American Journal of Analytical Chemistry
Vol.08 No.05(2017), Article ID:76144,11 pages
10.4236/ajac.2017.85025
The Structure of Insoluble Pectinates and Alginates of Polyvalent Metals Based on IR Spectra Data
Nelli Shalikovna Kajsheva, Alexander Shalikovich Kajshev, Anna Borisovna Samoryadova, Sergey Vasilyevich Volokitin, Christina Nicolaevna Gulbjakova, Ekaterina Alexandrovna Maslovskaya
Pyatigorsk Medical and Pharmaceutical Institute―A Branch of Federal State-Funded Educational Institution of Higher Vocational Education “Volg SMU”, Pyatigorsk, Russia

Copyright © 2017 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY 4.0).
http://creativecommons.org/licenses/by/4.0/



Received: April 3, 2017; Accepted: May 12, 2017; Published: May 15, 2017
ABSTRACT
Using the method of IR spectroscopy it was ascertained that in pectinates and alginates of polyvalent metals the coordination bonds between cations Pb2+, Cu2+, Zn2+, Cr3+, Mn2+, Fe2+, Co2+, Ni2+ and the oxygen atoms of carboxyl and hydroxyl groups, pyranose cycle and glycosidic linkage of polyuronides, the water molecules are formed. It was also ascertained that Cu2+ cations form asymmetrical structures with carboxyl groups of polyuronides (monodentate ligands) and cations of other metals―symmetrical structures with carboxyl groups of polyuronides (bidentate ligands).
Keywords:
Coordination Compounds, Pectinates of Metals, Alginates of Metals, Structure, IR Spectra

1. Introduction
The structure of coordination compounds of polyuronides with ions of polyvalent metals (polyuronates of metals) in solid state does not have an unambiguous interpretation due to the presence of several electron-donating atoms of oxygen able to coordinate with the ions of metals and due to the inclination to form coordination compounds of different forms (tetrahedrons, octahedrons) by the pyranose cycles of polyuronides [1] [2] . We believe that the most acceptable method of the ascertainment of the structure of insoluble polyuronates of metals is the method of IR spectroscopy while using which certain atomic bunching of polyuronates may cause the appearance of a great number of informative discrete lines and bands in vibrational spectra.
The goal of this research is to study the structure of the pectinates and alginates Cu2+, Zn2+, Pb2+, Cr3+, Mn2+, Fe2+, Co2+, Ni2+ which are insoluble in water using the method of IR spectroscopy.
2. Experimental
2.1. Materials
The objects of this research were pectin, the sodium alginate and the products of their interaction with inorganic salts: acetates Cu2+, Pb2+, Cr3+, sulphates Fe2+, Zn2+, Mn2+, chloride Co2+, nitrate Ni2+.
The pectin produced by experimental engineering technological bureau “Mars” (Nalchik) from the sugar beet marc and corresponding to the regulations of temporary pharmacopoeic article Temporary Pharmacopoeia article 42-3433- 99 “Pectin” is a polymer featuring average molar mass of 3200 kg/mol consisting of 1 → 4-bound remnants of α-D-galacturonic acid containing 14.4% of free carboxyl groups, 9.2% of methylated carboxyl groups. The content of total ash is 1.4%, the content of ash insoluble in 10% hydrochloric acid is 0.4%, рН of 2% water solution is 3.5, the water dissociation constant is 3.2 × 10−4 [3] .
Sodium alginate produced by experimental algal plant (Arkhangelsk) from the fronds of sacchariferous laminaria and corresponding to the regulations of State all-Russian standard 26185-84 “Seaweeds, sea grass and the products of their processing. The methods of analysis” is polymer featuring average molar mass of 89700 kg/mol consisting of alternating blocks of 1 → 4-bound remnants of β-L- guluronic acid and α-D-mannuronic acid with molar ratio 1:2 [4] [5] . The content of total ash is 25.7%, the content of ash insoluble in 10% hydrochloric acid is 0.8%, рН of 1% water solution is 7.6, the water dissociation constant is 2.5 × 10−8 [4] .
Inorganic salts with Pro Analysi (p.a.) purity qualification were used in the research.
2.2. The Method of Obtaining Polyuronates Metals
The jellous sediments of polyuronates of metals were obtained by mixing water solutions of pectin (5.0 × 10−3 mol/l) previously neutralized by 2.0 mol/l water solution of ammonia up to рН ~ 8, or solutions of sodium alginate (5.0 × 10−3 mol/l) with 4.5 × 10−2 water solutions of inorganic salts with volume ratio 1:1. For almost complete sedimentation reaction mixtures were treated with 96% ethanol (1:2), kept at indoor temperature (2 hours), the sediments were extracted by filtration, flushed by water till the neutral reaction of rinsing water and dried at temperature 60˚C ± 5˚C till fixed mass [6] .
2.3. Conditions of the Analysis by the Method of IR-Spectroscopy
IR absorption spectra of 10% suspensions of polyuronides and polyuronates of metals in liquid petrolatum were registered at 4000 - 400 cm−1 at Infrared Spectrometer-40 spectrophotometer [7] - [12] . The observed shifts of absorption stripes, the change of intensity of absorption bands and the energy of hydrogen bond were the objects of study. The relative error of definitions (n = 7) is 3.4% - 4.8%.
3. Results and Discussion
The pectinates of polyvalent metals. The characteristic absorption bands in IR spectra of pectinates of metals in comparison with pectin are given in Table 1.
Table 1. Characteristic absorption bands (cm−1) in IR spectra of metal pectinates.
Notes: “s.”, strong absorption intensity; “w.”, weak absorption intensity; “wi.”, wide stripe; “s”, symmetrical vibrations; “as”, asymmetrical vibrations; “χ”, inner vibrations. * The data received by the authors are especially reliable as initially they had been studying the IR spectra of monosaccharide metal complexes the structure of which was defined by the method of X-ray structural analysis, and they applied the received assignments to the crystalline structures which are isomorphic with the initial ones.
The most characteristic spectral region in which one can distinguish substantial differences in the character and intensity of absorption bands in IR spectra of pectin and pectinates refers to 3400 - 3100 см−1 range belonging to the valence vibrations of hydroxyl groups. Besides that, the absorption in the more long- wave area was observed for the pectinates of metals which is characteristic for different in energy two- and three-centered donor-acceptor hydrogen bonds of hydroxyl with hydroxyl or substituted hydroxyl [7] :
 (1)
(1)
The vibration interaction of oscillators of bound pectins can be the cause of band splitting in 4000 - 3000 cm−1 area. The hydroxyl groups associated by donor-acceptor hydrogen bonds form a system of bound oscillators which are very sensitive to the external field disturbance. While introducing metal cations onto the pectin molecule in IR spectra the bands shifts of valence vibration of hydroxyl groups into the long-wafe area is observed. That means that a bond breakage or a weakening of hydrogen bonds by metal ions took place while forming the coordination particles. Such spectral changes were observed for the pectinates of all metals. The strongest shift noted took place in the presence of Cu2+ cations.
A great number of stripes of valence vibrations of hydroxyl groups, a sufficient frequency range which all these stripes appear in testify a wide set of energy-wise unequivalent hydrogen bonds in the studied pectinates. Long-wave stripes ν(ОН) experience the most sufficient shift. Taking into account that a high frequency shift of valence vibrations of hydroxyl groups associated by hydrogen groups (νi) concerning the vibration frequency of free hydroxyl groups (νсв = 3636 cm−1) is proportional to the energy of hydrogen bonds (UВС) [7] :
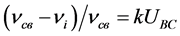 (2)
(2)
in which k is the coefficient of proportionality which for hexapyranosides is 9 × 10−3 mol/kJ [7] ), we have calculated the values of the energy of hydrogen bonds in molecules of the pectinates under study (Table 2).
In order to reveal the correlation between the absorption bands of hydroxyl groups associated by hydrogen bond and the energy values of hydrogen bonds in the pectinates of metals in the graphic system “νi = f(−UВС)” linear relationship y = a + bx, где а = 111.11, b = −3.06 × 10−2 was obtained. This testifies a reliable correlation of energy of hydrogen bonds with the experimental sizes of absorption bands of hydroxyl groups of pectinates which form associates. The series of metal ions in pectinates in the order of their influence on the attenuation of energy of hydrogen bonds is as follows: Cu2+ > Co2+ > Zn2+ > Ni2+ > Cr3+ > Mn2+ > Pb2+ > Fe2+.
The most significant band sift at 3350 cm−1 is caused by Cr3+, Zn2+, Co2+, Ni2+ ions; the band shift at 3275 cm−1 is caused by Cu2+, Cr3+, Pb2+, Mn2+ ions; the band shift at 3180 см−1 is caused by Cu2+, Cr3+, Zn2+ ions.
The second observed area of changes in IR spectra of metal pectin and pectinates refers to 1710 cm−1, this band is due to the absorption of valence vibrations
Table 2. Characteristics of hydrogen bonds formed by the pectinates of metals.
of carbonyl of ester groups. For pectinates this band was observed to shift to the long-wave area for 10 - 15 см−1. Concerning the absorption intensity this band is the weakest for all the substances under study.
The third observed area of changes in IR spectra of pectinates refers to the changes of absorption bands of valent asymmetrical and symmetrical vibrations of ionized carboxyl in 1630 - 1600 and 1430 - 1400 cm−1 areas which are the most susceptible to the nature of the metal cation. In this area the absorption bands of carboxyls are overlapped by the bands of deformative two-dimensional vibrations of water δ(Н2О). While in 1630 - 1600 cm−1 area it is characteristic of pectinates to demonstrate the shift of bands in the direction of low-frequency wavelengths, in 1430 - 1400 см−1 area they are shifted in the direction of high- frequence bands in comparison with pectin (Table 3). With the growth of the radius of hydrated metal ions and as a consequence of it with the decrease of the electrostatic field and the polarizing effect of cations, Δν(as-s СОО−) value is decreasing. Δν(as-s СОО−) value is £200 cm−1 for the studied metal pectinates except for Cu2+ pectinate, which testifies the formation of symmetrical structures of metal ions with carboxyl groups of pectin acting as bidentate ligands [9] :
Table 3. Shift of characteristic absorption bands in IR spectra of metal pectinates.
Примечание: “s”, symmetrical vibrations; “as”, asymmetrical vibrations; *СО of pyranose cycle; **СО of pyranose cycle in comparison with pectin; ***СО of α, glycosidic linkage; ****СО of α, glycosidic linkage in comparison with pectin; Аr, relative atomic mass of metal cation; r, thermochemical radius of hydrated metal cation; ε, polarizing effect of metal cation; Е, electrostatic field of metal cation; c, relative electronegativity of metal cation.
 (3)
(3)
while bonding with Cu2+ ions the carboxyl groups of pectin act as monodentate ligands which is testified by high values of Δν (215), and an asymmetrical structure of carboxylates is formed [9] :
 (4)
(4)
The next area of changes in IR spectra of pectinates refers to the changes of absorption band of valent vibrations of CO of pyranose cycle (924 cm−1): the most noticeable are the high frequency shifts (36 - 45 cm−1) for pectinates Zn2+, Mn2+, Cu2+, Co2+, which is due to the coordination of ions of the mentioned metals to the oxygen atoms of pyranose cycle characteristic to monomeric hexuronic acid as well [16] .
The shift of the absorption band of valent vibrations of α-glycosidic linkage (844 cm−1) into the long-wave area is noticeable only for pectinates Cr3+ и Co2+; evidently, the ions of these metals are coordinated to the oxygen atoms of glycosidic centre.
The position of the described bands and the difference of Δν frequencies are complex functions of relative atomic masses, thermochemical radii of hydrated ions, the polarizing effect of cations, the electrostatic field and the relative electronegativity of metal ions.
Comparing the intensity of the absorption band at 1600 cm−1 one can notice a significant weakening of the intensity of that band at pectinates which can be referred to the insignificant quantity or absence of crystallized water competing with pectins for the coordination with the metal ion.
It is evident that from two functional groups of pectins (carboxyl and hydroxyl) the stronger coordination of metal cations is in carboxyl group as salts are formed in the process. Besides, the carboxyl group from the point of conception of hard and soft acids and bases [17] is a less hard base in comparison with the hydroxyl group and it forms firm bonds with acids taking intermediate position on hardness (mainly with the ions of d-metals (II)) and less firm bonds with hard acids (mainly with the ions of d-metals (III)).
Thus, comparing the bands shift in IR spectra of pectinates and pectins in general, we draw the conclusion that the most sufficient shift of bands is observed for the hydroxyls associated by hydrogen bond. It is explained by the fact that in pyranose cycles the atoms of hydrogen and oxygen are fixed while the atoms of hydrogen of hydroxyl groups have more freedom of movement and only hydrogen binding holds them back from rotating around CO ties.
Alginates of polyvalent metals. The characteristic absorption bands in IR spectra of alginates of polyvalent metals in comparison with sodium alginate are given in Table 4, the shift of these bands and the change of energy of hydrogen bonds are given in Table 5. In 3925 - 3700 см−1 area the band splitting due to the vibration interaction of connected oscillators is observed, especially significant it is for Co2+, Zn2+, Pb2+ alginates. The band splitting in the IR spectra of alginates
Table 4. Characteristic absorption bands (cm−1) in IR spectra of alginates.
Table 5. The shift of absorption bands and the change of energy of hydrogen ties of alginates of polyvalent metals.
Note: * in comparison with sodium alginate.
of polyvalent metals is observed in a less degree than for pectinates. With the introduction of cations of polyvalent metals into the alginate molecule in IR spectra the band shift of valent vibrations of hydroxyl groups into the long-wave area is observed, thus testifying the rupture or weakening of hydrogen ties by the ions of polyvalent metals [11] [12] . The specified spectral changes were observed at all polyvalent metals under study.
While determining the correlation between the absorption bands of hydroxyl groups associated by hydrogen tie and the energy values of hydrogen ties in metal alginates in “νi = f(−UВС)” graphic system y = a + bx linear relationship was obtained in which а = 111.18, b = −2.86 × 10−2. The interaction of vibrations of oscillators of alginate ions with the ions of polyvalent metals is the cause of high-frequency shifts of bands and the energy decrease. In case of comparison with the alginic acid the differences given in Table 5 would have been more sufficient. The row of the metal ions in the order of their influence on the decrease of energy of hydrogen ties is as follows: Zn2+ > Cu2+ = Mn2+ > Ni2+ = Fe2+ > Co2+ > Cr3+ > Pb2+. The received data make it possible to assume that the ions of the above-mentioned metals, except for the ions of Pb2+, form coordination ties with the oxygen atoms of hydroxyl groups of alginates.
The value of Δν(as-s COO−) quantity is more than 200 cm−1 (219 cm−1) in 1660 - 1600 cm−1 and 1470 - 1440 cm−1 areas, characteristic for asymmetrical and symmetrical vibrations of ionized carboxyls in the molecule of Cu2+ alginate, testifies the formation of asymmetrical structures of Cu2+ ions with carboxyl groups of alginate acting as monodentate ligands. For the products of interaction of alginate ions with the ions of other polyvalent metals Δν value is less than 200 см−1, which proves the formation of symmetrical structures in which carboxyl groups of alginate behave as bidentate ligands. In comparison with the pectinates Ni2+, Co2+, Mn2+, Fe2+ alginates form more symmetrical structures.
In 1320 см−1 area characteristic for the absorption of carboxyl groups of mannuronic blocks of alginic acid, for alginates of all metals the shift of the band into the long-wave part of the spectrum was observed: for 40 cm−1 for Na+ ions and for 58 - 62 см−1 for the ions of other metals; in comparison with Na+ alginate this shift is 12 - 22 cm−1. Most intensively the mentioned band appeared with Cu2+ и Fe2+ alginates. In 1290 см−1 spectral area, which is characteristic for the absorption of carboxyl groups of guluronic blocks of alginic acid, the shift of the band into the long-wave part of the spectrum for 16 - 17 cm−1 for the alginates of all metals was observed; along with it the intensity of this band is mostly expressed for Fe2+ and Ni2+ alginates. The obtained data make it possible to draw the conclusion about the coordination of the metal cations of carboxyl groups of both blocks.
The shift of the band in 949 см−1 area, characteristic for CO vibrations of pyranose cycle, into the long-wave part of the spectrum is mostly expressed (18 - 28 см−1) in the presence of Fe2+, Pb2+, Zn2+, Ni2+ cations. Apparently, the ions of these metals are additionally coordinated by the oxygen atoms of pyranose cycle.
For Cu2+ и Fe2+ alginates a band in 1600 см−1 area was revealed, which proves that they contain crystallization water. The insolubility of alginates as well as that of pectinates, polyvalent metals in water testifies the formation of three- dimensional net structures.
Thus, just as in the case with pectinates, in the molecules of the alginates of polyvalent metals the metal ions are coordinated to the oxygen atoms of hydroxyl groups, carboxyl groups, pyranose cycle and water.
4. Summary
1) Comparison of IR absorption spectra of the 10% suspensions polyuronides out in the presence of cations of polyvalent metals (Pb2+, Cu2+, Zn2+, Cr3+, Mn2+, Fe2+, Co2+, Ni2+) has allowed to identify the influence of metals the displacement of the spectral bands characteristic of specific functional groups and structural fragments polyuronides. Such groups and sections are (listed in descending order of size of shift):
・ hydroxyl groups, including hydrogen bonds associated with free or substituted hydroxyl groups (shift of bands in the longwave region); between the absorption bands of associates and the decrease of energy of hydrogen bonds revealed significant correlation;
・ ionized carboxyl group (shear bands, asymmetric characteristic fluctuation in the short wavelength region, and bands characteristic of the symmetrical variation in the long-wave region); polyuronates Pb2+, Zn2+, Cr3+, Mn2+, Fe2+, Co2+, Ni2+ formed a symmetrical structure of metal ions with carboxyl groups (bidentate ligands), polyuronates Cu2+―asymmetric structures with carboxyl groups (monodentate ligands);
・ water molecules;
・ the oxygen atoms of the pyranose cycle;
・ the oxygen atoms of carbonyl groups of ester groups;
・ α-glycosidic bond (affect only Cr3+ and Co2+).
2) A significant shift of the absorption bands in the IR spectra polyuronates metals compared to polyuronide, the decrease of energy of hydrogen bonds associated hydroxyl groups, reducing the intensity of the absorption bands characteristic of water, is evidence of the formation of polyuronide coordination bonds with metal ions. It is established that metal ions in polyuronates contact with oxygen atoms mainly hydroxyl and carboxyl groups and oxygen atoms of water molecules, pyranose cycle of the carbonyls of the ester groups of the α- glycoside bond center. In coordination of metal cations by alginates are involved both units of the polymer: polyguluronate and polymannuronate.
3) Characteristic changes in the IR spectra of pectinates are more pronounced than in the IR spectra of alginates.
4) Used methodological approach to the study of the structure polyuronates metals can be extended to the metallic derivatives of the various other classes of organic substances.
Cite this paper
Kajsheva, N.S., Kajshev, A.S., Samoryadova, A.B., Volokitin, S.V., Gulbjakova, C.N. and Maslovskaya, E.A. (2017) The Structure of Insoluble Pectinates and Alginates of Polyvalent Metals Based on IR Spectra Data. American Journal of Analytical Chemistry, 8, 334- 344. https://doi.org/10.4236/ajac.2017.85025
References
- 1. Alekseev, Y.Y., Garnovsky, А.D. and Zhdanov, Y.А. (1998) Complexes of Natural Carbohydrates with Metal Cations. Achievements of Chemistry, 67, 723-744.
- 2. Kajsheva, N.S. (2012) Study the Configuration of Metal Centers in Polyuronatic Method of EPR Spectroscopy. Chemical-Pharmaceutical Journal, 46, 51-53.
- 3. Kajsheva, N.S. (2004) The Study of Natural Polyuronides and the Production of Medicinal Agents on Their Basis. Doctor’s Thesis, Pyatigorsk State Pharmaceutical Academy, Pyatigorsk, 47 p.
- 4. Kompantsev, V.А., Kajsheva, N.S., Samokish, I.I., et al. (2002) The Method of Extracting Biologically Active Substances from Laminaria for Medical Use. USSR Patent No. 2194525.
- 5. Knutson, C.A. and Jeans, A.A. (1968) New Modification of the Carbazole Analysis: Application to Heteropolysaccharides. Analytical Biochemistry, 24, 470-481.
- 6. Lakatosh, B., Mayzel, Y. and Varyu, М. (1981) The Method of Producing the Complex of Metal Ion with Oligo- or Polygalacturonic Acids. USSR Patent No. 886750.
- 7. Panov, V.P. and Zhbankov, R.G. (1988) Intra- and Intermolecular Interactions in Carbohydrates (Nonvalent Interactions and Conformations). Science and Technics, Minsk, 359 p.
- 8. Usov, А.I. (1999) Alginic Acids and Alginates: Analytical Methods Used for Their Estimation and Characterisation of Composition and Primary Structure. Achievements of Chemistry, 68, 1051-1061.
- 9. Deiana, S., Erre, I. and Micera, G., et al. (1980) Download Iron(III) Reduction by D-Galacturonic Acid. Part II. Influence of Uranyl(VI), Lead(II), Nickel(II), and Cadmium(II) Complexes Formation. Analytica Chimica Acta, 46, 249-258.
- 10. Debongnir, P. (1987) An E. P. R. and Potentiometric Study of the Complexes of Copper Ions by Galacturonic Acid and Galacturonates. Carbohydrate Research, 170, 137-148.
- 11. Tajmir-Riahi, H.A. (1986) Triadimefon Protects Bean Plants from Water Stress through Its Effects on Abscisic Acid. Journal of Inorganic Biochemistry, 26, 23-33.
- 12. Tajmir-Riahi, H.A. (1985) Complexes of Dendrimers with Bovine Serum Albumin. Journal of Inorganic Biochemistry, 24, 127-136.
- 13. Filippov, M.P. (1990) The Nature of Ties in the Plant Tissue, the Structure and Infrared Spectra as the Basis of Classification of Pectic Substances. Doctor’s Thesis, Kishinev State University, Odessa, 38 p.
- 14. Lurye, Y.Y. (1989) Manual of Analytical Chemistry. Khimiya, Moscow, 448 p.
- 15. Batsanov, S.S. (1971) Electronegativity and Effective Atomic Charges. Znaniye, Moscow, 83 p.
- 16. Zhdanov, Y.А. and Alekseev, Y.Y. (2002) The Main Achievements of the Coordination Chemistry of Modified Polysaccharides. Achievements of Chemistry, 71, 1090-1102.
- 17. Garnovsky, А.D., Vasilchenko, I.S. and Garnovsky, D.А. (2000) Modern Aspects of Metal Complex Synthesis. Main Ligands and Methods. Rostov-on-Don, 355 p.


