23], damaging the wood. The highest mycelial density decreasing of P. ostreatus isolates, together with growth rate and dry mass reduction clearly shows that P. ostreatus is more sensitive to Se than P. eryngii (Figure 1). Therefore, utilization of Se as antifungal preservative should be more effective to this fungus.
The interest to produce Se-enriched mushrooms has increased in the last years [7,24,25]. One of the most important steps for mushrooms production is the mycelial development in the substrate. As faster the fungus grown, less time will be available for contaminants development, which increases the probability to have a high mushrooms yield. Thus, decreasing the mycelial growth rate is not desirable and should be avoid or minimized. We observed that the addition of 50.9 mg∙L−1 decreased drastically the fungal grown rate of the isolates (Figure 1) what can be reflecting in the reduction of P. ostreatus
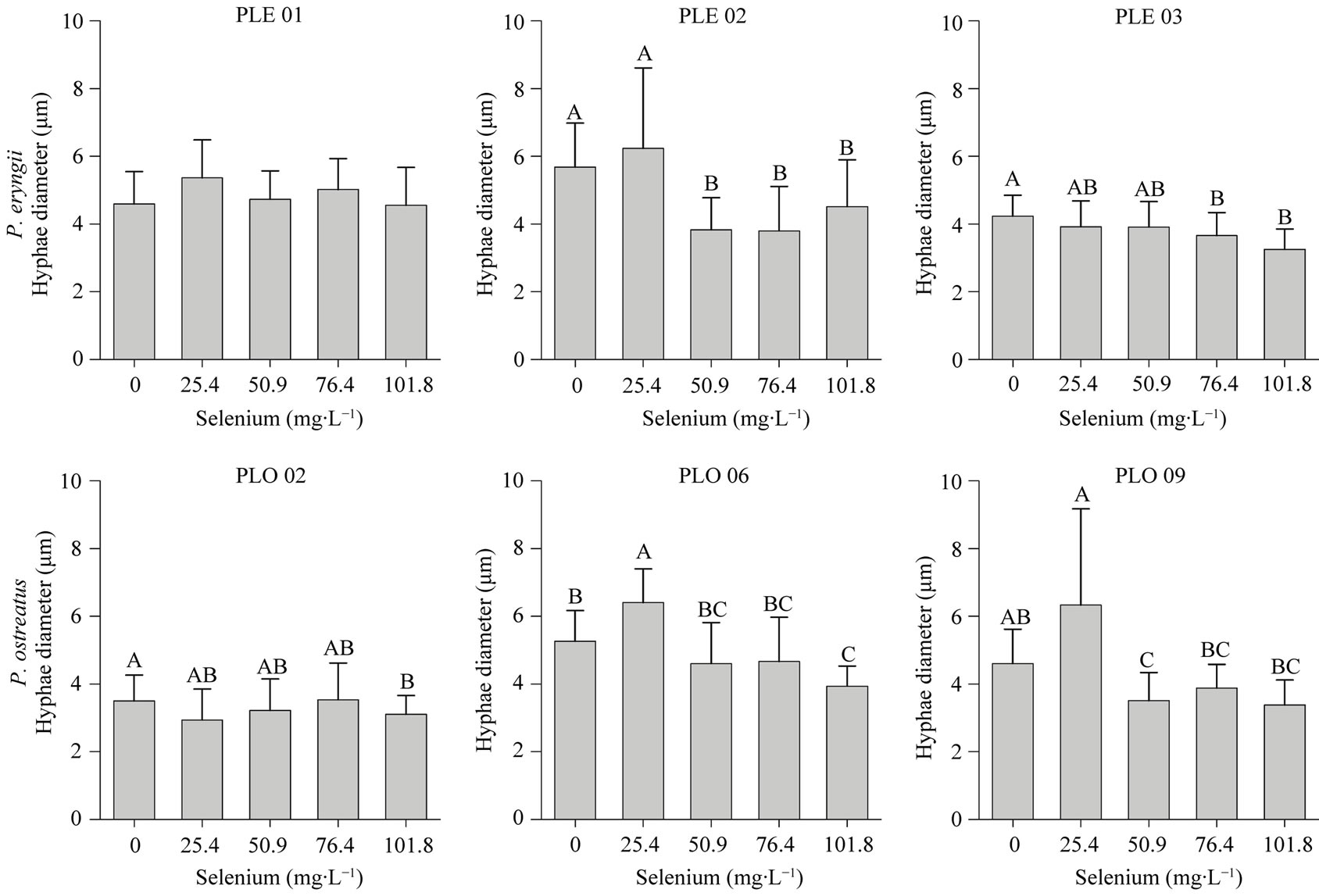
Figure 2. Hyphae diameter of Pleurotus eryngiii (PLE 01, PLE 02 and PLE 03) and Pleurotus ostreatus (PLO 02, PLO 06 and PLO 09) cultivated in different selenium concentrations. Means followed by different letters differ at Tukey’s test (p < 0.05).

Figure 3. Septa distance of Pleurotus eryngiii (PLE 01, PLE 02 and PLE 03) and Pleurotus ostreatus (PLO 02, PLO 06 and PLO 09) cultivated in different selenium concentrations. Means followed by different letters differ at Tukey’s test (p < 0.05).
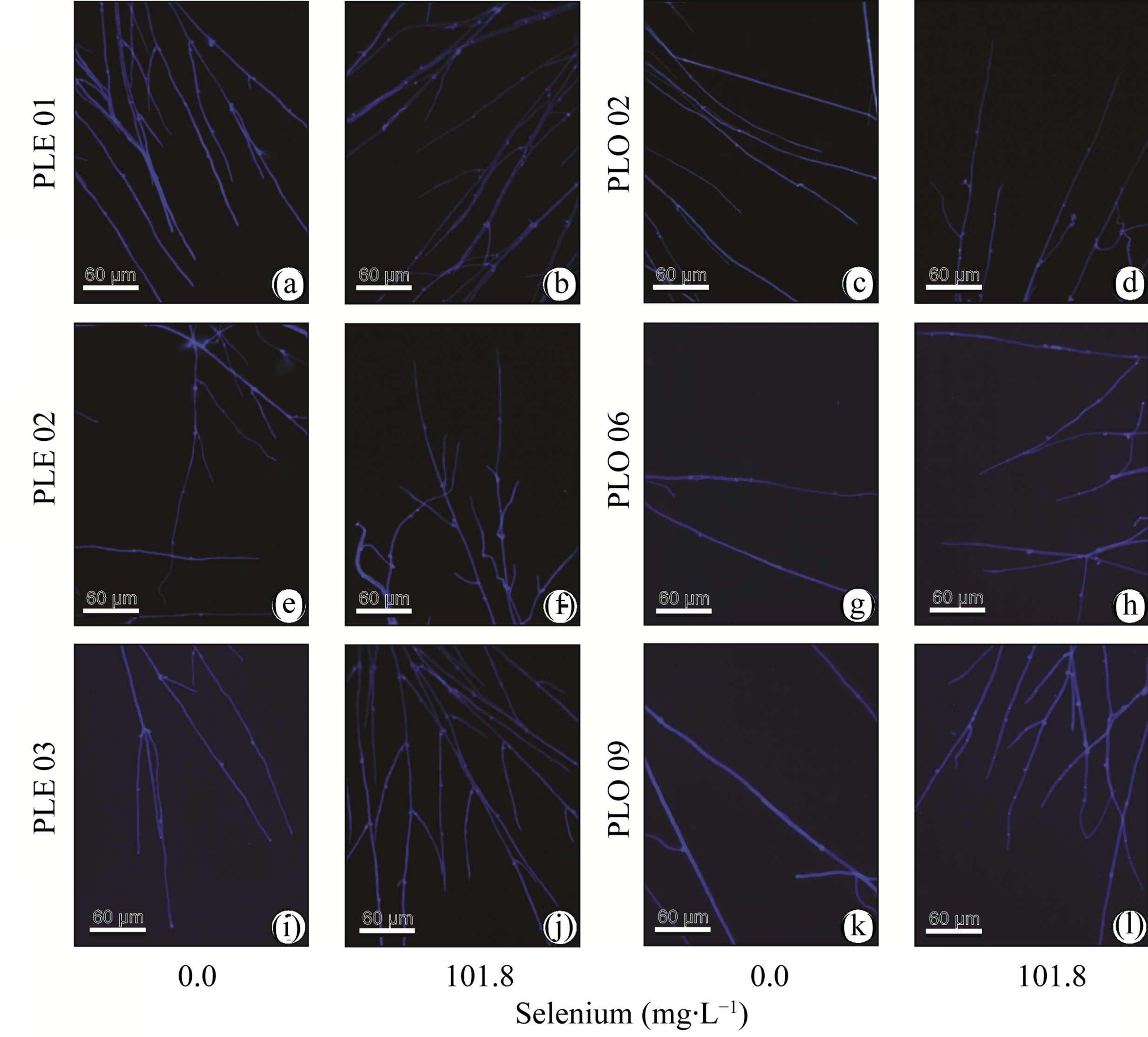
Figure 4. Hyphae of Pleurotus eryngiii (PLE 01, PLE 02 and PLE 03) and Pleurotus ostreatus (PLO 02, PLO 06 and PLO 09) growth in media without selenium ((a), (c), (e), (g), (i), (k)) and with selenium ((b), (d), (f), (h), (j), (l)) stained with calcofluor.
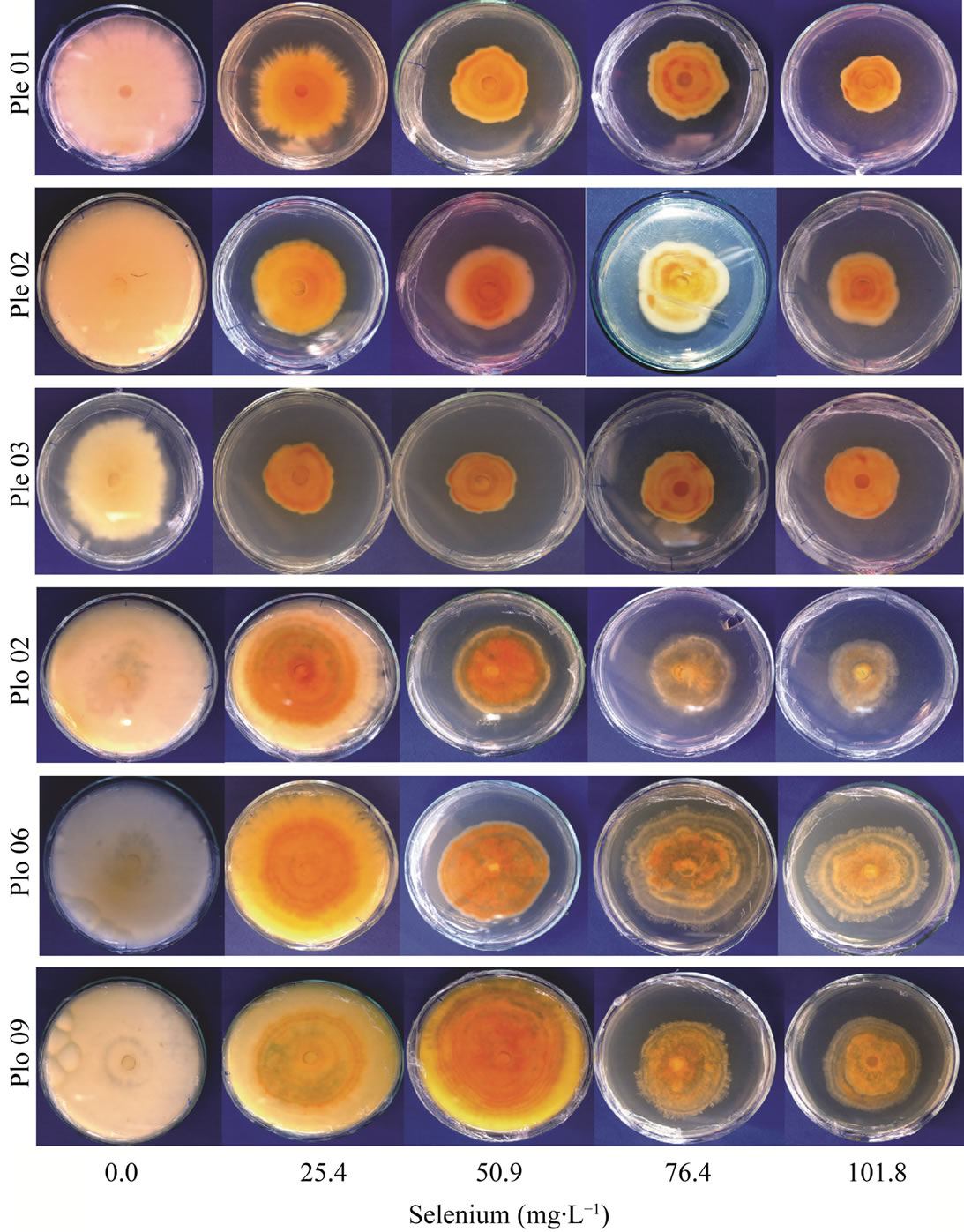
Figure 5. Mycelial growth of Pleurotus eryngiii (PLE 01, PLE 02 and PLE 03) and Pleurotus ostreatus (PLO 02, PLO 06 and PLO 09) cultivated in different selenium concentrations.
mushroom production, when 25.4 mg∙L−1 of Se was added to the substrate for [7]. However, the same authors observed an increase in mushroom yield using the concentration of 12.7 mg∙Kg−1. Therefore, we suggested that Se-enrichment of Pleurotus sp with concentrations higher than 25.4 mg∙L−1 of Se should be avoided.
Hyphae shrinkage is also a morphological change that some fungi perform when growing in a not favorable condition (Figure 2). An increasing of Cu concentration in culture medium decreased the hyphae diameter of Amycolatopsis eurytherma [26]. Fusarium oxysporum decreased their hyphae diameter when grown in the presence of fungicide oil [27]. However, other fungi can increase their hyphae diameter when growing in a stressful condition [28,29], showing that there is no standard behavior for the fungi. Hyphae diameter decreasing could be related to energy saving, since the reduction in the cell diameter decrease the energy necessary to cell growth. This energy could be relocated to the fungal radial growth, producing effuse mycelium that is more appropriated for resource exploration.
The decreasing of the septum distance (Figure 3) has been also observed as a fungus response to adverse conditions [28,30,31]. Turner and Harris [31] observed that a mutant of Aspergillus nidulas was grown in the presence of Calcofluor white had decreased their septum distance. Pythium ultimum and Rhizoctonia solani when exposure to viscosinamide, an antifungal compound, increased their hyphal septation and branching [31]. When a gene require for Fusarium oxysporum grown was knockout, it was observed an increase in hyphal septation [29]. Septa serve to compartmentalize hyphae, but permit communication between cells. However, septa pores can be blocked. Thereby, if a cell suffers any damage the fungus can immediately block the septa preventing other cells to be affected. So, the increase of the hyphae septation can reduce the portion of the colony affected by an eventually damage. Thus, it should be reasonable to conclude that filamentous fungi can increase their hyphal septation in a stressful condition as a way to protect the colony, increasing the survive chance of the fungi.
The colony color change from white to orange indicates that the fungi are producing some intracellular compound due to the Se (Figure 5). These compounds may be related to the capacity of the fungi to adapt and growth in environments with high Se levels. Therefore, further metabolic studies should be done to identify and evaluate if the production of these compounds are related to Se level and if it brings some adaptive advantage to the fungi.
Other mechanism that may be related to fungal tolerance to Se is alterations in the enzymatic activity. Nunes et al., [32] showed increase in laccase activity of Lentinula edodes in medium with Se. This is important because this enzyme has been used in many detoxification process [33-35].
The color change of the colony could also be related to volatile compounds. The volatile compounds nonadecanoic acid, 9,12-octadecadien-1-ol, cis-Linoleic acid methyl ester, hexadecanoic acid-palmitic acid, palmitic acid, (2-tetradecyloxy) ethyl ester, and others, had already been reported to be produced by Pleurotus sp. [36]. Some of those compounds are colorful, for example, yellow. Due to the strong smell coming from fungal colonies and the culture medium color change, we suggested that the fungi are producing volatile compounds to avoid or minimized the Se uptake and toxicity. From this starting point, further studies should be realized to elucidate the mechanism used by the fungi to adapt to environments with high Se concentration.
5. Conclusions
Selenium can be toxic to fungi. The fungi respond to this compound changing their macroscopic and microscopic morphology. The main changes are the colony color, increasing hyphae septation and hyphae shrinkage. All those changes can help to elucidate the strategy of this microorganism to adapt to high Se concentration.
Determination of Se concentration that impairs fungal mycelium growth is very important, because there is the need to know which concentration of this compound is more appropriate to be used to produce Se-enriched Pleurotus spp mushrooms.
Due to the selenium toxicity, high Se concentration can be used for fungicide development. Determination of the minimal inhibitory concentration of Se for different fungi may help to clarify the potential of this mineral as fungicide.
REFERENCES
- C. Allmang and A. Krol, “Selenoprotein Synthesis: UGA Does not End the Story,” Biochimie, Vol. 88, No. 11, 2006, pp. 1561-1571. http://dx.doi.org/10.1016/j.biochi.2006.04.015
- L. R. Ferguson, N. Karunasinghe, S. Zhua and H. A. Wang, “Selenium and Its’ Role in the Maintenance of Genomic Stability,” Mutation Research, Vol. 733, No. 1-2, 2012, pp. 100-110. http://dx.doi.org/10.1016/j.mrfmmm.2011.12.011
- G. F. J. R. Combs, “Selenium in Global Food Systems,” British Journal of Nutrition, Vol. 85, No. 5, 2001, pp. 517-547. http://dx.doi.org/10.1079/BJN2000280
- L. V. Papp, J. Lu, A. Holmgren and K. K. Khanna, “From Selenium to Selenoproteins: Synthesis, Identity, and Their Role in Human Health,” Antioxidants & Redox Signaling, Vol. 9, No. 7, 2007, pp. 775-806. http://dx.doi.org/10.1089/ars.2007.1528
- K. Sieja and M. Talercszyk, “Re: Selenium as Element in the Treatment of Ovarian Cancer in Women Receiving Chemotherapy,” Gynecology and Oncology, Vol. 96, No. 2, 2004, pp. 559-561. http://dx.doi.org/10.1016/j.ygyno.2004.11.016
- D. J. Hart, S. J. Fairweather-Tait, M. R. Broadley, S. J. Dickinson, I. Foot, P. Knott, S. P. McGrath, H. Mowat, K. Norman, P. R. Scott, J. L. Stroud, M. Tucker, P. J. White, F. J. Zhao and R. Hurst, “Selenium Concentration and Speciation in Biofortified Flour and Bread: Retention of Selenium during Grain Biofortification, Processing and Production of Se-Enriched Food,” Food Chemistry, Vol. 126, No. 4, 2011, pp. 1771-1778. http://dx.doi.org/10.1016/j.foodchem.2010.12.079
- M. C. S. da Silva, J. Naozuka, J. M. R. da Luz, L. S. de Assunção, P. V. Oliveira, M. C. D. Vanetti, D. M. S. Bazzolli and M. C. M. Kasuya, “Enrichment of Pleurotus Ostreatus Mushrooms with Selenium in Coffee Husks,” Food Chemistry, Vol. 131, No. 2, 2012, pp. 558-563. http://dx.doi.org/10.1016/j.foodchem.2011.09.023
- M. R. Spolar, E. M. Schaffer, R. B. Beelman and J. A. Milner, “Selenium-Enriched Agaricus bisporus Mushrooms Suppress 7,12-Dimethlybenz[a]anthracene Bioactivation in Mammary Tissue,” Cancer Letters, Vol. 138, No. 1, 1999, pp. 145-150. http://dx.doi.org/10.1016/S0304-3835(99)00003-8
- L. Cocchi, L. Vescovi, L. E. Petrini and O. Petrini, “Heavy Metals in Edible Mushrooms in Italy,” Food Chemistry, Vol. 98, No. 2, 2006, pp. 277-284. http://dx.doi.org/10.1016/j.foodchem.2005.05.068
- A. Rodriguez Estrada, H. J. Lee, R. Beelman, M. D. Jimenez-Gasco and D. Royse, “Enhancement of the Antioxidants Ergothioneine and Selenium in Pleurotus eryngii var. eryngii Basidiomata through Cultural Practices,” World Journal of Microbiology and Biotechnology, Vol. 25, No. 9, 2009, pp. 1597-1607. http://dx.doi.org/10.1007/s11274-009-0049-8
- D. Shang, J. Zhang, L. Wen, Y. Li and Q. Cui, “Preparation, Characterization, and Antiproliferative Activities of the Se-Containing Polysaccharide SeGLP-2B-1 from SeEnriched Ganoderma lucidum,” Journal of Agricultural and Food Chemistry, Vol. 57, No. 17, 2009, pp. 7737- 7742. http://dx.doi.org/10.1021/jf9019344
- R. G. F. L. Nunes, J. M. R. Luz, R. B. Freitas, A. Higushi, M. C. M. Kasuya and M. C. D. Vanetti, “Selenium Bioaccumulation in Shiitake Mushrooms: A Nutritional Alternative Source of This Element,” Journal of Food Science, Vol. 77, No. 9, 2012, pp. 983-986. http://dx.doi.org/10.1111/j.1750-3841.2012.02837.x
- R. Sanmee, B. Dell, P. Lumyong, K. Izumori and S. Lumyong, “Nutritive Value of Popular Wild Edible Mushrooms from Northern Thailand,” Food Chemistry, Vol. 82, No. 4, 2003, pp. 527-532. http://dx.doi.org/10.1016/S0308-8146(02)00595-2
- E. Bernaś, G. Jaworska, Z. Lisiewska, “Edible Mushrooms as a Source of Valuable Nutritive Constituents,” Acta Scientiarum Polonorum—Technologia Alimentaria, Vol. 5, No. 1, 2006, pp. 5-20.
- Institute of Medicine, “Dietary Reference Intakes for Vitamin C, Vitamin E, Selenium, and Carotenoids,” National Academy Press, Washington DC, 2000, p. 506.
- L. Zhao, G. Zhao, B. Hui, Z. Zhao, J. Tong and X. Hu, “Effect of Selenium on Increasing the Antioxidant Activity of Protein Extracts from a Selenium-Enriched Mushroom Species of the Ganoderma Genus,” Journal of Food Science, Vol. 69, No. 3, 2004, pp. 184-188.
- S. H. Lee, B. Y. Park, S. S. Lee, N. J. Choi, J. H. Lee, J. M. Yeo, J. K. Ha, W. J. Maeng and W. Y. Kim, “Effects of Spent Composts of Selenium-Enriched Mushroom and Sodium Selenite on Plasma Glutathione Peroxidase Activity and Selenium Deposition in Finishing Hanwoo Steers,” Asian-Australasian Journal Animal Science, Vol. 19, No. 7, 2006, pp. 984-991.
- M. Stajić, I. Milenković, I. Brčeski, J. Vukojević and S. Duletić-Lausević, “Mycelial Growth of Edible and Medicinal Oyster Mushroom [Pleurotus ostreatus (Jacq.: Fr.) Kumm.] on Selenium-Enriched Media,” International Journal of Medicinal Mushrooms, Vol. 4, No. 3, 2002, pp. 241-244. http://dx.doi.org/10.1615/IntJMedMushr.v4.i3.70
- M. Stajić, I. Brčeski, S. P. Wasser and E. Nevo, “Screening of Selenium Absorption Ability of Mycelia of Selected Pleurotus Species,” Agro Food Industry High-Tech, Vol. 17, No. 3, 2006, pp. 33-35.
- J. E. Spallholz, “On the Nature of Selenium Toxicity and Carcinostatic Activity,” Free Radical Biology and Medicine, Vol. 17, No. 1, 1994, pp. 45-64. http://dx.doi.org/10.1016/0891-5849(94)90007-8
- P. Baldrian, “Interactions of Heavy Metals with White- Rot Fungi,” Enzyme and Microbial Technology, Vol. 32, No. 1, 2003, pp. 78-91. http://dx.doi.org/10.1016/S0141-0229(02)00245-4
- K. Kannan, G. Oblisami and B. G. Loganathan, “Enzymology of Ligno-Cellulose Degradation by Pleurotus sajorcaju during Growth on Paper-Mill Sludge,” Biological Wastes, Vol. 33, No. 1, 1990, pp. 1-8. http://dx.doi.org/10.1016/0269-7483(90)90116-A
- A. T. Martı́nez, S. Camarero, A. Gutiérrez, P. Bocchini and G. C. Galletti, “Studies on Wheat Lignin Degradation by Pleurotus Species Using Analytical Pyrolysis,” Journal of Analytical and Applied Pyrolysis, Vol. 58-59, 2001, pp. 401-411. http://dx.doi.org/10.1016/S0165-2370(00)00116-9
- P. Bhatia, F. Aureli,, M. D’Amato, R. Prakash, S. S. Cameotra, T. P. Nagaraja and F. Cubadda, “Selenium Bioaccessibility and Speciation in Biofortified Pleurotus Mushrooms Grown on Selenium-Rich Agricultural Residues,” Food Chemistry, Vol. 140, No. 1-2, 2013, pp. 225- 230. http://dx.doi.org/10.1016/j.foodchem.2013.02.054
- O. Cremades, M. M. Diaz-Herrero, P. Carbonero-Aguilar, J. F. Gutierrez-Gil, E. Fontiveros, B. Rodríguez-Morgado, J. Parrado and J. Bautista, “Preparation and Characterisation of Selenium-Enriched Mushroom Aqueous Enzymatic Extracts (MAEE) Obtained from the White Button Mushroom (Agaricus bisporus),” Food Chemistry, Vol. 133, No. 4, 2012, pp. 1538-1543. http://dx.doi.org/10.1016/j.foodchem.2012.02.046
- J. S. Dávila Costa, V. H. Albarracín and C. M. Abate, “Responses of Environmental Amycolatopsis Strains to Copper Stress,” Ecotoxicology and Environmental Safety, Vol. 74, No. 7, 2011, pp. 2020-2028. http://dx.doi.org/10.1016/j.ecoenv.2011.06.017
- A. Tripathi, N. Sharma and V. Sharma, “In Vitro Efficacy of Hyptis suaveolens L. (Poit.) Essential Oil on Growth and Morphogenesis of Fusarium oxysporum f. sp. Gladioli (Massey) Snyder & Hansen,” World Journal of Microbiology and Biotechnology, Vol. 25, No. 3, 2009, pp. 503-512. http://dx.doi.org/10.1007/s11274-008-9916-y
- M. McIntyre, J. Dynesen and J. Nielsen, “Morphological Characterization of Aspergillus nidulans: Growth, Septation and Fragmentation,” Microbiology, Vol. 147, 2001, pp. 239-246.
- Y. Denisov, S. Freeman and O. Yarden, “Inactivation of Snt2, a BAH/PHD-Containing Transcription Factor, Impairs Pathogenicity and Increases Autophagosome Abundance in Fusarium oxysporum,” Molecular Plant Pathology, Vol. 12, No. 5, 2011, pp. 449-461. http://dx.doi.org/10.1111/j.1364-3703.2010.00683.x
- C. Thrane, S. Olsson, T. Harder Nielsen and J. Sørensen, “Vital Fluorescent Stains for Detection of Stress in Pythium ultimum and Rhizoctonia solani Challenged with Viscosinamide from Pseudomonas fluorescens DR54,” FEMS Microbiology Ecology, Vol. 30, No. 1, 1999, pp. 11-23. http://dx.doi.org/10.1111/j.1574-6941.1999.tb00631.x
- G. Turner and S. D. Harris, “Genetic Control of Polarized Growth and Branching in Filamentous Fungi,” The fungal colony, Cambridge University Press, Cambridge, 1999, pp. 229-260. http://dx.doi.org/10.1017/CBO9780511549694.011
- R. G. F. L. Nunes, J. M. R. Luz, E. Fantuzzi, M. C. M. Kasuya and M. C. D. Vanetti, “Regulation of Respiratory and Ligninolytic Enzyme Activity of Lentinula edodes by Selenium,” Advances in Microbiology, in press.
- M. C. M. Kasuya, J. M. R. da Luz, L. P. S. Pereira, J. S. da Silva, H. C. Montavani and M. T. Rodrigues, “BioDetoxification of Jatropha Seed Cake and Its Use in Animal Feed,” In: Z. Fang, Ed., Feedstocks, Production and Applications, Intech, Croatia, Vol. 1, 2012, p. 487.
- J. M. R. da Luz, S. A. Paes, D. P. Torres, M. D. Nunes, M. C. S. da Silva, H. C. Mantovani and M. C. M. Kasuya, “Production of Edible Mushroom and Degradation of Antinutritional Factors in Jatropha Biodiesel Residues,” Food Science and Technology, Vol. 50, No. 2, 2013, pp. 575-580.
- G. S. Nyanhongo, A. Erlacher, M. Schroeder and G. M. Gubitz, “Enzymatic Immobilization of 2,4,6-Trinitrotoluene (TNT) Biodegradation Products onto Model Humic Substances,” Enzyme and Microbial Technology, Vol. 39, No. 6, 2006, pp. 1197-1204. http://dx.doi.org/10.1016/j.enzmictec.2006.03.004
- N. Çağlarırmak, “The Nutrients of Exotic Mushrooms (Lentinula edodes and Pleurotus Species) and an Estimated Approach to the Volatile Compounds,” Food Chemistry, Vol. 105, No. 3, 2007, pp. 1188-1194. http://dx.doi.org/10.1016/j.foodchem.2007.02.021
NOTES
*Corresponding author.

