CellBio
Vol.1 No.2(2012), Article ID:25505,4 pages DOI:10.4236/cellbio.2012.12005
Optimized Conditions for the Delivery of Small Membrane Impermeable Compounds into Human Cells Using Hypotonic Shift
Institute for Glycomics, Griffith University Gold Coast Campus, Griffith University, Queensland, Australia
Email: *j.tiralongo@griffith.edu.au
Received October 14, 2012; revised November 20, 2012; accepted November 30, 2012
Keywords: Hypotonic Shift; Cell-Based Assay; Membrane Impermeable Compound Delivery; Osmolarity; Endocytosis
ABSTRACT
Cell-based assays represent a major end point of high throughput screening (HTS) but a key limitation of such assays is the potentially poor membrane permeability of test compounds. In this study, we optimized the conditions for the delivery of the membrane impermeable compound 8-hydroxypyrene-1,3,6-trisulfonic acid trisodium salt (HPTS) into human cells using hypotonic shift; a method that can promote the uptake of molecules from the extracellular fluid into cell cytoplasm via endocytosis. We showed that uptake of HPTS by cells was a function of hypotonic buffer osmolarity and that delivery was highly efficient with almost 100% of cells displaying uptake. Delivery of HPTS was equally effective at 25˚C and 37˚C, with delivery of compound proportional to incubation time and concentration of HPTS within the loading medium. The experimental conditions identified in this study could be applied to HTS drug discovery studies providing an effective method of delivering small membrane impermeable compounds into cells.
1. Introduction
A variety of end-point assays are implemented to assess the effects of compounds on target molecules during high throughput screening (HTS), and these can be broadly divided into two classes: biochemical assays and cellbased assays [1]. Cell-based assays provide a means to investigate putative activity-modifying compounds within an in vitro cellular environment and end points for analysis include second messenger assays, reporter gene assays and cellular process assays (i.e. proliferation) [1,2]. The establishment of a simple and efficient method to deliver membrane impermeant compounds during HTS could help increase the pool of lead compounds for further investigation helping to identify compounds for potential therapeutic use or aid in the discovery of molecular tools for research purposes.
In this study, the optimal conditions for the delivery of hydrophilic 8-hydroxypyrene-1,3,6-trisulfonic acid trisodium salt (HPTS) into mammalian cells using hypotonic shift were investigated. Hypotonic mediated delivery of compounds has already proven useful for molecular biology, cellular biology and in vivo research (e.g. [3-10]) and has a potential application in drug screening assays, where panels of compounds could be assessed in cellbased assays irrespective of their membrane permeability. The conditions studied included hypotonic buffer osmolarity, loading temperature and duration, rounds of loading and concentration of HPTS within the loading medium.
2. Materials and Methods
2.1. Cell Culture and Hypotonic Loading
HT-29 colorectal adenocarcinoma cells (HTB-38) were maintained in Dulbecco’s Modified Eagle Medium (DMEM) (Invitrogen) containing 1% penicillin/streptomycin solution (v/v) (Invitrogen) and 10% fetal bovine serum (v/v) (Invitrogen). For hypotonic shift mediated loading, HT-29 cells were detached from culture flasks via incubation in 0.025% trypsin (Invitrogen), 2 mM EDTA in 1 × PBS. Cells were pelleted via centrifugation, washed once in 1 × PBS and counted. For each sample, 3 × 105 cells were transferred to 1.5 mL microfuge tubes, the cells were pelleted via centrifugation and the supernatant discarded. The cells were then resuspended in 200 µL of isoosmolar or hypoosmolar buffers containing 0.5 mM HPTS (Sigma-Aldrich) unless otherwise indicated. The formulations of the buffers used in the study are listed in Table 1 and the buffers were adjusted to pH 7.4 using 1 M NaOH. Cells were incubated in buffers for 30 min
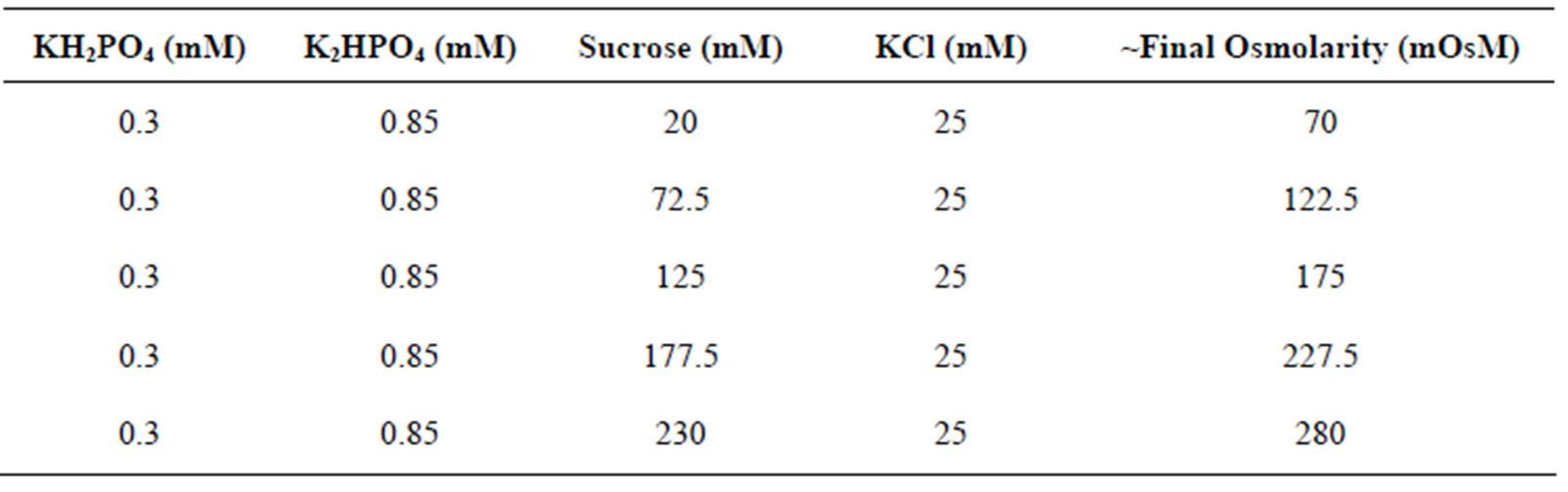
Table 1. Formulations and approximate osmolarities of the different buffers used in this study.
at room temperature (~25˚C) unless stated otherwise. After incubation, cells were washed three times with one volume of staining buffer (1 × PBS containing 1% fetal bovine serum (v/v)) and then lysed in 50 µL of lysis solution (50 mM Tris-Cl pH 8.0, 150 mM NaCl, 1% Triton X-100 (v/v)). Fluorescence levels of test samples were measured using the Victor 3 plate reader (PerkinElmer) using excitation at 485 nm and emission detection at 535 nm. The concentration of HPTS within the test samples was quantitated by comparing sample fluorescence levels to a standard curve generated from a 1 in 2 serial dilution series of 0.1 M HPTS.
2.2. Fluorescence Imaging
For fluorescence imaging, 3 × 105 HT-29 cells were resuspended in either isoosmolar buffer or 122.5 mOsM buffer containing 0.5 mM HPTS for 30 minutes. Cells were washed three times in staining buffer and seeded into the wells of 6-well culture plates in total volumes of 5 mL complete medium. The cells were allowed to adhere to the bottom of the culture vessels for two hours prior to Hoffman modulation contrast and fluorescence imaging on a Nikon Eclipse Ti inverted microscope system.
2.3. Statistical Analysis
Differences between the means of two groups were assessed by Student’s t-tests and for the testing of differences between the means of three or more groups analysis of variance with LSD post-hoc tests were used. Significant differences were denoted by p < 0.05. Errors bars in graphs represent a standard error of the mean. The number of samples assessed for each experimental condition (group) was at least three (n = 3).
3. Results and Discussion
The delivery of membrane impermeant molecules into mammalian cells via hypotonic shift requires the use of a hypoosmolar buffer to invoke the regulatory volume decrease (RVD) associated with hypotonic shock. RVD is characterized by the opening of K+ channels and anion channels and a reduction of membrane surface through intensive endocytosis. During endocytosis, molecules present within the extracellular fluid are able to enter cells [11-13]. We set out to determine the optimal osmolarity of hypotonic buffer used to mediate the RVD and thus facilitate the entry of HPTS into HT-29 cells. Figure 1(a) shows that dose responsive increase in HPTS uptake was observed with decreasing buffer osmolarity. Peak delivery of HPTS was observed at a buffer osmolarity of 122.5 mOsM. This buffer osmolarity was used for all subsequent optimisation experiments. In order to rule out that cell stress from the hypoosmolar conditions used was contributing to the observed fluorescence increase, a control experiment where HPTS was omitted was performed. We found that a buffer osmolarity of 122.5 mOsM was well tolerated by the cells, with no fluorescence observed in the absence of HPTS. To quantitate the efficiency of HPTS uptake fluorescence imaging was carried out on cells incubated in isoolsmolar and hypoosmolar buffers. Figure 1(b) reveals that only a small proportion of cells incubated in isoosmolar buffer took up HPTS. On the other hand, almost 100% of cells incubated in hypoosmolar buffer were positive for HPTS uptake, indicating the delivery of molecules through hypotonic shift was highly efficient. This clearly confirms that cell swelling and RVD represents a global response such that each cell attempts to counteract the differences between intracellular and extracellular osmolarity when exposed to hypoosmolar buffer.
We next investigated the effect of incubation duration (period of time cells were exposed to hypoosmolar solution) on the delivery of HPTS into HT-29 cells (Figure 1(c)). Incubation periods beyond 10 min showed time dependent increases in the uptake of HPTS, with maximal delivery at 60 min. Incubation of cells in hypotonic buffer for 60 min was well tolerated and no significant increase in cell death was observed compared to cells maintained in complete growth medium (data not shown). The impact of incubation temperature (4˚C, 25˚C and 37˚C) on the uptake of HPTS was also investigated. Figure 1(d) shows that incubation at 4˚C significantly reduced HPTS uptake compared to incubation at 25˚C.
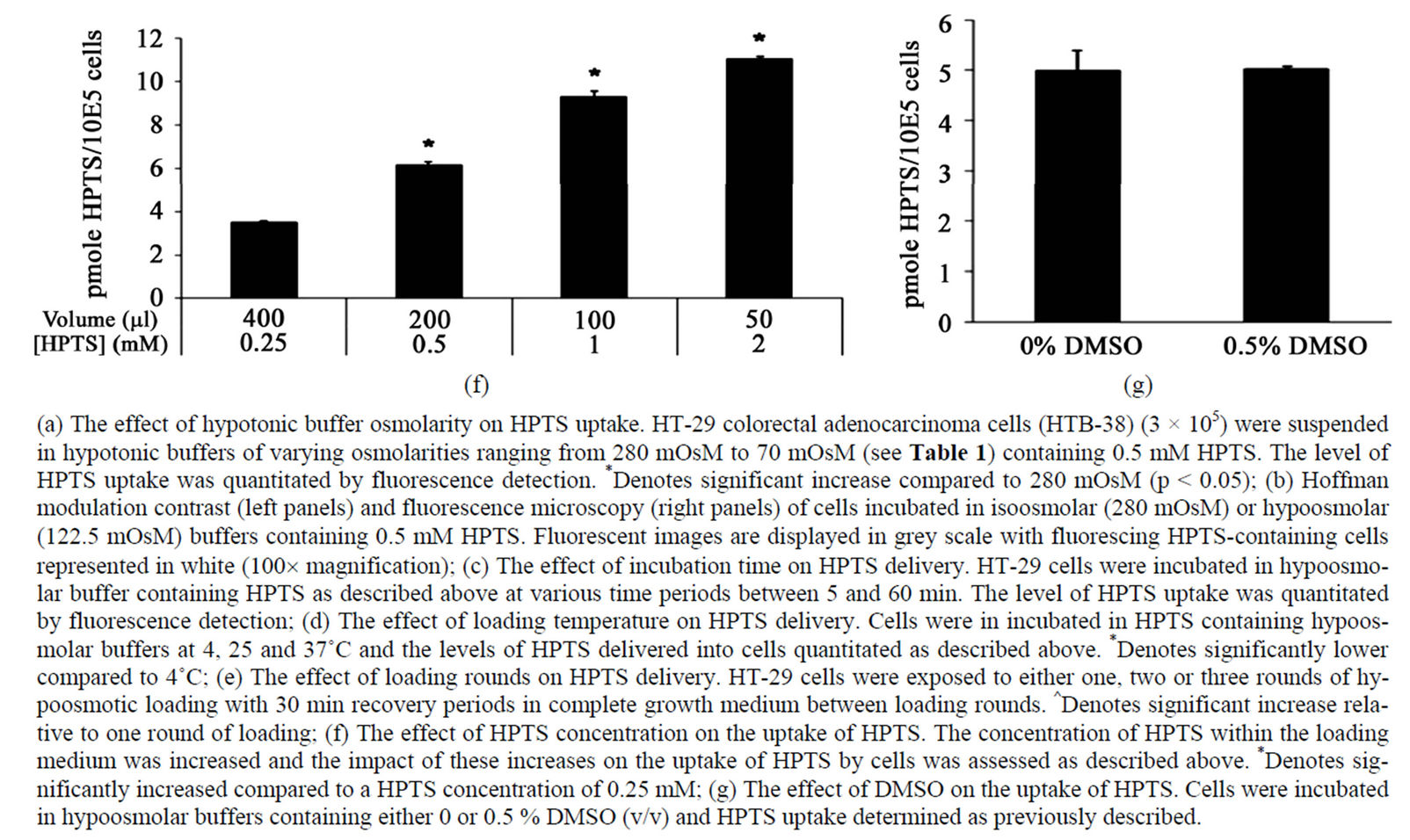
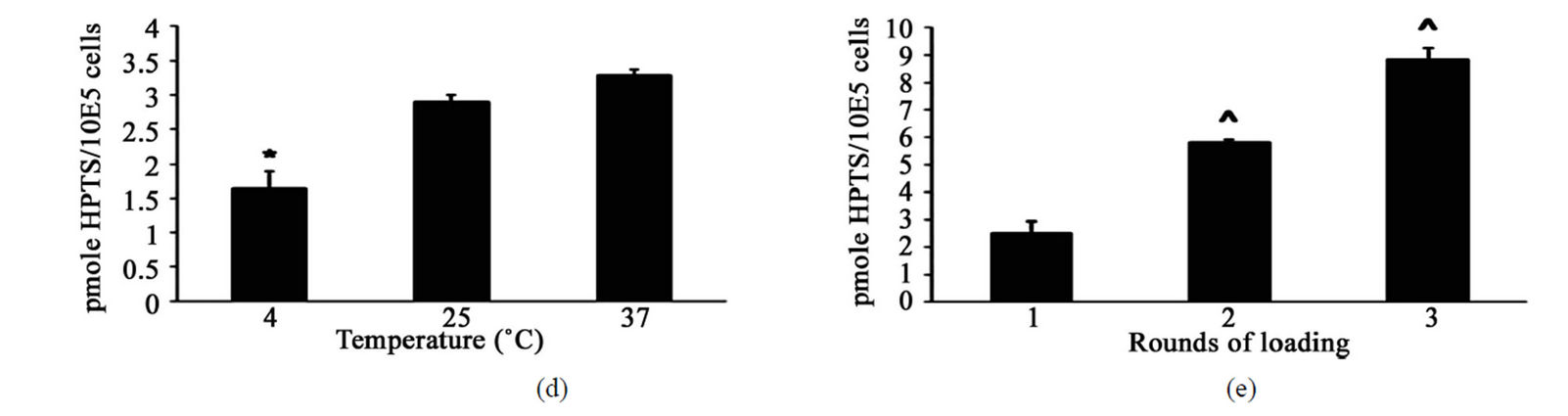
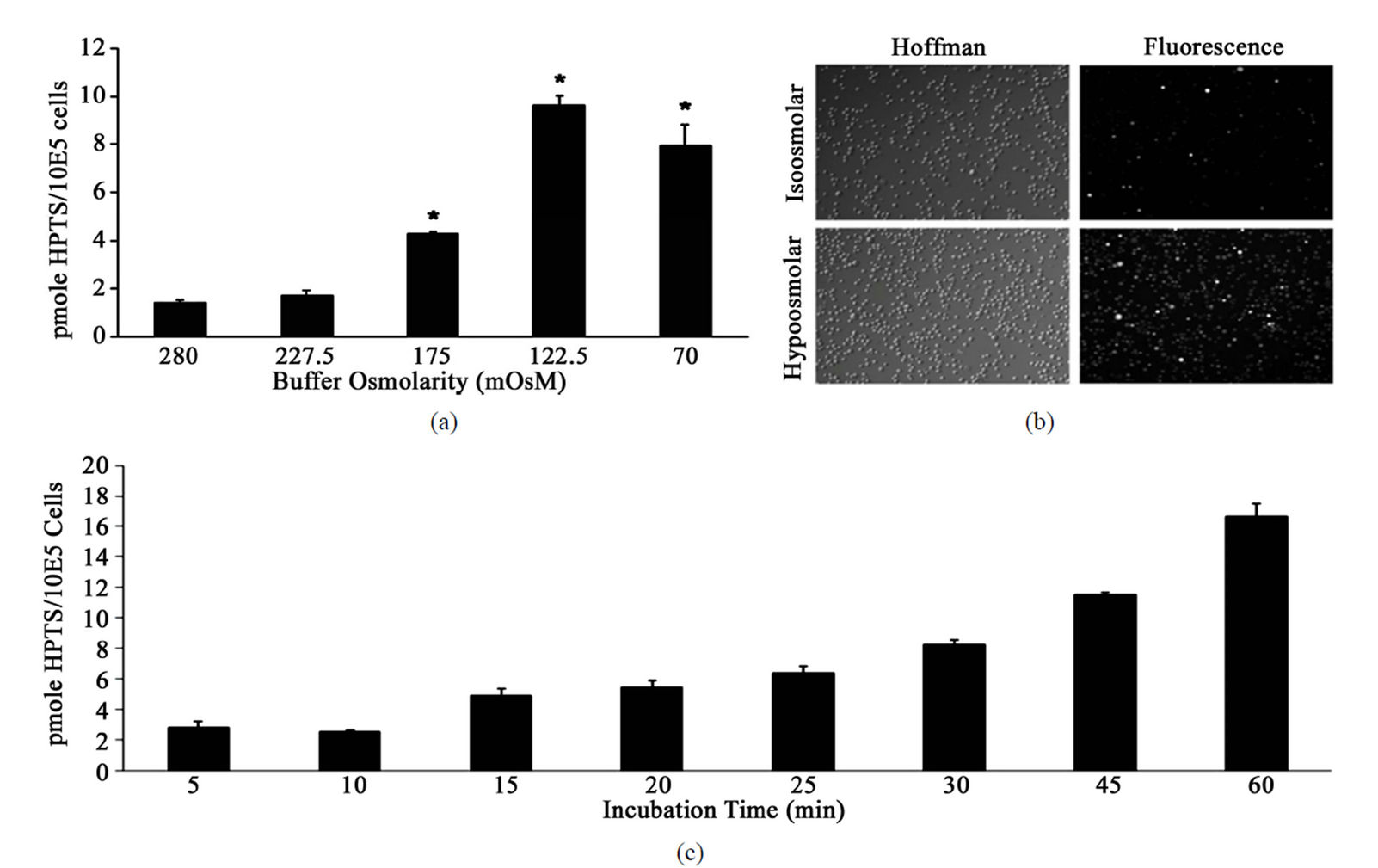
Figure 1. Optimisation of HPTS uptake into HT-29 cells.
Most likely, cellular responses linked to hypotonic challenge require higher temperatures to function effectively. On the other hand, incubation at 37˚C only produced a marginal, non-significant increase in the uptake of HPTS relative to incubation at 25˚C. Notably, this indicates that loading at 25˚C was as effective as loading at normal physiological body temperature, therefore eliminating the need to pre-warm buffers or use temperature controlling equipment such as water baths or incubators to effectively load compounds. The influence of multiple rounds of hypotonic loading on the delivery of HPTS (Figure 1(e)) was investigated by exposing cells to consecutive rounds (1 - 3) of hypoosmolar loading with a 30 min recovery period in complete growth medium in between loading rounds. Assessment of HPTS uptake showed that multiple rounds of loading were able to increase the amounts of compound delivered in a manner that was proportional to the number of times cells were loaded.
The capacity to deliver membrane impermeable HPTS into HT-29 cells was likely to be a function of the concentration of HPTS within the hypoosmolar solution. To this end, we tested the effect of increasing the concentration of HPTS in the hypoosmolar solution on the delivery of HPTS into cells (Figure 1(f)). The concentration of HPTS was effectively increased by decreasing the incubation volume. As such, the same moles of compound were used in the hypotonic buffers containing different final concentrations of HPTS. The incubation volumes ranged from 400 µL to 50 µL with the final concentrations of HPTS ranging from 0.25 mM up to 2 mM. Analysis of fluorescence levels revealed that HPTS uptake was proportionally related to the concentration of HPTS in solution and that increasing HPTS concentration by reducing final incubation volumes provided an effective means to deliver molecules while preserving compound. Finally the effect of adding DMSO, a solvent commonly used to dissolve drugs and compounds due to its amphipathic quality, on the delivery of HPTS into HT-29 cells was assessed. Cells were incubated in hypotonic solution supplemented with or without 0.5% DMSO and HPTS uptake measured (Figure 1(g)). HPTS levels were not significantly altered by the presence of DMSO indicating that DMSO could be used as a solvent for compounds.
In conclusion, we describe the conditions for efficient hypotonic shift mediated delivery of a model cell-impermeable compound HPTS into a human cell line. The study clearly showed that HPTS delivery displayed an inverse relationship to loading buffer osmolarity, could be performed at 25˚C with no lose of efficiency, was proportionally related to incubation time, and was increased through multiple rounds of loading. Hypotonic shift mediated delivery represents a highly efficient method of loading membrane impermeable compounds into mammalian cells. The technique described herein has a potential application in HTS drug discovery studies where the activities of compounds against target molecules could be assessed irrespective of membrane permeability, thus increasing the pool of lead compounds identified for drug discovery. Lead compounds identified that prove to be membrane impermeable could then be further studied or modified to improve membrane permeability. Ultimately, hypotonic shift mediated delivery of compounds could aid in the discovery of molecules for therapeutic use or as tools for biomedical research.
4. Acknowledgements
JT acknowledges the Association for International Cancer Research (UK) and the Cancer Council Queensland (Australia) for funding.
REFERENCES
- E. A. Martis, R. Radhakrishnan and R. R. Badve, “HighThroughput Screening: The Hits and Leads of Drug Discovery—An Overview,” Journal of Applied Pharmaceutical Science, Vol. 1, No. 1, 2011, pp. 2-10.
- W. F. An and N. Tolliday, “Cell-Based Assays for HighThroughput Screening,” Molecular Biotechnology, Vol. 45, No. 2, 2010, pp. 180-186. doi:10.1007/s12033-010-9251-z
- B. S. Gan, E. Krump, L. D. Shrode and S. Grinstein, “Loading Pyranine via Purinergic Receptors or Hypotonic Stress for Measurement of Cytosolic pH by Imaging,” American Journal of Physiology, Vol. 275, No. 4, 1998, pp. C1158-C1166.
- K. Koberna, D. Stanek, J. Malinsky, M. Eltsov, A. Pliss, V. Ctrnacta, S. Cermanova and I. Raska, “Nuclear Organization Studied with the Help of a Hypotonic Shift: Its Use Permits Hydrophilic Molecules to Enter into Living Cells,” Chromosoma, Vol. 108, No. 5, 1999, pp. 325-335. doi:10.1007/s004120050384
- I. Kuriyama, T. Mizuno, K. Fukudome, K. Kuramochi, K. Tsubaki, T. Usui, N. Imamoto, K. Sakaguchi, et al., “Effect of Dehydroaltenusin-C12 Derivative, a Selective DNA Polymerase Alpha Inhibitor, on DNA Replication in Cultured Cells,” Molecules, Vol. 13, No. 12, 2008, pp. 2948-2961. doi:10.3390/molecules13122948
- Y. H. Lee and C. A. Peng, “Effect of Hypotonic Stress on Retroviral Transduction,” Biochemical and Biophysical Research Communications, Vol. 390, No. 4, 2009, pp. 1367- 1371. doi:10.1016/j.bbrc.2009.10.161
- J. L. Lemoine, R. Farley and L. Huang, “Mechanism of Efficient Transfection of the Nasal Airway Epithelium by Hypotonic Shock,” Gene Therapy, Vol. 12, No. 16, 2005, pp. 1275-1282. doi:10.1038/sj.gt.3302548
- J. Sharif, M. Muto, S. Takebayashi, I. Suetake, A. Iwamatsu, T. A. Endo, J. Shinga, Y. Mizutani-Koseki, et al., “The SRA Protein Np95 Mediates Epigenetic Inheritance by Recruiting Dnmt1 to Methylated DNA,” Nature, Vol. 450, No. 7171, 2007, pp. 908-912. doi:10.1038/nature06397
- D. Stanek, K. Koberna, A. Pliss, J. Malinsky, M. Masata, J. Vecerova, M. C. Risueno and I. Raska, “Non-Isotopic Mapping of Ribosomal RNA Synthesis and Processing in the Nucleolus,” Chromosoma, Vol. 110, No. 7, 2001, pp. 460-470. doi:10.1007/s00412-001-0172-2
- S. Takebayashi, T. Tamura, C. Matsuoka and M. Okano, “Major and Essential Role for the DNA Methylation Mark in Mouse Embryogenesis and Stable Association of DNMT1 with Newly Replicated Regions,” Molecular and Cellular Biology, Vol. 27, No. 23, 2007, pp. 8243-8258. doi:10.1128/MCB.00899-07
- E. K. Hoffmann, I. H. Lambert and S. F. Pedersen, “Physiology of Cell Volume Regulation in Vertebrates,” Physiological Reviews, Vol. 89, No. 1, 2009, pp. 193-277. doi:10.1152/physrev.00037.2007
- W. C. O’Neill, “Physiological Significance of VolumeRegulatory Transporters,” American Journal of Physiology, Vol. 276, No. 5, 1999, pp. C995-C1011.
- T. van der Wijk, S. F. Tomassen, A. B. Houtsmuller, H. R. de Jonge and B. C. Tilly, “Increased Vesicle Recycling in Response to Osmotic Cell Swelling. Cause and Consequence of Hypotonicity-Provoked ATP Release,” The Journal of Biological Chemistry, Vol. 278, No. 41, 2003, pp. 40020-40025. doi:10.1074/jbc.M307603200
NOTES
*Corresponding author.

