American Journal of Analytical Chemistry
Vol.10 No.05(2019), Article ID:92342,14 pages
10.4236/ajac.2019.105015
Photocatalytic Degradation of Paraquat Herbicide Using a Fixed Bed Reactor Containing TiO2 Nanoparticles Coated onto β-SiC Alveolar Foams
Ignace Christian M’Bra1,2*, Grah Patrick Atheba2, Didier Robert1, Patrick Drogui3, Albert Trokourey2
1Institut de Chimie et Procédés pour l’Energie, l’Environnement et la Santé (ICPEES), CNRS-UMR7515 Université de Strasbourg, antenne de Saint-Avold, Université de Lorraine, Saint-Avold, France
2Laboratoire de Chimie Physique, UFR Sciences des Structures de la Matière et de la Technologie (SSMT), Université Félix Houphouët Boigny d’Abidjan, Côte d’Ivoire
3Institut National de la Recherche Scientifique (INRS-Eau, Terre et environnement), Université du Québec, Québec, Canada

Copyright © 2019 by author(s) and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY 4.0).
http://creativecommons.org/licenses/by/4.0/



Received: March 4, 2019; Accepted: May 7, 2019; Published: May 10, 2019
ABSTRACT
Photocatalytic degradation of paraquat (PQ) aqueous solutions was studied in a fixed bed photoreactor under UV irradiation at 368 nm. This contained β-SiC alveolar foams coated with TiO2 P25 by dip-coating method. SEM analyses revealed that the surface of the film did not exhibit cracks in the presence of TTIP as a binder in the TiO2 P25 suspension. The following parameters were studied in continuous mode operation: the flow rate in the reactor, the initial concentration of the paraquat, the pH of the solution, the weight of photocatalytic material with the number of foams in the reactor and the weight of the catalyst deposited onto the support. The results showed that by working under optimal operating conditions at natural pH (pH = 6.7), low paraquat (Co = 10 ppm), and flow (26 mL/min), we recorded approximately (43.16 ± 1.00)% oxidation of paraquat and a decrease in total organic carbon (TOC) of (27.13 ± 1.00)% after about 70 minutes. The apparent rate constant is in the order of (0.0656 ± 0.0010) min−1. In addition, by increasing the amount of β-SiC foams coated with TiO2, we improve the degradation of paraquat in the same order. The study of aging of the material showed its stability over time. However, photocatalytic activity was limited after 20 minutes of UV irradiation due to the limitation of the diffusion of the paraquat molecules towards the surface of the photocatalyst. As an outcome, we obtained an efficient TiO2/β-SiC material for photocatalytic degradation of organic compounds in water.
Keywords:
Photocatalytic Degradation, Paraquat, β-SiC Foam, TiO2 P25, Water

1. Introduction
Paraquat (PQ) is an herbicide belonging to the family of bipyridines, previously marketed as dichloride and widely used in agriculture especially in developing countries. It is used for the control of aquatic weeds, weeds in cereals (corn, wheat, barley, rye, rice …), soybean, potato, fruits (apple, orange, banana …), plants for the manufacture of beverages (coffee, tea, cocoa) and treated crops (cotton, palm oil, sugar cane and rubber) [1] . Although it has enormous benefits for farmers, its use can be a source of pollution for surface water due to its high adsorption capacity in soils [2] . This situation represents a real hazard for our environment and especially for the human health. The absorption of paraquat is dangerous because of its acute and chronic toxicity, by ingestion even at low doses (10 ml or 2 coffee spoons). Research has shown a link between paraquat and Parkinson’s disease [3] .
It is well known that many pesticides are recalcitrant organic compounds [4] [5] . This has prompted the scientific community to effectively develop elimination technologies, which are known as Advanced Oxidation Processes (AOPs) [6] . These processes are based on the production of hydroxyl (OH˚) radicals which are strong oxidizing agents for mineralizing organic pollutants. Among the various AOPs, attention has been paid to semi-conductor photocatalysts (e.g. TiO2) [7] since twenty years because of their ability to mineralize a wide range of recalcitrant organic compounds at room temperature and atmospheric pressure into harmless substances [8] .
Previous studies have been carried out using suspended photocatalysts semi-conductors (suspension of TiO2 nanopowder) [9] . However, post-treatment recovery of TiO2 is an arduous process due to the size of the catalyst particles in the order of the nanometer and the cost involved [10] . Therefore, filtration and resuspension of photocatalyst powder should be avoided if possible, in a wastewater treatment process.
Over the past two decades, the idea of immobilizing a photocatalyst on an adequate and chemically inert support has begun to emerge because it may help to avoid the process of separating expensive phases [11] . Many studies have been published on the photocatalyst particle deposition onto different supports that are easily removable. Several researchers have immobilized the photocatalysts on a variety of surfaces, such as glass, silica gel, metal, ceramics, polymers, fibers, zeolite, clay alumina, coal cellulose, foams [12] [13] [14] . However, the surface of the photocatalyst is active only when illuminated by solar or artificial light with an appropriate wavelength. Thus, supported photocatalytic systems often suffer from mass transfer limitation due to the reduction in surface area relative to suspended photocatalysts [15] . However, the immobilization of the catalysts on substrates remains at the moment a promising alternative for the use of heterogeneous photocatalysis in the field of the industrial water treatment.
In this work, β-SiC foams were used as a photocatalytic support due to their high chemical resistance, outstanding thermal stability and their macroporosity providing a high internal surface enabling the immobilization of large amounts of photocatalysts [16] [17] . The objective of this work is to immobilize TiO2 P25 nanoparticles onto β-SiC alveolar foams for the treatment of industrial effluents. The obtained TiO2 films were characterized by scanning electron microscopy. Thus, we have chosen to study and optimize the treatment of water containing a pesticide by heterogeneous photocatalysis under artificial UV-A lamps irradiation (368 nm). We built a simple photoreactor to remove the paraquat from the water in continuous mode.
2. Materials and Methods
2.1. Reactants
Paraquat (C12H14Cl2N2, methyl-violen or 1,1’-dimethyl-4,4’-bipyridinium according to IUPAC, from Sigma-Aldrich), TiO2 P25 (average size 20 nm, purity 97%, surface area 50 m2∙g−1 and 80% anatase, 20% rutile, Evonik industries) were used as received. Ethanol anhydride 99.8% and titanium tetra isopropoxide (TTIP, C12H28O4Ti, 97%) were purchased from respectively Fluka and Sigma-Aldrich. Distilled water was used to prepare all the solutions.
2.2. Preparation of TiO2 P25 Suspension
TiO2 suspension was prepared by adding 10 g of Titanium dioxide P25 in 200 mL of ethanol used as solvent. The resulting concentration of TiO2 was 50 g/L. Then, 4 mL of TTIP was added in the suspension and used as binder to join the TiO2 nanoparticles together. TiO2 slurry is stirred for 24 h with a magnetic stirrer.
2.3. Deposition of TiO2 P25 onto β-SiC Foams by Dip-Coating
The β-SiC alveolar foams were synthesized and supplied by SICAT Company (Willstätt, Germany) [18] . The β-SiC foams were presented in form of parallelepipedic substrates with a dimension of 9.5 cm (length) × 6 cm (width) × 1 cm (thickness), each having a weight of 20 g.
Each piece of β-SiC foam was completely immersed in the TiO2 suspension for three min at 5 rpm in order to immobilize the TiO2 photocatalysts on β-SiC. This action was repeated five times. Then the TiO2/β-SiC foams were dried at room temperature for 20 min. The TiO2/β-SiC foams were placed in an oven at 110˚C overnight to evaporate the residual solvent. Subsequently, TiO2/β-SiC foams were brought to the furnace at 450˚C during 2 h at a rise rate of 5˚C∙min−1. The material was calcined to remove all traces of organic matter and to increase the TiO2 grip on the foam. The average wt % of TiO2 per foam was 7.5%. Figure 1 shows the material photocatalytic β-SiC before and after coating.
2.4. SEM Analysis
Scanning Electron Microscopy (SEM) images of TiO2 P25 films onto the β-SiC foams were recorded using a JEOL scanning electron microscope (JSM-6390) at an accelerating voltage of 25 kV.
2.5. Photocatalytic Experiments
Photocatalytic treatments were carried out in a continuous flow-through photoreactor made of polypropylene material with a dimension of 20 cm (length) × 7.5 cm (width) × 2.5 cm (depth). One or two TiO2/β-SiC foams were placed inside the photoreactor to perform the photocatalytic tests. The photoreactor was covered with a quartz plate to filter the UV radiation. Two UV-A lamps (Philips 18 W, China) were placed horizontally 2 cm above the reactor to illuminate the TiO2/β-SiC photocatalysts. Irradiation wavelength was around 368 ± 20 nm with an irradiance of about 60 W∙m−2 using a UV-A radiometer (spectral range: 315 - 400 nm; HD 9021; Delta OHM; Italy). Figure 2 shows the photocatalytic experimental device. Paraquat’s solution was obtained with distilled water. The treated effluent left the reactor through overflow mode. The effluent containing the pollutant (paraquat herbicide) was stored in a 1 litre tank immersed in an ice bath (about 0˚C) to avoid evaporation of the solution in the reactor. The effluent circulated through the photocatalytic system in a single pass using a peristaltic pump. The inlet and outlet flow rates were quite the same and ranged between 26 and 87 mL∙min−1 with a retention time varying from 4 to 12 min about. The feed solution contained 10 to 30 ppm of paraquat herbicide.
Preliminary tests were carried out to compare the direct UV-A photolysis (direct irradiation of the solution without TiO2/β-SiC), the paraquat adsorption on
Figure 1. β-SiC foam uncoated (a) and coated (b).
Figure 2. Schematic view of the photocatalytic device: A (2 UV-A lamps); B (photoreactor); C (paraquat’s aqueous solution); D (ice bath).
TiO2/β-SiC (without UV-A) and the heterogeneous photocatalysis (UV-A and TiO2/β-SiC) processes. The experimental conditions are: flow (26 mL∙min−1); Co = 10 ppm; V (PQ) = 1 L, pH = 6.7 and two TiO2/β-SiC. Photocatalytic device was conducted at room temperature of the laboratory at about 20˚C.
2.6. Analytical Method for Paraquat Determination
Paraquat was chosen as a target pollutant [8] to evaluate the photocatalytic performances of the photocatalytic materials. During the irradiation procedure, 2 mL of the solutions were sampled at regular time intervals. The remaining concentration of paraquat was followed by a LIBRA S12 UV-Vis spectrophotometer at 257 nm (maximum absorption wavelength). The pH of the aqueous solutions of the paraquat was adjusted by HCl (0.1 M) and NaOH (0.1 M) aqueous solutions. pH values were determined by a pH-meter (labChem-CP) equipped with junction pH electrode (Model IJ44C). The evolution of TOC during the degradation kinetics of paraquat was followed by a SHIMADZU TOC-L (Total Organic Carbon Analyzer, Japan). Considering that the degradation follows a pseudo first order kinetics, the apparent kinetic constant kapp was calculated according to the following equations:
(1)
(2)
With
r: reaction rate;
C: residual concentration of the pollutant;
Co: initial concentration of the pollutant;
kapp: apparent kinetic constant and
t: UV irradiation time.
2.7. Study of the Aging of the Photocatalytic Material
Five subsequent experiments were carried out using the same sample of TiO2/ β-SiC photocatalysts. Between each experiment, the TiO2/β-SiC foams were treated at 450˚C for 2 hours to remove all traces of organic matter. The evaporation temperature of paraquat is about 300˚C. The operating conditions of the continuous aging study were as follows: pump flow rate (26 mL∙min−1), volume of paraquat to be treated (2 L), concentration of paraquat (10 ppm), two TiO2/SiC materials, duration of the experiment (70 min).
3. Results and Discussion
3.1. SEM Analysis of the TiO2 P25 Film on β-SiC Foams
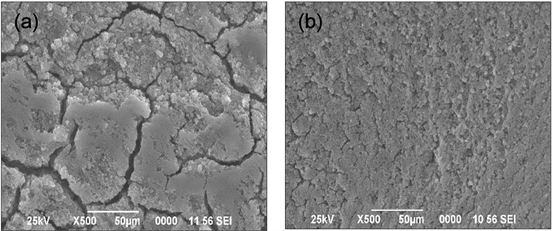
Figure 3 shows the morphologies of the TiO2 P25 film surfaces with and without TTIP. The analyses showed the presence of micro and macro cracks at the surface of the film prepared without the addition of the TTIP in the suspension (Figure 3(a)). These results were observed by some authors [19] . The size of the cracks varied from 3 to 7 μm. These cracks were due to the drying of the material after the dip-coating and during the heat treatment at 450˚C. However, no cracks are observed on the film surface when we added TTIP in the suspension (Figure 3(b)). This homogeneity of the film was due to the presence of the TTIP that plays the role of binder. This organic polymer made it possible to solder the molecules of the TiO2 P25 nanoparticles, thus avoiding cracks during temperature drying and calcination in the furnace.
3.2. Preliminary Study
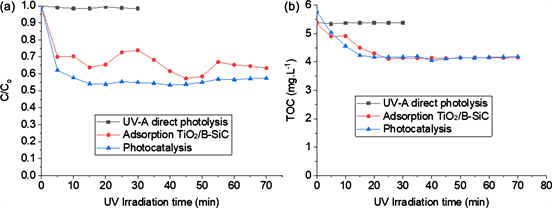
Photocatalytic study was carried out to compare the removal efficiency of paraquat by direct photolysis, adsorption and photocatalysis processes. Figure 4
Figure 3. SEM pictures of the surface of TiO2 P25 films coating β-SiC alveolar foams (a) without addition of TTIP and (b) with addition of TTIP.
shows that the direct photolysis process remains ineffective, only 1.50% of paraquat and 0.17% of TOC was eliminated after 70 min of irradiation. On the other hand, adsorption and photocatalytic processes remain effective with paraquat abatement rates of 36.65% and 42.68% respectively (Figure 4(a)) and 22.34% and 27.45% of TOC eliminated (Figure 4(b)). A competition between the two processes occured. However, the photocatalytic process remained as effective as adsorption process [20] . The difference between the two processes can be explained by the fact that during the photocatalytic process, OH˚ radicals were released to destroy some Paraquat molecules adsorbed on the surface of TiO2 P25.
Beyond 20 min of irradiation, we observed a limit of the diffusion of the paraquat molecules to the surface of photocatalyst. This was due to the plug flow reactor used during these experiments. Kouamé et al. [21] showed that the foam played the role of mixer. Due to the size of the foams, some molecules of the paraquat remain at the bottom of the unlit reactor.
3.3. Optimization of Operating Parameters on Photocatalytic Degradation
3.3.1. Influence of TTIP
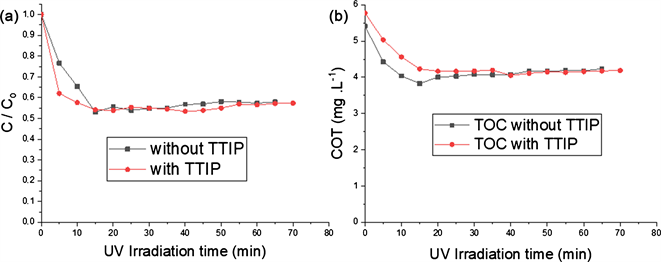
The addition of TTIP in the TiO2 P25 suspension has interesting advantages. It was important to know whether the TTIP had an influence on the kinetics of paraquat degradation. Figure 5(a) shows that the two TiO2/β-SiC materials made with or without TTIP had the same photocatalytic activities. Their apparent reaction rate constants were respectively 0.0632 min−1 and 0.0446 min−1 with and without TTIP. The results were similar for the elimination of TOC. 26.9% and 24.44% were respectively registered with and without TTIP (Figure 5(b)). The presence of TTIP has a little influence on the paraquat degradation kinetics on a single test. The TTIP has no influence on the kinetics of degradation of paraquat. Thus, TiO2/β-SiC material containing TTIP was selected for the next step of the experiments because the homogeneous films obtained could mechanically stabilize the material during the photocatalytic experiments.
3.3.2. Influence of the Flow in the Reactor
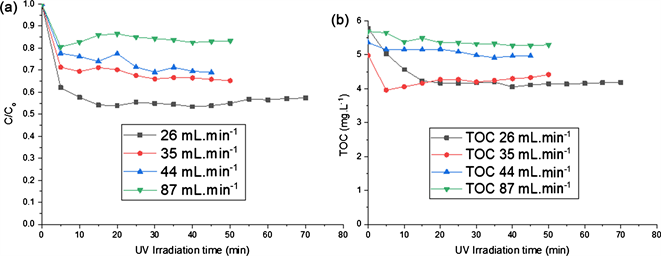
The flow rate of the Paraquat aqueous solution was evaluated from 26 to 87 mL∙min−1 in the reactor. Figure 6 shows the photocatalytic degradation of the
Figure 4. Preliminary tests: UV-A direct photolysis, adsorption and photocatalysis.
Figure 5. Influence of TTIP.
Figure 6. Influence of flow rate in the reactor.
Paraquat with variation of the flow rate. The results showed that the flow rate had an influence on the degradation kinetics. The Paraquat and TOC abatement rates decreased respectively from 42.68% to 16.74% (Figure 6(a)) and from 27.45% to 6.93% (Figure 6(b)) when the flow rate increased (Figure 6(a)). The reason resulted in shorter residence time that reduced the degradation rates of paraquat. Indeed, the shorter residence time negatively affected the adsorption process on TiO2 surface and photocatalytic degradation of paraquat was also affected. Yu et al. observed a similar effect. These authors reported that the photocatalytic degradation of formaldehyde using TiO2 catalyst was affected by the flow rate, when it was increased from 0.0001 to 0.1 ms−1 [22] . The apparent kinetics constant decreased from 0.0632 to 0.0273 min−1 when the flow rate of the solution increased. Indeed, at the beginning of the process, we observed a degradation of the paraquat molecules. Beyond 10 min, the diffusion of paraquat molecules on the surface of TiO2 P25 supported on the foams is limited. Supported TiO2 photocatalysts suffer from mass transfer limitation. For the next step of the experiments, the flow rate was set at 26 mL∙min−1.
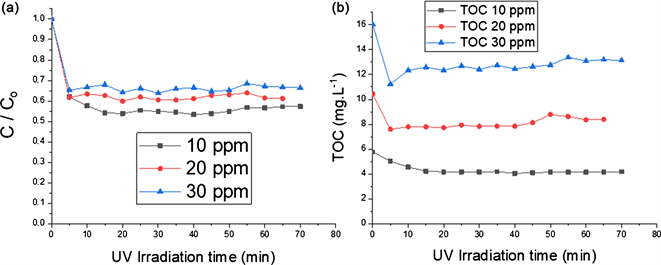
3.3.3. Influence of the Initial Concentration of Paraquat
The influence of the pollutant concentration on photocatalytic degradation and TOC removal are respectively shown in Figure 7(a) and Figure 7(b). Three concentrations were chosen: 10 ppm, 20 ppm and 30 ppm. The paraquat degradation rate decreased from 42% to 33.57% when the concentration increased. The same trend was also recorded for TOC removal (ranging from 27.45% to 18.08% when the concentration of paraquat increased). These results were consistent with those recorded by some authors [23] . The results showed that kapp decreased slowly from 0.0632 min−1 to 0.0495 min−1 when the initial concentration of paraquat increased. Beyond 10 min, the variation of the apparent kinetics constant Δkapp remains zero. Due to the limited diffusion of paraquat molecules towards the TiO2 P25 surface, the reaction intermediates disturbed paraquat degradation. When the concentration of the paraquat increased, there was a significant production of intermediate products. For the next experiments, the initial paraquat concentration was set at 10 ppm.
3.3.4. Influence of pH
Figure 8 shows the effects of pH on the photocatalytic degradation of paraquat.
Figure 7. Influence of paraquat initial concentration.
Figure 8. Influence of pH: evolution of apparent kinetic constant.
The natural pH was 6.7 and pH values ranging between 5 and 10 were tested. To study the influence of pH on the pollutant oxidation, it was necessary to know the isoelectric point (ZPC) of the photocatalyst. For TiO2 P25, the pHzpc value is 6.25 [13] . The maximum rate of paraquat degradation was obtained at a neutral pH value close to pHzpc. In this condition, the apparent average kinetic constant was 0.0510 min−1. The pH effect on Paraquat photocatalysis can be attributed to the surface properties of the photocatalyst TiO2 P25. For pH values below pHZPC, the surface of the photocatalyst was positively charged and an electrostatic repulsion towards the cationic compounds predominates. When the pH was higher than pHZPC, the photocatalyst surface charges negatively and electrostatic repulsion towards the anionic compounds dominates. Similarly, depending on the pKa = 9 - 9.5 value [24] [25] , the cationic form of Paraquat is dominant for pH values below pKa. The paraquat was stable in acidic and neutral media and was hydrolyzed in a basic medium (Equation (3)).
(3)
Thus, the phenomena of repulsion between the positive surface of TiO2 P25 and the positively charged Paraquat molecules could explain the decrease in photocatalytic activity at pH values below 6.7. Atheba et al. observed the same phenomenon while studying the pH effect on the photodegradation of butylparaben by using supported TiO2 photocatalyst [20] .
This is an important result for industrial applications because it will be no need to adjust pH during treatment. For the subsequent experiments, a pH value around 6.7 was selected.
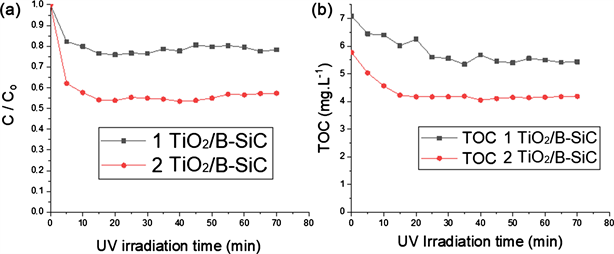
3.3.5. Influence of Number of β-SiC Foams Coated with TiO2 P25
The photoreactor used for photodegradation of the paraquat could contain up to two β-SiC foams. It was important to know the influence of the number of foams and consequently the mass of catalyst, on the paraquat degradation kinetics. The amount of TiO2 P25 were 1.97 g and 3.29 g on average respectively for one foam and two foams. Figure 9 shows that the amount of photocatalytic materials in the reactor had an important influence on paraquat degradation. The percentages
Figure 9. Influence of the number of foams.
of paraquat degradation were respectively 21.63% and 42.68% (Figure 9(a)) respectively for one foam and two foams coated with TiO2 P25. When the amount of photocatalytic materials increases, the TiO2 P25 nanoparticles occupied a much larger reaction area. However, the level of TOC removed during the reaction increased slowly (from 20.01% to 27.45%) (Figure 9(b)).
3.3.6. Influence of the Photocatalyst’s Weight onto the β-SiC Foam
In this section, we studied the variation of TiO2 P25 weight onto one foam and two β-SiC foams to evaluate the photocatalytic degradation of the paraquat. Figure 10 shows the kinetic degradation rate of the paraquat for each sample. The obtained results were 15.74%, 16.75% and 21.63% respectively for 0.6 g; 1 g and 1.97 g of TiO2 P25 onto one foam. On the other hand, the photocatalytic activity of TiO2 P25 distributed over two foams: 0.6 g + 1 g TiO2 (19.51%) is practically the same when we have 1.97 g of TiO2 P25 onto one foam (21.63%). However, with 3.29 g of TiO2 P25, we had 42.68% degradation of paraquat. The number of foams has an influence on the photocatalytic degradation of the paraquat, it is recommended to coat an enough TiO2 P25 on a foam (on average 2 g of TiO2 P25).
3.4. Study of the Aging of the TiO2/β-SiC Material
The paraquat kinetics of disappearance after reuse of the TiO2/β-SiC catalytic material is studied. The results showed that the photocatalytic activity of TiO2/β-SiC remained constant after five cycles of reuse of photocatalyst [26] . Their photocatalytic properties remained unchanged. This shows that the remaining degradation by-products at the material surface are eliminated after the heat treatment between two tests.
Table 1 shows the percentages of paraquat removal (43.16 ± 1.00)%, TOC
Figure 10. Influence of TiO2 P25 weight onto one foam and two foams in presence of TTIP.
Table 1. Percentages of paraquat removal, TOC removal and the apparent rate constant of the photoctalytic reaction during the five reuse tests using TiO2/β-SiC.
removal (27.13 ± 1.00)% and the apparent rate constant (0.0656 ± 0.0010) min−1 during the five reuse tests. These results are very encouraging because the study of the aging of the photocatalytic material remains a necessity in the step of application of the heterogeneous photocatalysis on a large-scale application.
4. Conclusion
The titanium dioxide nanoparticles were immobilized on β-SiC foam by dip-coating method. The addition of TTIP in the TiO2 P25 suspension made it possible to weld the nanoparticles together and to be well-fixed on the support. The studied parameters allowed us to have a photocatalytic activity on the degradation of the paraquat. The results obtained are encouraging while working in continuous mode operation. This revealed the photocatalytic efficiency of TiO2/β-SiC photocatalyst. It can be easily reused after several cycles of treatment. The filtration step could be avoided using TiO2/β-SiC supported photocatalyst. However, the photocatalytic activity is limited by the low diffusion of the pollutant towards the surface of the photocatalyst after 20 minutes of UV irradiation. In addition, the foams can have any shape to be adapted to the industrial scale applications.
Acknowledgements
Thanks to Agence Universitaire de la Francophonie (AUF) for the funding granted under the project “Development of advanced technologies for the remediation of waste water and effluents adapted to non-collective sanitation” led by Patrick DROGUI (Full Professor) of INRS, Eau Terre et Environnement, University of Québec. The authors also wish to thank SICAT Company (Germany) for the availability of β-SiC alveolar foams and Henri VAHABI (associate Professor) IUT Saint-Avold, University of Lorraine for SEM images.
Conflicts of Interest
The authors declare no conflict of interest.
Cite this paper
M’Bra, I.C., Atheba, G.P., Robert, D., Drogui, P. and Trokourey, A. (2019) Photocatalytic Degradation of Paraquat Herbicide Using a Fixed Bed Reactor Containing TiO2 Nanoparticles Coated onto β-SiC Alveolar Foams. American Journal of Analytical Chemistry, 10, 171-184. https://doi.org/10.4236/ajac.2019.105015
References
- 1. Grillo, R., Pereira, A.E., Nishisaka, C.S., de Lima, R., Oehlke, K., Greiner, R. and Fraceto, L.F. (2014) Chitosan/Tripolyphosphate Nanoparticles Loaded with Paraquat Herbicide: An Environmentally Safer Alternative for Weed Control. Journal of Hazardous Materials, 278, 163-171. https://doi.org/10.1016/j.jhazmat.2014.05.079
- 2. Gondar, D., López, R., Antelo, J., Fiol, S. and Arce, F. (2012) Adsorption of Paraquat on Soil Organic Matter: Effect of Exchangeable Cations and Dissolved Organic Carbon. Journal of Hazardous Materials, 235, 218-223. https://doi.org/10.1016/j.jhazmat.2012.07.044
- 3. Kab, S., Spinosi, J., Chaperon, L., Dugravot, A., Singh-Manoux, A., Moisan, F. and Elbaz, A. (2017) Agricultural Activities and the Incidence of Parkinson’s Disease in the General French Population. European Journal of Epidemiology, 32, 203-216. https://doi.org/10.1007/s10654-017-0229-z
- 4. Jeirani, Z., Sadeghi, A., Soltan, J., Roshani, B. and Rindall, B. (2015) Effectiveness of Advanced Oxidation Processes for the Removal of Manganese and Organic Compounds in Membrane Concentrate. Separation and Purification Technology, 149, 110-115. https://doi.org/10.1016/j.seppur.2015.05.009
- 5. Rodriguez-Mozaz, S., Ricart, M., Köck-Schulmeyer, M., Guasch, H., Bonnineau, C., Proia, L., de Alda, M.L., Sabater, S. and Barceló, D. (2015) Pharmaceuticals and Pesticides in Reclaimed Water: Efficiency Assessment of a Microfiltration-Reverse Osmosis (MF-RO) Pilot Plant. Journal of Hazardous Materials, 282, 165-173. https://doi.org/10.1016/j.jhazmat.2014.09.015
- 6. Tichonovas, M., Krugly, E., Jankunaite, D., Racys, V. and Martuzevicius, D. (2017) Ozone-UV-Catalysis Based Advanced Oxidation Process for Wastewater Treatment. Environmental Science and Pollution Research, 24, 17584-17597. https://doi.org/10.1007/s11356-017-9381-y
- 7. Haynes, V.N., Ward, J.E., Russell, B.J. and Agrios, A.G. (2017) Photocatalytic Effects of Titanium Dioxide Nanoparticles on Aquatic Organisms—Current Knowledge and Suggestions for Future Research. Aquatic Toxicology, 185, 138-148. https://doi.org/10.1016/j.aquatox.2017.02.012
- 8. Marien, C.B.D., Marchal, C., Koch, A., Robert, D. and Drogui, P. (2017) Sol-Gel Synthesis of TiO2 Nanoparticles: Effect of Pluronic P123 on Particle’s Morphology and Photocatalytic Degradation of Paraquat. Environmental Science and Pollution Research, 24, 12582-12588. https://doi.org/10.1007/s11356-016-7681-2
- 9. León, D.E., Zúñiga-Benítez, H., Peñuela, G.A. and Mansilla, H.D. (2017) Photocatalytic Removal of the Antibiotic Cefotaxime on TiO2 and ZnO Suspensions under Simulated Sunlight Radiation. Water, Air, and Soil Pollution, 228, 361. https://doi.org/10.1007/s11270-017-3557-4
- 10. Mahmoodi, N.M. and Arami, M. (2006) Bulk Phase Degradation of Acid Red 14 by Nanophotocatalysis Using Immobilized Titanium(IV) Oxide Nanoparticles. Journal of Photochemistry and Photobiology A: Chemistry, 182, 60-66. https://doi.org/10.1016/j.jphotochem.2006.01.014
- 11. Li, P., Wei, Y., Tan, X., Li, X., Wang, Y., Zhao, Z., Yuan, Z. and Liu, A. (2016) Effective Optimization of Emitters and Surface Passivation for Nanostructured Silicon Solar Cells. RSC Advances, 6, 104073-104081. https://doi.org/10.1039/C6RA20945A
- 12. Robert, D., Keller, V. and Keller, N. (2013) Immobilization of a Semiconductor Photocatalyst on Solid Supports: Methods, Materials, and Applications. Wiley-VCH, Weinheim, 145-178. https://doi.org/10.1002/9783527645404.ch6
- 13. Kanakaraju, D., Kockler, J., Motti, C.A., Glass, B.D. and Oelgemöller, M. (2015) Titanium Dioxide/Zeolite Integrated Photocatalytic Adsorbents for the Degradation of Amoxicillin. Applied Catalysis B: Environmental, 166, 45-55. https://doi.org/10.1016/j.apcatb.2014.11.001
- 14. Trabelsi, H., Atheba Grah, P., Hentati, O., Mariette Yehe, D., Robert, D. and Drogui, P. (2016) Solar Photocatalytic Decolorization and Degradation of Methyl Orange Using Supported TiO2. City, 19, 79. https://doi.org/10.1515/jaots-2016-0110
- 15. Parra, S., Elena Stanca, S., Guasaquillo, I. and Ravindranathan Thampi, K. (2004) Photocatalytic Degradation of Atrazine Using Suspended and Supported TiO2. Applied Catalysis B: Environmental, 51, 107-116. https://doi.org/10.1016/j.apcatb.2004.01.021
- 16. M’Bra, I.C., García-Muñoz, P., Drogui, P., Keller, N., Trokourey, A. and Robert, D. (2019) Heterogeneous Photodegradation of Pyrimethanil and Its Commercial Formulation with TiO2 Immobilized on SiC Foams. Journal of Photochemistry and Photobiology A: Chemistry, 368, 1-6. https://doi.org/10.1016/j.jphotochem.2018.09.007
- 17. Marien, C.B., Le Pivert, M., Azaïs, A., M’Bra, I.C., Drogui, P., Dirany, A. and Robert, D. (2018) Kinetics and Mechanism of Paraquat’s Degradation: UV-C Photolysis vs UV-C Photocatalysis with TiO2/SiC Foams. Journal of Hazardous Materials, 370, 164-171. https://doi.org/10.1016/j.jhazmat.2018.06.009
- 18. Nguyen, P. and Pham, C. (2011) Innovative Porous SiC-Based Materials: From Nanoscopic Understandings to Tunable Carriers Serving Catalytic Needs. Applied Catalysis A: General, 391, 443-454. https://doi.org/10.1016/j.apcata.2010.07.054
- 19. Chen, L., Zheng, K. and Liu, Y. (2017) Geopolymer-Supported Photocatalytic TiO2 Film: Preparation and Characterization. Construction and Building Materials, 151, 63-70. https://doi.org/10.1016/j.conbuildmat.2017.06.097
- 20. Atheba, P., Drogui, P., Seyhi, B. and Robert, D. (2013) Photo-Degradation of Butyl Parahydroxybenzoate by Using TiO2-Supported Catalyst. Water Science and Technology, 67, 2141-2147. https://doi.org/10.2166/wst.2013.117
- 21. Kouamé, A.N., Masson, R., Robert, D., Keller, N. and Keller, V. (2013) β-SiC Foams as a Promising Structured Photocatalytic Support for Water and Air Detoxification. Catalysis Today, 209, 13-20. https://doi.org/10.1016/j.cattod.2012.12.008
- 22. Yu, H., Zhang, K. and Rossi, C. (2007) Theoretical Study on Photocatalytic Oxidation of VOCs Using Nano-TiO2 Photocatalyst. Journal of Photochemistry and Photobiology A: Chemistry, 188, 65-73. https://doi.org/10.1016/j.jphotochem.2006.11.021
- 23. Marien, C.B.D., Cottineau, T., Robert, D. and Drogui, P. (2016) TiO2 Nanotube Arrays: Influence of Tube Length on the Photocatalytic Degradation of Paraquat. Applied Catalysis B: Environmental, 194, 1-6. https://doi.org/10.1016/j.apcatb.2016.04.040
- 24. Tantriratna, P., Wirojanagud, W., Neramittagapong, S., Wantala, K. and Grisdanurak, N. (2011) Optimization for UV-Photocatalytic Degradation of Paraquat over Titanium Dioxide Supported on Rice Husk Silica Using Box-Behnken Design. Indian Journal of Chemical Technology, 18, 363-371.
- 25. Milman, B.L. (2003) Cluster Ions of Diquat and Paraquat in Electrospray Ionization Mass Spectra and Their Collision-Induced Dissociation Spectra. Rapid Communications in Mass Spectrometry, 17, 1344-1349. https://doi.org/10.1002/rcm.1056
- 26. Atheba, P., Robert, D., Trokourey, A., Bamba, D. and Weber, J.-V. (2009) Design and Study of a Cost-Effective Solar Photoreactor for Pesticide Removal from Water. Water Science and Technology, 60, 2187-2193. https://doi.org/10.2166/wst.2009.640