CellBio
Vol. 2 No. 2 (2013) , Article ID: 32589 , 9 pages DOI:10.4236/cellbio.2013.22006
The Expression of TIMP1, TIMP2, VCAN, SPARC, CLEC3B and E2F1 in Subcutaneous Adipose Tissue of Obese Males and Glucose Intolerance
1Department of Molecular Biology, Palladin Institute of Biochemistry, National Academy of Sciences of Ukraine, Kyiv, Ukraine
2Department of Pediatrics, National Bogomolets Medical University, Kyiv, Ukraine
Email: ominchenko@yahoo.com
Copyright © 2013 Dmytro Minchenko et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received December 1, 2012; revised January 2, 2013; accepted January 17, 2013
Keywords: Obesity; Glucose Intolerance; Men; Gene Expression; TIMP1; TIMP2; VCAN; SPARC; CLEC3B; E2F1
ABSTRACT
We investigated the expression of TIMP1, TIMP2, SPARC, VCAN, and CLEC3B genes, encoded matricellular proteins with pleiotropic functions, and glucose intolerance in obese male subjects with normal and impaired glucose tolerance. The purpose of this study was to examine the association between the gene expressions and glucose intolerance in obesity. The results indicate that obesity leads to significant increase of TIMP1, TIMP2, E2F1 and CLEC3B gene expressions in subcutaneous adipose tissue, especially TIMP2 gene. However, more significant increase of the expression of TIMP1 and TIMP2 was found in adipose tissue of obese patients with glucose intolerance. No significant changes were found in the expression of VCAN and SPARC genes in adipose tissue of obese subjects with normal glucose tolerance but increased in the group of obese subjects with glucose intolerance. At the same time, the E2F1 and CLEC3B gene expressions were decreased in adipose tissue of obese patients with glucose intolerance. Results of this study provide evidence that changes in the expression of genes encoded TIMP1, TIMP2, VCAN, SPARC, E2F1 and CLEC3B in subcutaneous adipose tissue of obese individuals associate with glucose intolerance.
1. Introduction
Obesity is one of the most profound public health problems. Much has been learned regarding the regulation of body weight and adiposity [1,2]. Like many other medical conditions, obesity, metabolic syndrome and type 2 diabetes are the results of interaction between environmental and genetic factors, including biological rhythms which control metabolism and are intrinsically and inter dependently linked [3,4].
Circadian rhythms govern or contribute to regulation of a large array of metabolic and physiological functions, including insulin sensitivity, energy homeostasis, satiety signaling, cellular proliferation, and cardiovascular function [5-7]. Recent studies have demonstrated relationships between the dysfunction of circadian clock system and the development of metabolic abnormalities, including adiposity and excessive levels of circulating lipids [8-11].
Adipose tissue growth is tightly associated with cell proliferation processes and angiogenesis which is an important component of different proliferative processes. In particular, fat tissue growth is regulated by different tightly interconnected factors [12,13]. Special interest deserve the matrix proteins with pleiotropic roles, such as the tissue inhibitor of matrix metalloproteinase (TIMP1 and TIMP2) [14,15], versican (VCAN) [16] and SPARC (secreted protein acidic and rich in cysteine, also known as osteonectin) [17]. These proteins are linked to human obesity and diabetes complications as they to reveal antiangiogenic properties. Moreover, they seem to regulate cell growth and apoptosis, particularly through affecting the vascular endothelial growth factor (VEGF), at resulting in VEGF resistance in diabetes mellitus despite the presence of the functionally active VEGF receptor 1 [18, 19].
TIMPs are multifunctional and can act either directly through cell surface receptors or indirectly through modulation of protease activity to direct cell fate [20]. Thus, TIMP1 is able to promote cell proliferation in a wide range of cell types, and may also have an anti-apoptotic function [14]. However, at the same time, TIMP1 is a potent inhibitor of tumor growth and angiogenesis and is involved in cell adhesion and migration [14]. TIMP1 is a negative regulator of adipogenesis both in mice and in humans [15]. TIMP2 thought to be a metastasis suppressor and has a unique role among TIMP family members in its ability to directly suppress the proliferation of endothelial cells [14]. Nevertheless, TIMP-2 has been shown to induce gene expression, to promote G(1) cell cycle arrest, and to inhibit cell migration [14]. It was shown that TIMP-2 involved in binding to the receptor integrin α3β1, and mediates angio-inhibitory and tumor suppressor activity [20].
It was also observed that enhanced neovascularization correlated with down regulation of anti-angiogenic connective tissue growth factor (CTGF). The latter binds VEGFA and its angiogenic activity are inhibited in the VEGFA-CTGF complex form [12]. But, stability of this complex as well as the angiogenic activity of VEGF depends from the matrix metalloproteinases and its inhibitors [12,21].
SPARC (secreted protein acidic and rich in cysteine), also known as osteonectin or BM-40, is a widely expressed profibrotic protein with pleiotropic roles, is linked to human obesity, insulin resistance, diabetes mellitus and diabetes complications such as diabetic retinopathy and nephropathy because it has anti-angiogenic properties and appears to regulate cell growth [17]. Elevated plasma levels of this protein were observed in patients with newly diagnosed type 2 diabetes mellitus [22]. Furthermore, evidence suggests that adipose tissue becomes increasingly fibrotic in obesity and SPARC as a regulator of the extracellular matrix also contributes to this adipose-tissue fibrosis [17,23]. Fibrosis of subcutaneous adipose tissue may restrict accumulation of triglycerides in this type of tissue. These are, therefore, deposited as ectopic lipids in other non-adipose tissues such as the liver or as intramyocellular lipids in skeletal muscle and predisposes to insulin resistance [17].
Versican (VCAN), also known as chondroitin sulfate proteoglycan 2 (CSPG2), is one of the main components of the extracellular matrix and is considered to be crucial to several key cellular processes involved in development and disease; i.e. cellular adhesion, proliferation, differentiation, migration, and angiogenesis, as well as, in intercellular signaling and connecting cells with the extracellular matrix [16,24,25]. Recently, the Wnt/β-catenin/TCF response elements in the human versican promoter, which is essential for activation of versican transcription, was identified [25]. Moreover, the versican was identified among six proteins at the center of the angiogenesis-associated network [26]. Hence, the angiogenesis phenomena are governed by complex signaling networks.
The transcription factor E2F1 regulates the expression of genes involved in a wide range of cellular processes; it is needed for regulation of nuclear receptor networks by retinoblastoma and via the CDK4-pRB-E2F1 regulatory pathway is involved in glucose homeostasis, defining a new link between cell proliferation and metabolism [27].
The endoplasmic reticulum stress is recognized as an important determinant of type 2 diabetes and contributes to the expression profile of many regulatory genes resulting in peripheral insulin resistance and diabetic complications, acting by inhibiting insulin receptor signaling [28,29]. However, detailed molecular mechanisms of the involvement of the endoplasmic reticulum stress in the development of obesity and its complications with different degree of dysglycemia are not yet clear.
Because the function of some matricellular proteins with pleiotropic roles is closely linked to metabolic homeostasis and these proteins can modulate the intracellular signaling network and lead to development of obesity and its complications (insulin resistance and glucose intolerance), the main goal of this work was to study the role of gene expressions encoding for the matricellular proteins with pleiotropic roles, in subcutaneous adipose tissue of obese individuals for evaluation of its possible significance to development of human obesity and glucose intolerance.
2. Materials and Methods
2.1. Patients
All participants gave written informed consent and the studies were approved by the local research ethics committees of Institute of Experimental Endocrinology Slovak Academy of Sciences. Patients clinical characteristics are shown in Table 1. The 18 male subjects participate in the study. They were divided into three groups (6 men in each group): 1) clinically healthy lean individuals, 2) patients with obesity and normal glucose tolerance (NGT) and 3) obesity and impaired glucose tolerance (IGT).
The lean (control) participant groups were individuals with mean age 44 ± 3 years and mean body mass index (BMI) 23 ± 0.6 kg/m2. The obese participant groups were individuals with mean age (45 ± 3 and 44 ± 3 years, respectively) and mean BMI (32 ± 0.6 and 34 ± 0.6 kg/m2, respectively). Thus, BMI in these groups of patients was significantly higher as compared to lean individuals (Table 1).
In the group of obese patients with impaired glucose tolerance 2 h glucose level in the blood was 7.8 mmol/l during an OGTT as compared with 5.3 mmol/l in obese participants (NGT) (Table 1). Moreover, in this group of obese patients with glucose intolerance is decreased insulin sensitivity index T (2.70 ± 0.19 vs 5.09 ± 0.67 mg/kg/min in an obese patients) and increased the level of fasting triglycerides (2.17 ± 0.44 vs 1.36 ± 0.2 mmol/l in an obese patients) as well as fasting insulin (15.2 ± 2.3 vs 9.37 ± 1.6 μIU/ml in an obese patients (NGT), as shown in Table 1.
2.2. RNA Isolation
RNasy Lipid Tissue Mini Kit (QIAGEN, Germany) was used for RNA extraction from subcutaneous adipose tissue.
2.3. Reverse Transcription and Quantitative Real-Time PCR Analysis
The expression levels of genes encoded TIMP metallopeptidase inhibitor 1 (TIMP1), TIMP2, SPARC (secreted protein, acidic, cysteine-rich) and versican (VCAN) were measured in subcutaneous fat tissue by real-time quantitative polymerase chain reaction of complementary DNA (cDNA). QuaniTect Reverse Transcription Kit
(QIAGEN, Germany) was used for cDNA synthesis. The 7900 HT Fast Real-Time PCR System (Applied Biosystems), Absolute QPCR SYBR Green Mix (Thermo Scientific, UK) and pair of primers specific for each studied gene (Sigma, USA) were used for quantitative real-time polymerase chain reaction (qPCR). The primers sequences are shown in Table 2. The expression of betaactin mRNA was used as control of analyzed RNA quantity.
An analysis of quantitative real-time polymerase chain reaction was performed using special computer program “Differential expression calculator” and statistical analysis—in Excel program. The values of different gene expressions were normalized to the expression of beta-actin mRNA and represent as percent of control (lean individuals, 100%) and are the means ± SEM for up to six different samples.
3. Results
In this study, we analyzed the expression levels of genes
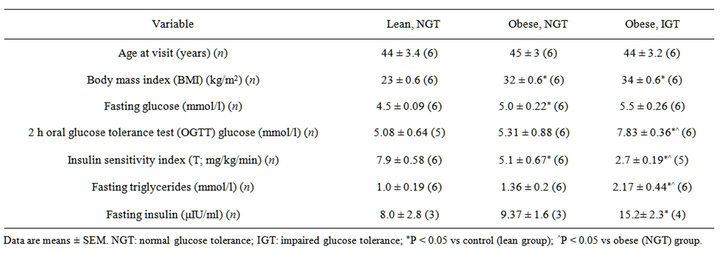
Table 1. Characteristics of the study participants.
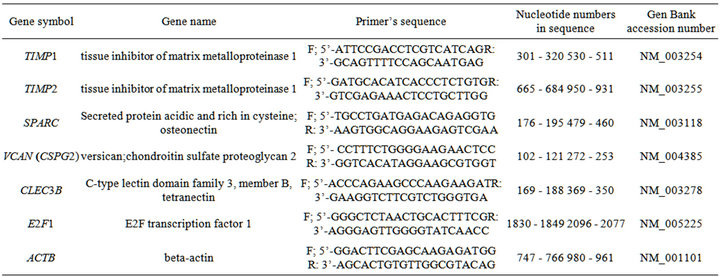
Table 2. Characteristics of the primers used for quantitative real-time polymerase chain reaction.
encoding tissue inhibitor of matrix metalloproteinase 1 and 2, SPARC, versican, CLEC3B and E2F1 in subcutaneous adipose tissue from three groups of participants: lean (control), obese with normal glucose tolerance and obese with impaired glucose tolerance. As shown in Figure 1, the expression level of TIMP1 and TIMP2 mRNA in subcutaneous adipose tissue of obese individuals with normal glucose tolerance increases as compared to the control (lean) subjects: +56% and +183%, correspondingly. Moreover, in obese patients with impaired glucose tolerance (IGT) the expression of these genes was significantly higher as compared both to the lean subjects and the obese individuals with normal glucose tolerance.
As shown in Figure 2, the expression level of SPARC and VCAN mRNA did not change significantly in subcutaneous adipose tissue of the obese individuals, compared with lean participants. At the same time, the expression level of SPARC and VCAN mRNA is increased in subcutaneous adipose tissue of the obese individuals with impaired glucose tolerance, when compared to the groups of obese subjects with normal glucose tolerance or lean participants, being much more intense for the SPARC gene (Figure 2).
As shown in Figure 3, the expression level of CLEC3B and E21F1 mRNA significantly increased in subcutaneous adipose tissue of the obese individuals with NGT, compared with lean participants, being much more intense for the CLEC3B gene. At the same time, the expression level both of these genes in adipose tissue of the obese individuals with impaired glucose tolerance was
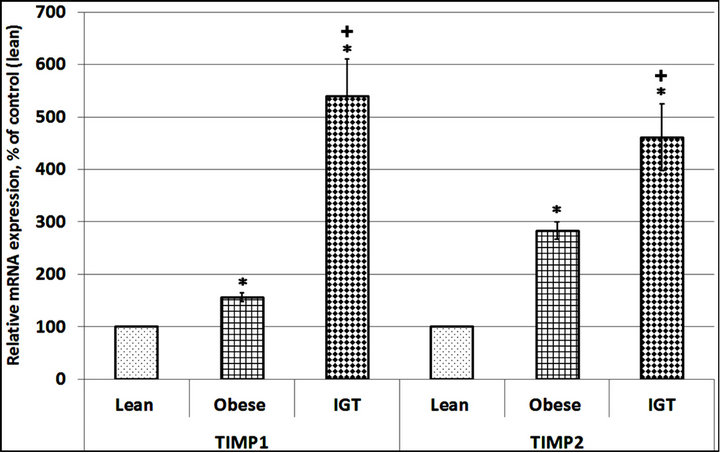
Figure 1. The expression level of TIMP metallopeptidase inhibitor 1 and 2 (TIMP1 and TIMP2) mRNA in subcutaneous adipose tissue of lean (Lean) and obese individuals with normal glucose tolerance (Obese) as well as in obese patients with glucose intolerance (IGT). The values of TIMP1 and TIMP2 mRNA expressions were normalized to the expression of beta-actin mRNA and are represented as a percent of control (Lean, 100%). Data is expressed as mean ± SEM of values from each group; *P < 0.05 vs group of lean individuals; +P < 0.05 vs group with obesity and normal glucose tolerance test (NGT).
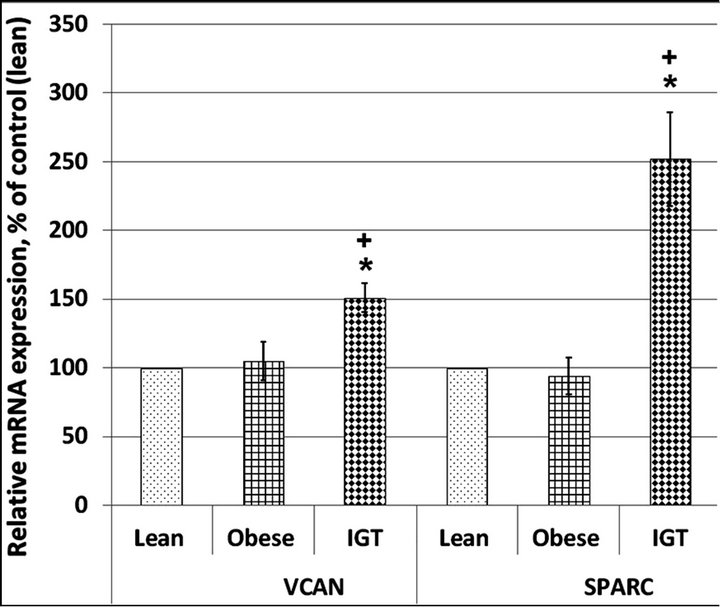
Figure 2. Versican (VCAN) and secreted protein acidic and rich in cysteine (SPARC) mRNA expression level in subcutaneous adipose tissue of lean and obese individuals with normal glucose tolerance (Obese) as well as in obese patients with impaired glucose tolerance (IGT). The values of VCAN and SPARC mRNA expressions were normalized to the expression of beta-actin mRNA and are represented as a percent of control (Lean, 100%). Data is expressed as mean ± SEM of values from each group; *P < 0.05 vs lean group; +P < 0.05 vs obese group.
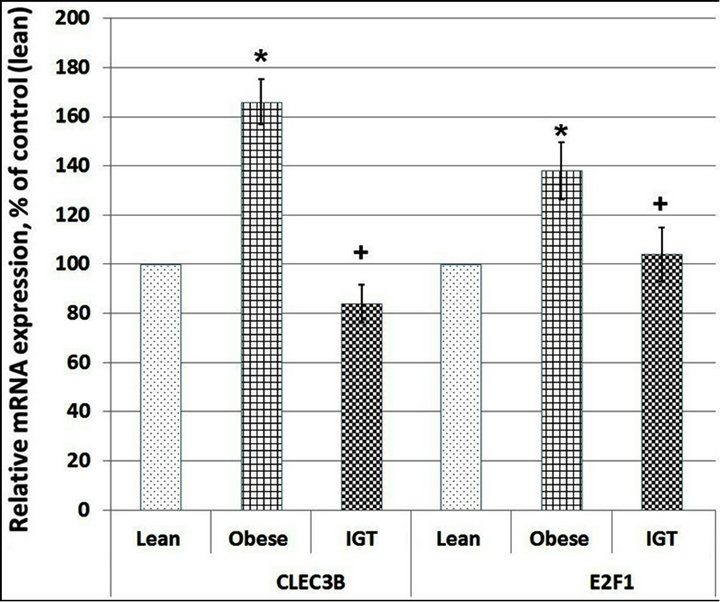
Figure 3. C-type lectin domain family 3, member B (CLEC3B) and transcription factor E2F1 mRNA expression level in subcutaneous adipose tissue of lean and obese individuals with normal glucose tolerance (Obese) as well as in obese patients with impaired glucose tolerance (IGT). The values of VCAN and SPARC mRNA expressions were normalized to the expression of beta-actin mRNA and are represented as a percent of control (Lean, 100%). Data is expressed as mean ± SEM of values from each group; *P < 0.05 vs lean group; +P < 0.05 vs obese group.
decreased when compared to the groups of obese subjects with normal glucose tolerance (Figure 3).
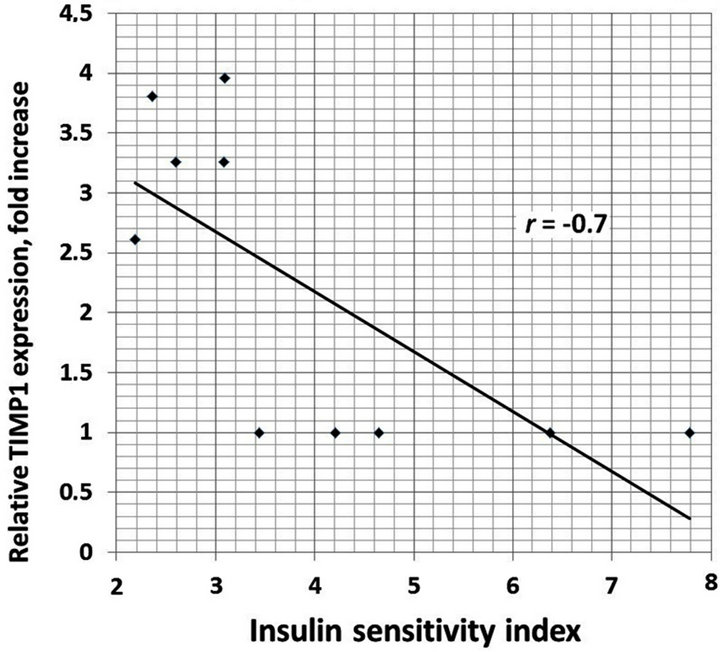
We also analyzed the correlation between different clinical characteristics and gene expressions. There is positive correlation between increased BMI and TIMP1 (r = 0.93), TIMP2 (r = 0.95), E2F1 (r = 0.89) and CLEC3B (r = 0.92) gene expressions in the obese men with normal glucose tolerance versus lean subjects (Figure 4). Moreover, as shown in Table 1, obese individuals with glucose intolerance versus obese subjects with normal glucose tolerance have significantly higher the 2 h oral glucose tolerance test (OGTT), fasting triglycerides and fasting insulin levels, but decreased insulin sensitivity index. We observed negative correlation between this insulin sensitivity index and TIMP1 (r = −0.70), TIMP2 (r = −0.64), SPARC (r = −0.64), VCAN (r = −0.66), and CLEC3B (r = 0.75) gene expressions in the obese individuals with glucose intolerance versus obese men with normal glucose tolerance (Figure 5).
4. Discussion
In this study, we have demonstrated that obesity is associated with dysregulation of the TIMP1, TIMP2, VCAN, CLEC3B, E2F1 and SPARC genes, which encode the important regulatory factors with pleiotropic functions, in particularly, control angiogenesis and growth processes [14,15,17,25]. Moreover, these factors are involved in the development of the obesity, glucose tolerance and type 2 diabetes, the most profound public health problems [15,17,27]. Angiogenesis is an important component of different proliferative processes, in particular, fat tissue growth; however, angiogenesis can also contribute to the development of complications associated with obesity, insulin resistance and glucose intolerance. In this study we have shown that obesity leads to a significant increase of TIMP1 and TIMP2 gene expressions in men subcutaneous adipose tissue, besides that, the most significant increase was shown for TIMP2 gene. It is possible that proteins encoded by these genes are involved the development of obesity, because both the TIMP1 and TIMP2 genes are implicated in direct regulation of cell growth, apoptosis and angiogenesis [14].
However, the tissue inhibitors of metalloproteinases
 (a)
(a)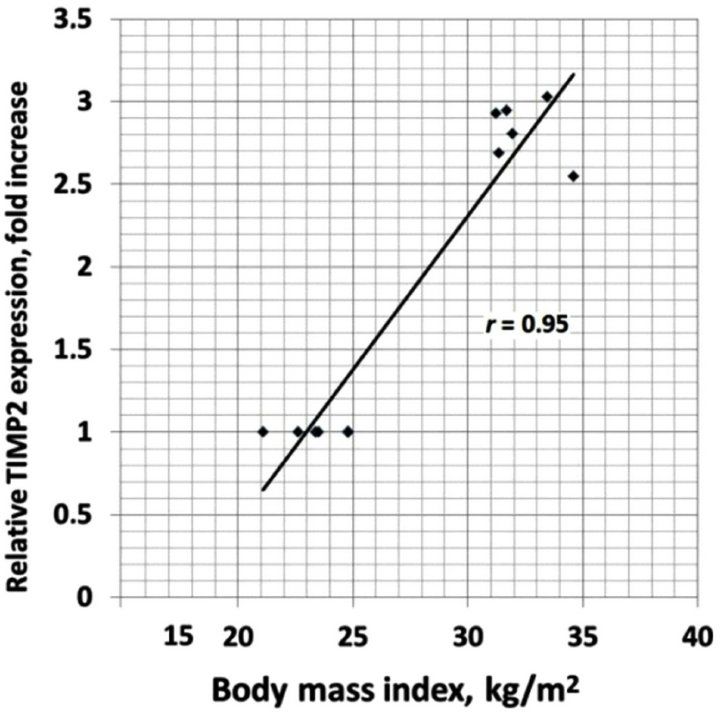 (b)
(b)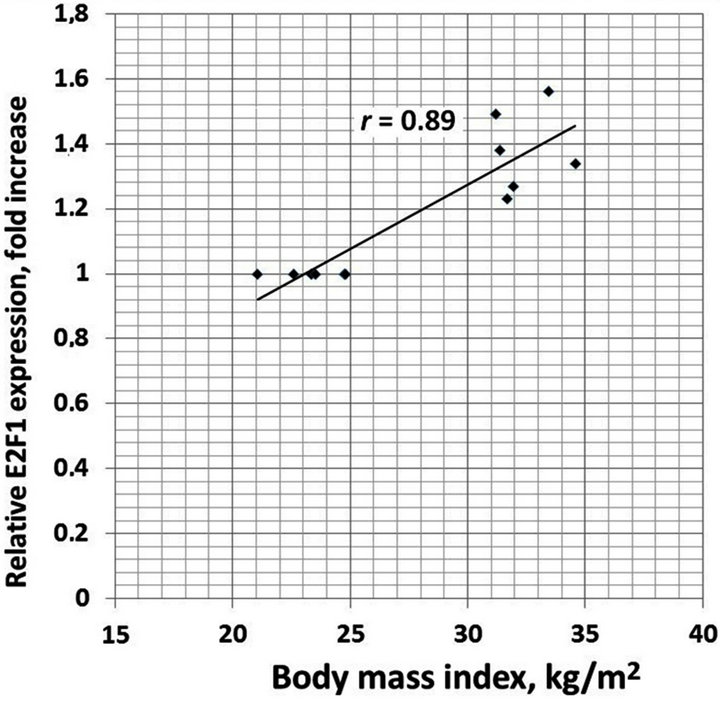 (c)
(c) (d)
(d)
Figure 4. The correlation between body mass index (BMI) and TIMP1 (a); TIMP2 (b); E2F1 (c) and CLEC3B (d) gene expressions in the obese men with normal glucose tolerance versus lean subjects.
 (a)
(a)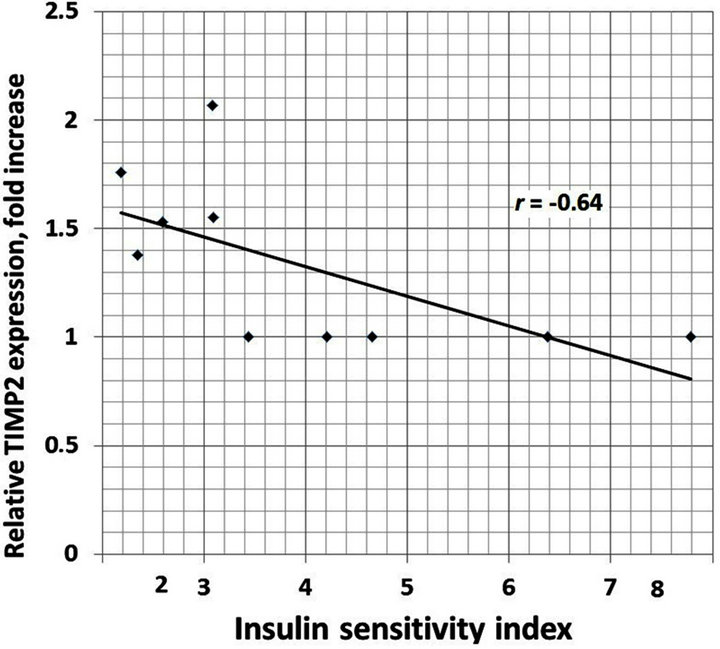 (b)
(b)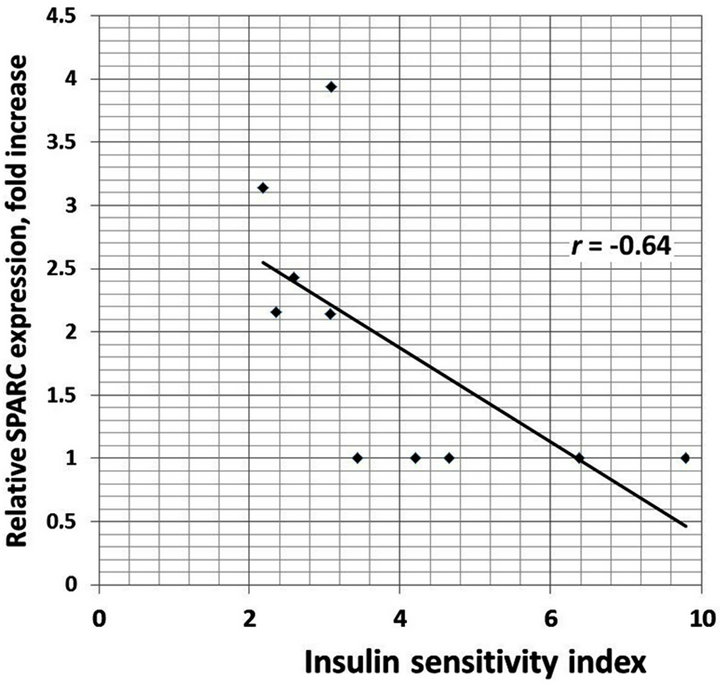 (c)
(c)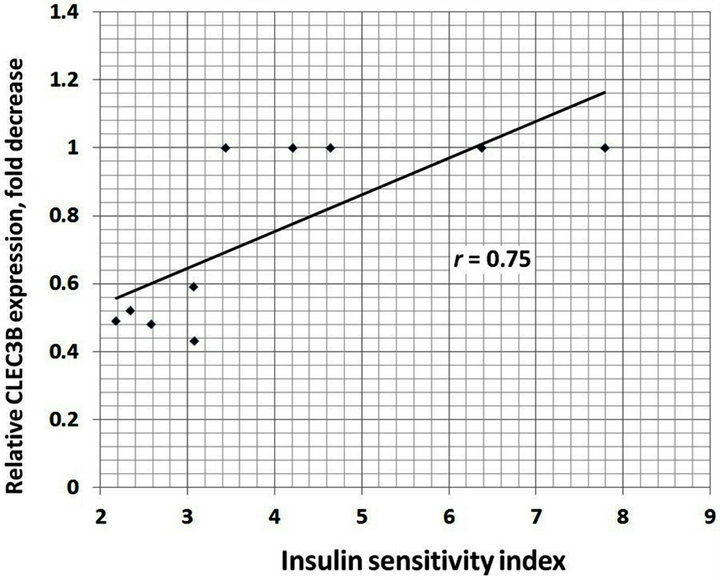 (d)
(d)
Figure 5. The correlation between insulin sensitivity index andTIMP1 (a); TIMP2 (b); SPARC (c) and CLEC3B (d) gene expressions in the obese individuals with glucose intolerance versus obese men with normal glucose tolerance.
(TIMPs) are multifunctional and can both promote and inhibit of cells growth in cell specific manner via different signaling pathways [14]. TIMPs can act either directly through cell surface receptors or indirectly through modulation of protease activity to direct cell fate. Thus, Meissburger et al. [15] have shown that tissue inhibitor of matrix metalloproteinase 1 controls adipogenesis in obesity in humans. At the same time, TIMP1 can inhibit cell growth and apoptosis via binding to CD63 [14]. Tissue inhibitors of metalloproteinases suppress matrix metalloproteinase activity critical for extracellular matrix turnover associated with both physiologic and pathologic tissue remodeling; however, anti-proliferative as well as anti-angiogenic effects of TIMP2 are independent of metalloproteinase inhibition [20]. It was also shown that these effects of TIMP2 require alpha 3 beta 1 integrinmediated binding of this tissue inhibitor of metalloproteinases to endothelial cells. Further, TIMP2 induces a decrease in total protein tyrosine phosphatase activity associated with beta1 integrin subunits as well as dissociation of the phosphatase SHP-1 from beta 1; however, TIMP2 also results in a concomitant increase in protein tyrosine phosphatase activity associated with tyrosine kinase receptors FGFR-1 and KDR [20]. Furthermore, TIMP2 has been shown to induce gene expression, to promote cell cycle arrest and to inhibit cell migration [14]. Thus, these findings establish an unexpected, metalloproteinase-independent mechanism for TIMP2 inhibition of endothelial cell proliferation and reveal an important component of the anti-angiogenic effect of this metalloproteinase inhibitor [20].
Moreover, it is possible that these genes are also involved the development of obesity associated complications, like glucose intolerance, because we have shown significant up-regulation of both TIMP1 and TIMP2 as well as SPARC and VCAN gene expressions in adipose tissue of subjects with impaired glucose intolerance versus the obese individuals with normal glucose tolerance. This observation agrees with data which recently has been shown that transcription factor retinoid-related orphan receptor gamma as a negative regulator of adipocyte differentiation modulates insulin sensitivity in obesity through expression of metalloproteinase-3 and its inhibitors [30]. It is also known that SPARC expression is predominant in subcutaneous adipose tissue, its expression contributing to metabolic dysregulation in obesity and is associated with insulin resistance and glucose intolerance [17,22]. The augmented expression of SPARC gene in the obese participants with glucose intolerance is possibly associated with hyperinsulinemia, because insulin as well as leptin can induce its expression [23].
It is possible that changes in TIMP1, TIMP2, SPARC, CLEC3B, E2F1, and VCAN gene expressions in obese individuals with impaired glucose tolerance are probably a result, at least partly, of endoplasmic reticulum stress mediated by the insulin resistance and elevated level of glucose. Recently, the endoplasmic reticulum stress is recognized as an important determinant of obesity and type 2 diabetes as well as a central feature of peripheral insulin resistance and glucose homeostasis [28,31,32].
Furthermore, SPARC as a regulator of the extra cellular matrix may contributes to metabolic dysregulation in obesity, insulin resistance and diabetes complications such as diabetic retinopathy and nephropathy, conditions that are ameliorated in the Sparc-knockout mouse model, via adipose-tissue fibrosis [17]. Hence, our results agree with this data that the SPARC protein is associated with diabetes complications, because SPARC protein plays an important role in angiogenesis [33]. At the same time, the primary function of SPARC in angiogenesis remains unclear, because SPARC activity in some circumstances promotes angiogenesis, while in others is more consistent with an anti-angiogenic activity [34]. Undoubtedly, the mercurial nature of SPARC belies a redundancy of functional proteins in angiogenesis. Moreover, fibrosis of subcutaneous adipose tissue may restrict accumulation of triglycerides in this type of tissue which are, therefore, diverted and deposited as ectopic lipids in other tissues such as the liver or as intramyocellular lipids in skeletal muscle, which predisposes to insulin resistance [17].
Results of this study have also shown enhanced level of VCAN expression in subcutaneous adipose tissue of obese individuals with glucose intolerance, which possibly contributes to proliferative processes, angiogenesis, metabolic dysregulation, and insulin resistance, because this chondroitin sulfate proteoglycan as an one of the main components of the extracellular matrix participates in cell adhesion, proliferation, migration, apoptosis and angiogenesis [16,25]. Most of these effects of versican are possibly mediated through the Wnt/β-catenin/TCF response elements in the VCAN promoter [35].
We also observed significant reduction of E2F1 gene expression in adipose tissue of obese patients with glucose intolerance. This data is consistent with results of Blanchet et al. [36] concerning functional activity of transcription factor E2F1 which regulate both proliferative and metabolic genes and coordinates cellular responses by acting as a regulatory switch between cell proliferation and metabolism. This reduction of the expression of E2F1 gene is possibly associated with activation of pro-proliferative and suppression of anti-proliferative gene expressions. Thus, tumor suppressor kinase LKB 1 is turning out to be a key regulator of the body’s metabolic activities, including its handling of glucose body’s level via activation of AMPK [37]. Moreover, knockout of AMPK related gene NUAK2 leads to developing of obesity, metabolic syndrome as well as cancer [38].
It is interesting to note that angiogenesis like many other biological processes is regulated by complex network of different factors which are tightly interconnected [12,19,21,39]. Thus, thrombospondin-1, a matrix-bound adhesive glycoprotein, has been shown to modulate tumor progression and up-regulates tissue inhibitor of metalloproteinase-1 production in human tumor cells and also up-regulates matrix metalloproteinases MMP-2 and MMP-9 [39]. This data suggested that the balance between matrix metalloproteinases and tissue inhibitors of metalloproteinases is a key determinant in different biological effects of THBS1, including tumor cell invasion, and may provide an explanation for the divergent activeties reported for thrombospondin-1 in tumor progression. Thus, the THBS1 is involved in influencing the critical balance between MMPs and their inhibitors, maintaining the controlled degradation of the extracellular matrix needed to support metastasis and possibly in obesity as well as in obesity with different degree of dysglycemia. Moreover, different cells express a lot of pro-angiogenic and anti-angiogenic genes (e.g., TIMP1, TIMP2); however, detailed analysis of melanoma cells from different patients do not show a significantly higher median number of expressed pro-angiogenic or anti-angiogenic genes, but 97% of these cell samples aberrantly express at least one of the angiogenic factors [40]. Thus, theangiogenesis is regulated by complex network of pro-angiogenic and anti-angiogenic factors and aberrant expression at least one of these factors can modify an angiogenesis.
It is possible that TIMP1, TIMP2, VCAN, CLEC3B, E2F1 and SPARC are also included in this network and the balance between different regulatory factors which participate in the control of angiogenesis, including vascular endothelial growth factor, a key pro-angiogenic factor, insulin, insulin-like growth factors, leptin and others, really determinate an angiogenesis both in obesity and in obesity with glucose intolerance.
Results of this study also provide strong evidence that expression of TIMP1, TIMP2, VCAN and SPARC genes encoded the key regulatory factors with pleiotropic functions in subcutaneous adipose tissue of the obese individuals with glucose intolerance is deregulated and that these changes can be determined both by insulin resistance and endoplasmic reticulum stress. Collectively, the results of this study underscore the crucial role of TIMP1, TIMP2, SPARC and VCAM regulatory factors with pleiotropic function in the developing the obesity and its complications, especially glucose intolerance via participation in the intracellular signaling network, responsible for regulation of angiogenesis and glucose metabolism.
5. Acknowledgements
We thank Prof. Iwar Klimes (Institute of Experimental Endocrinology, Slovakia) for support and interest in this work as well as the gift of RNA from men’s adipose tissue and clinical characteristics of patients.
REFERENCES
- M. S. Bray and M. E. Young, “Circadian Rhythms in the Development of Obesity: Potential Role for the Circadian Clock within the Adipocyte,” Obesity Reviews, Vol. 8, No. 2, 2007, pp. 169-181.
- M. S. Bray and M. E. Young, “The Role of Cell-Specific Circadian Clocks in Metabolism and Disease,” Obesity Reviews, Vol. 10, No. 2, 2009, pp. 6-13. doi:10.1111/j.1467-789X.2009.00684.x
- J. Kovac, J. Husse and H. Oster, “A Time to Fast, a Time to Feast: The Crosstalk between Metabolism and the Circadian Clock,” Molecules and Cells, Vol. 282, No. 2, 2009, pp. 75-80. doi:10.1007/s10059-009-0113-0
- E. M. Scott, A. M. Carter and P. J. Grant, “Association between Polymorphisms in the Clock Gene, Obesity and the Metabolic Syndrome in Man Clock Polymorphisms and Obesity,” International Journal of Obesity, Vol. 32, 2008, pp. 658-662. doi:10.1038/sj.ijo.0803778
- C. B. Green, J. S. Takahashi and J. Bass, “The Meter of Metabolism,” Cell, Vol. 134, No. 5, 2008, pp. 728-742. doi:10.1016/j.cell.2008.08.022
- K. M. Ramsey, B. Marcheva, A. Kohsaka and J. Bass, “The Clock Work of Metabolism,” Annual Review of Nutrition, Vol. 27, 2007, pp. 219-240. doi:10.1146/annurev.nutr.27.061406.093546
- M. S. Bray and M. E. Young, “Regulation of Fatty Acid Metabolism by Cell Autonomous Circadian Clocks: Time to Fatten Up on Information?” The Journal of Biological Chemistry, Vol. 286, 2011, pp. 11883-11889. doi:10.1074/jbc.R110.214643
- H. Ando, T. Takamura, N. Matsuzawa-Nagata, K. R. Shima, T. Eto, H. Misu, M. Shiramoto, T. Tsuru, S. Irie, A. Fujimura and S. Kaneko, “Clock Gene Expression in Peripheral Leucocytes of Patients with Type 2 Diabetes,” Diabetologia, Vol. 52, No. 2, 2009, pp. 329-335. doi:10.1007/s00125-008-1194-6
- H. Ando, M. Kumazaki, Y. Motosugi, K. Ushijima, T. Maekawa, E. Ishikawa and A. Fujimura, “Impairment of Peripheral Circadian Clocks Precedes Metabolic Abnormalities in ob/ob mice,” Endocrinology, Vol. 152, No. 4, 2011, pp. 1347-1354. doi:10.1210/en.2010-1068
- W. Huang, K. M. Ramsey, B. Marcheva and J. Bass, “Circadian Rhythms, Sleep, and Metabolism,” Journal of Clinical Investigation, Vol. 121, No. 6, 2011, pp. 2133- 2141. doi:10.1172/JCI46043
- S. Shimba, T. Ogawa, S. Hitosugi, Y. Ichihashi, Y. Nakadaira, M. Kobayashi, M. Tezuka, Y. Kosuge, K. Ishige, Y. Ito, K. Komiyama, Y. Okamatsu-Ogura, K. Kimura and M. Saito, “Deficient of a Clock Gene, Brain and Muscle Arnt-Like Protein-1 (BMAL1), Induces Dyslipidemia and Ectopic Fat Formation,” PLoS One, Vol. 6, 2011, e25231. doi:10.1371/journal.pone.0025231
- G. Hashimoto, I. Inoki, FujiiY, T. Aoki, E. Ikeda and Y. Okada, “Matrix Metalloproteinases Cleave Connective Tissue Growth Factor and Reactivate Angiogenic Activity of Vascular Endothelial Growth Factor 165,” The Journal of Biological Chemistry, Vol. 277, 2002, pp. 36288- 36295. doi:10.1074/jbc.M201674200
- I. Inoki, T. Shiomi, G. Hashimoto, H. Enomoto, H. Nakamura, K. Makino, E. Ikeda, S. Takata, K. Kobayashi and Y. Okada, “Connective Tissue Growth Factor Binds Vascular Endothelial Growth Factor (VEGF) and Inhibits VEGF-Induced Angiogenesis,” FASEB Journal, Vol. 16, 2002, pp. 219-221. doi:10.1096/fj.01-0332fje
- W. G. Stetler-Stevenson, “Tissue Inhibitors of Metalloproteinases in Cell Signaling: Metalloproteinase-Independent Biological Activities,” Science Signal, Vol. 1, No. 27, 2008. doi:10.1126/scisignal.127re6
- B. Meissburger, L. Stachorski, E. Roder, G. Rudofsky and C. Wolfrum, “Tissue Inhibitor of Matrix Metalloproteinase 1 (TIMP1) Controls Adipogenesis in Obesity in Mice and in Humans,” Diabetologia, Vol. 54, No. 6, 2011, pp. 1468-1479. doi:10.1007/s00125-011-2093-9
- W. W. Du, B. B. Yang, B. L. Yang, Z. Deng, L. Fang, S. W. Shan, Z. Jeyapalan, Y. Zhang, A. Seth and A. J. Yee, “Versican G3 Domain Modulates Breast Cancer Cell Apoptosis: A Mechanism for Breast Cancer Cell Response to Chemotherapy and EGFR Therapy,” PLoS One, Vol. 6, 2011, Article ID:E26396. doi:10.1371/journal.pone.0026396
- K. Kos and J. P. Wilding, “SPARC: A Key Player in the Pathologies Associated with Obesity and Diabetes,” Nature Reviews Endocrinology, Vol. 6, 2010, pp. 225-235. doi:10.1038/nrendo.2010.18
- V. Tchaikovski, S. Olieslagers, F. D. Böhmer and J. Waltenberger, “Diabetes Mellitus Activates Signal Transduction Pathways Resulting in Vascular Endothelial Growth Factor Resistance of Human Monocytes,” Circulation, Vol. 120, 2009, pp. 150-159. doi:10.1161/CIRCULATIONAHA.108.817528
- J. Waltenberger, “VEGF Resistance as a Molecular Basis to Explain the Angiogenesis Paradox in Diabetes Mellitus,” Biochemical Society Transactions, Vol. 37, 2009, pp. 1167-1170. doi:10.1042/BST0371167
- D. W. Seo, H. Li, L. Guedez, P. T. Wingfield, T. Diaz, R. Salloum, B. Y. Wei and W. G. Stetler-Stevenson, “TIMP- 2 Mediated Inhibition of Angiogenesis: An MMP-Independent Mechanism,” Cell, Vol. 114, No. 2, 200, pp. 171- 180. doi:10.1016/S0092-8674(03)00551-8
- M. Dews, A. Homayouni, D. Yu, D. Murphy, C. Sevignani, E. Wentzel, E. E. Furth, W. M. Lee, G. H. Enders, J. T. Mendell and A. Thomas-Tikhonenko, “Augmentation of Tumor Angiogenesis by a Myc-Activated MicroRNA Cluster,” Nature Genetics, Vol. 38, 2006, pp. 1060-1065. doi:10.1038/ng1855
- D. Wu, L. Li, M Yang, H. Liu and G. Yang, “Elevated Plasma Levels of SPARC in Patients with Newly Diagnosed Type 2 Diabetes Mellitus,” European Journal of Endocrinology, Vol. 165, 2011, pp. 597-601. doi:10.1530/EJE-11-0131
- K. Kos, S. Wong, B. Tan, A. Gummesson, M. Jernas, N. Franck, D. Kerrigan, F. H. Nystrom, L. M. Carlsson, H. S. Randeva, J. H. Pinkney and J. P. Wilding, “Regulation of the Fibrosis and Angiogenesis Promoter SPARC/Osteonectin in Human Adipose Tissue by Weight Change, Leptin, Insulin, and Glucose,” Diabetes, Vol. 58, No. 8, 2009, pp. 1780-1788. doi:10.2337/db09-0211
- P. S. Zheng, J. Wen, L. C. Ang, W. Sheng, A. ViloriaPetit, Y. Wang, Y. Wu, R. S. Kerbel and B. B. Yang, “Versican/PG-M G3 Domain Promotes Tumor Growth and Angiogenesis,” FASEB Journal, Vol. 18, 2004, pp. 754-756,. doi:10.1096/fj.03-0545fje
- M. Rahmani, B. W. Wong, L. Ang, C. C. Cheung, J. M. Carthy, H. Walinski and B. M. McManus, “Versican: Signaling to Transcriptional Control Pathways,” Canadian Journal of Physiology and Pharmacology, Vol. 84, 2006, pp. 77-92. doi:10.1139/y05-154
- C. G. Rivera, J. S. Bader and A. S. Popel, “AngiogenesisAssociated Crosstalk between Collagens, CXC Chemokines, and Thrombospondin Domain-Containing Proteins,” Annals of Biomedical Engineering, Vol. 39, No. 8, 2011, pp. 2213-2222. doi:10.1007/s10439-011-0325-2
- J. S. Annicotte, E. Blanchet, C. Chavey, I. Iankova, S. Costes, S. Assou, J. Teyssier, S. Dalle, C. Sardet and L. Fajas, “The CDK4-pRB-E2F1 Pathway Controls Insulin Secretion,” NatCellBiol, Vol. 11, 2009, pp. 1017-1023. doi:10.1038/ncb1915
- A. Lombardi, L. Ulianich, A. S. Treglia, C. Nigro, L. Parrillo, D. D. Lofrumento, G. Nicolardi, C. Garbi, F. Beguinot, C. Miele and B. Di Jeso, “Increased Hexosamine Biosynthetic Pathway Flux Dedifferentiates INS-1E Cells and Murine Islets by an Extracellular Signal-Regulated Kinase (ERK)1/2-Mediated Signal Transmission Pathway,” Diabetologia, Vol. 55, No. 1, 2012, pp. 141-153. doi:10.1007/s00125-011-2315-1
- U. Ozcan, Q. Cao, E. Yilmaz, A. H. Lee, N. N. Iwakoshi, E. Ozdelen, G. Tuncman, C. Gorgun, L. H. Glimcher and G. S. Hotamisligil, “Endoplasmic Reticulum Stress Links Obesity, Insulin Action, and Type 2 Diabetes,” Science, Vol. 306, No. 5695, 2004, pp. 457-461. doi:10.1126/science.1103160
- B. Meissburger, J. Ukropec, E. Roeder, N. Beaton, M. Geiger, D. Teupser, B. Civan, W. Langhans, P. P. Nawroth, D. Gasperikova, G. Rudofsky and C. Wolfrum, “Adipogenesis and Insulin Sensitivity in Obesity Are Regulated by Retinoid-Related Orphan Receptor Gamma,” EMBO Molecular Medicine, Vol. 3, 2011, pp. 637-651. doi:10.1002/emmm.201100172
- S. W. Park, Y. Zhou, J. Lee, J. Lee and U. Ozcan, “Sarco(endo)plasmic Reticulum Ca2+-ATPase 2b Is a Major Regulator of Endoplasmic Reticulum Stress and Glucose Homeostasis in Obesity,” Proceedings of the National Academy of Sciences of USA, Vol. 107, 2010, pp. 19320-19325. doi:10.1073/pnas.1012044107
- Y. Zhou, J. Lee, C. M. Reno, C. Sun, S. W. Park, J. Chung, J. Lee, S. J. Fisher, M. F. White and S. B. Biddinger, U. Ozcan, “Regulation of Glucose Homeostasis through a XBP-1-FoxO1 Interaction,” Nature Medicine, Vol. 17, 2011, pp. 356-365. doi:10.1038/nm.2293
- G. P. Nagaraju and D. Sharma, “Anti-Cancer Role of SPARC, an Inhibitor of Adipogenesis,” Cancer Treatment Reviews, Vol. 37, No. 7, 2011, pp. 559-566. doi:10.1016/j.ctrv.2010.12.001
- A. D. Bradshaw, “Diverse Biological Functions of the SPARC Family of Proteins,” The International Journal of Biochemistry & Cell Biology, Vol. 44, No. 3, 2012, pp. 480-488. doi:10.1016/j.biocel.2011.12.021
- M. Rahmani, J. M. Carthy and B M. McManus, “Mapping of the Wnt/β-Catenin/TCF Response Elements in the Human Versican Promoter,” Methods in Molecular Biology, Vol. 836, 2012, pp. 35-52. doi:10.1007/978-1-61779-498-8_3
- E. Blanchet, J. S. Annicotte, S. Lagarrigue, V. Aguilar, C. Clapé, C. Chavey, V. Fritz, F. Casas, F. Apparailly, J. Auwerx and L. Fajas, “E2F Transcription Factor-1 Regulates Oxidative Metabolism,” Nature Cell Biology, Vol. 13, 2011, pp. 1146-1152. doi:10.1038/ncb2309
- R. J. Shaw, “LKB1 and AMP-Activated Protein Kinase Control of mTOR Signalling and Growth,” Acta Physiologica, Vol. 196, 2009, pp. 65-80. doi:10.1111/j.1748-1716.2009.01972.x
- K. Tsuchihara, T. Ogura, R. Fujioka, S. Fujii, W. Kuga, M. Saito, T. Ochiya, A. Ochiai and H. Esumi, “Susceptibility of Snark-Deficient Mice to Azoxymethane Induced Colorectal Tumorigenesis and the Formation of Aberrant Crypt Foci,” Cancer Science, Vol. 99, No. 4, 2008, pp. 677-682. doi:10.1111/j.1349-7006.2008.00734.x
- A. S. John, X. Hu, V. L. Rothman and G. P. Tuszynski, “Thrombospondin-1 (TSP-1) Up-Regulates Tissue Inhibitor of Metalloproteinase-1 (TIMP-1) Production in Human Tumor Cells: Exploring the Functional Significance in Tumor Cell Invasion,” Experimental and Molecular Pathology, Vol. 87, No. 3, 2009, pp. 184-188. doi:10.1016/j.yexmp.2009.09.002
- D. Hose, J. Moreaux, T. Meissner, A. Seckinger, H. Goldschmidt, A. Benner, K. Mahtouk, J. Hillengass, T. Rème, J. De Vos, M. Hundemer, M. Condomines, U. Bertsch, J. F. Rossi, A. Jauch, B. Klein and T. Möhler, “Induction of Angiogenesis by Normal and Malignant Plasma Cells,” Blood, Vol. 114, No. 1, 2009, pp. 128-143. doi:10.1182/blood-2008-10-184226
NOTES
The Expression of TIMP1, TIMP2, VCAN, SPARC, CLEC3B and E2F1 in Subcutaneous Adipose Tissue of Obese Males and Glucose Intolerance
*Corresponding author.

