Journal of Biosciences and Medicines
Vol.05 No.02(2017), Article ID:74012,17 pages
10.4236/jbm.2017.52003
Role of Signal-Regulated Changes in Microtubule Stability Dynamics through α-Tubulin Phosphorylation on Ser/Tyr in Modulation of Salivary Gland Matrix Metalloproteinase-9 (MMP-9) Secretion in Response to Porphyromonas gingivalis and Ghrelin
Bronislaw L. Slomiany, Amalia Slomiany
Research Center, Rutgers School of Dental Medicine, Rutgers, The State University of New Jersey, Newark, NJ, USA

Copyright © 2017 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY 4.0).
http://creativecommons.org/licenses/by/4.0/



Received: January 12, 2017; Accepted: February 6, 2017; Published: February 9, 2017
ABSTRACT
Up-regulation in salivary gland acinar cell MMP-9 secretion in response to proinflammatory challenge by periodontopathic bacterium, P. gingivalis relays heavily on the factors that influence the protein processing at the level of endoplasmic reticulum-to-Golgi trafficking, and occurs in concert with the changes in the stability dynamics of the major cytoskeleton polymeric structures, microtubules (MTs). In this study, we report that P. gingivalis LPS- elicited induction in the acinar cell MMP-9 secretion is accompanied by the enhancement in MT stabilization, while the modulatory influence of peptide hormone, ghrelin, is associated with MT destabilization. Further, we reveal that the changes in MT dynamics induced by the LPS and ghrelin occur through signal-regulated α-tubulin phosphorylation on Ser/Tyr. The LPS effect is reflected in a marked increase in PKCδ-mediated α-tubulin phosphorylation on Ser, whereas the modulatory influence of ghrelin on MT dynamics is manifested in by SFK-dependent phosphorylation of α-tubulin on Tyr. More- over, we show that that the changes in MT dynamics, conferred by the LPS and ghrelin, affect the Golgi localization of GTP-Arf1 and the recruitment and activation of PKD2. The findings underscore the role of signal-regulated changes in MT stability dynamics through PKCδ/SFK-mediated α-tubulin phosphorylation on Ser/Tyr in controlling the salivary gland acinar cell MMP-9 secretion in response to P. gingivalis LPS as well as its modulation by ghrelin.
Keywords:
P. gingivalis, Oral Mucosa, Ghrelin, MMP-9, α-Tubulin Ser/Tyr Phosphorylation

1. Introduction
Porphyromonas gingivalis is a potent periodontopathic pathogen implicated in the etiology of periodontitis, a chronic inflammatory disease that affects about 15% of population and leads to progressive destruction of teeth-supporting tissue, and is a major cause of adult tooth loss [1] [2] [3] . The oral mucosal responses to P. gingivalis and its key endotoxin, cell-wall lipopolysaccharide (LPS), are characterized by the disturbances in nitric oxide synthase and cyclooxygenase systems, up-regulation in EGFR and MAPK activation, and induction in the secretion of highly glycosylated endopeptidase, metalloproteinase-9 (MMP-9) [4] [5] [6] [7] [8] . Similarly, to other regulated secretory proteins, the processing of MMP-9 along the endoplasmic reticulum (ER), Golgi, and trans-Golgi network (TGN) remains under a strict control of factors that affect the membrane recruitment and activation of various coat and cargo proteins, including ADP- ribosylation factors (Arfs) and protein kinase D (PKD), [9] [10] [11] [12] .
Arfs are small 20 kDa GDP/GTP-binding proteins belonging to the Ras superfamily of GTPases that control trafficking and sorting of secretory cargo through Golgi-TNG [9] . The Arfs are active when bound to GTP and inactive when bound to GDP, and the activation status is controlled by the guanine nucleotide exchange factors (GEFs) [9] [13] . While GDP-bound inactive Arfs are cytosolic, the stimulus activated GTP-bound class I Arfs (Arf1, Arf2, and Arf3) rapidly translocate to the Golgi membrane compartments and assume the principal role in the recruitment of various cytosolic coat and cargo adaptor proteins, exchange factors, and lipid modifying enzymes that are essential for regulation of ER-to-Golgi traffic [11] [12] . Of particular significance to the MMP-9- containing secretory cargo processing is the role of Arf1 in the recruitment from the cytoplasm to the TGN of PKD2 [14] [15] . The PKD family of serine/threo- nine kinases (PKD1, PKD2, and PKD3), plays a crucial role in the regulation of Golgi structure and function by phosphorylation of the TGN-localized substrates required for subsequent shedding of cargo-containing vesicles [10] .
Moreover, the literature data indicate that the activation of various coat and cargo proteins involved in MMP-9 processing occurs in concert with the changes in dynamics of the major cytoskeleton polymeric structures, microtubules (MTs) [16] [17] [18] . The MTs polymers, assembled from heterodimers of α- and β-tubulin proteins in a head-to-tail manner, form a dense network of filamentous tubes that undergo frequent alternating periods of growth (polymerization) and shortening (depolymerization) termed “dynamic instability” [18] [19] . The diverse aspects of MT dynamics arise from accumulation of a variety of posttranslational modifications on the tubulin subunits, including acetylation, detyrosination, polyglutamylation, polyglycylation and phosphorylation, and are further tuned and refined through binding of MT-associated proteins [19] [20] [21] [22] . Indeed, changes in MT stability dynamics and the increase in α-tubulin acetylation have been directly linked to the increase in MMP-9 secretion elicited by MT stabilizing agents [17] , and we have demonstrated previously that MT destabilizing agents that inhibit microtubule polymerization exert the inhibitory effect on the increase in salivary gland acinar cell MMP-9 secretion elicited by P. gingivalis LPS [23] . Other findings point to the role of tubulin phosphorylation in MT stabilization, and suggest the involvement of protein kinase C (PKC) and Src family tyrosine kinase (SFK) in this process [24] [25] [26] [27] .
Therefore, considering that PKC and SFKs play a major role in propagation of oral mucosal inflammatory response to P. gingivalis associated with the enhancement in MMP-9 production, as well as are the targets of modulatory influence of a peptide hormone, ghrelin, in this study we investigated the influence of P. gingivalis LPS and ghrelin on tubulin phosphorylation in salivary gland acinar cells and the MMP-9 secretory processes affected by the changes in MT dynamics. Since the elevated levels of MMP-9 are routinely detected in gingival tissue of patients with periodontitis, a chronic inflammatory disease that leads to progressive destruction of teeth-supporting tissue [1] [3] [6] , the results of our findings point to factors affecting MT dynamics as a tempting target for therapeutic intervention in the secretory processes of MMP-9.
2. Materials and Methods
2.1. Salivary Gland Cell Incubation
The acinar cells of rat sublingual salivary gland were suspended in five volumes of ice-cold Dulbecco’s modified (Gibco) Eagle’s minimal essential medium (DMEM), supplemented with fungizone (50 µg/ml), penicillin (50 U/ml), streptomycin (50 µg/ml), and 10% fetal calf serum, and gently dispersed by trituration with a syringe and settled by centrifugation [28] . The cells were then resuspended in the medium to a concentration of 2 × 107 cell/ml, and transferred in 1 ml aliquots to DMEM in culture dishes and incubated under 95% O2 and 5% CO2 at 37˚C for up to 16 h in the presence of 0 - 100 ng/ml P. gingivalis LPS [23] . P. gingivalis used for LPS preparation was cultured from clinical isolates obtained from ATCC No. 33277 [4] . In the experiments evaluating the effect of ghrelin (rat) and wide spectrum PKC inhibitor, GF109203X (Sigma), Src family protein tyrosine kinase (SFK-PTK) selective inhibitor, PP2, ER-to-Golgi transport inhibitor, Brefeldin A (BFA), microtubule stabilizing agent, tubacin (Tb) and microtubule destabilization agent, nocodazole (Noc), (Calbiochem), the cells were first preincubated for 30 min with the indicated dose of the agent or vehicle before the addition of the LPS.
2.2. MMP-9 Western Blot Analysis
The measurement of P. gingivalis LPS effect on the acinar cell secretion of MMP-9, the cell incubates were centrifuged at 1200 × g for 5 min and the resulting supernatant was collected. The samples were then boiled in SDS sample buffer for 5 min, separated on 8% SDS-PAGE and transferred to nitrocellulose membranes. Blots were blocked for 1 h in 5% skim milk in Tris-buffered Tween [17] , and the membranes were incubated overnight at 4˚C with the specific anti- MMP-9 antibody (Calbiochem).
2.3. Arf1-GEF Activity Assay
The measurement of Arf1 activation (GTP-Arf1) was carried out with Arf1 Pull-Down and Detection Kit (EMD Millipore). The acinar cells from the control and experimental treatments were lysed in the lysis/binding/wash buffer (25 mM Tris-HCl, pH 7.2, 0.15 M NaCl, 5 mM MgCl2, 1% Nonidet P-40, and 5% glycerol), containing protease inhibitor cocktail (10 µg/ml leupeptin, 10 µg/ml aprotinin, 1 mM sodium orthovanadate, 1 mM PAF, and 1 mM NaF), at 4˚C for 30 min and centrifuged at 12,000 × g for 10 min. The supernatants were precleared with GST beads and the beads were incubated with a 100 µg of GST- GGA3-PBD proteinsPAK1 PBD-agarose for 1 h at 4˚C. The beads were washed three times in the lysis buffer, resuspended in Laemmli reducing sample buffer, resolved on 12% SDS-PAGE, and immunoblotted for GTP-bound Arf1 using anti-Arf1 antibody [23] .
2.4. Golgi Membranes
An intact Golgi apparatus fraction of the salivary gland acinar cells was prepared essentially according to the previously described procedure [29] . The acinar cells were homogenized for 10 s at 600 rpm in 3 volumes of 50 mM Tris-HCl buffer, pH 7.4, containing 0.25 M sucrose, 5 mM EDTA, 25 mM KCl, 1 mM PMSF, 10 mM aprotinin, 10 mM leupeptin, and 10 mM chymostatin. The homogenate was filtered through a layer of gauze and centrifuged at 10,000 rpm for 20 min. The supernatant was collected, layered onto 1.25 M sucrose, buffered with 10 mM Tris-HCl, pH 7.4, and centrifuged at 25,000 rpm for 90 min in SW28 rotor. The crude membranes sedimented above the interface with the sucrose layer were collected by aspiration, adjusted to 1.2 M sucrose, and overlaid with 1.1, 1.0, and 0.5 M buffered (10 mM Tris-HCl, pH 7.4) sucrose. Following centrifugation for 2.5 h at 25,000 rpm in the SW28 rotor, the Golgi-enriched membranes were collected from the 0.5/1.0 sucrose interface.
2.5. Immunoprecipitation and Immunoblotting
The acinar cells from various experimental treatments were collected by centrifugation and resuspended for 30 min in ice-cold lysis buffer (20 mM Tris-HCl, pH 7.4, 150 mM NaCl, 10% glycerol, 1% Triton X-100, 2 mM EDTA, 1 mM sodium orthovanadate, 4 mM sodium pyrophosphate, 1 mM PMSF, and 1 mM NaF), containing 1 µg/ml leupeptin and 1 µg/ml pepstatin [5] . Following brief sonication, the lysates were centrifuged at 10,000 g for 10 min, and the supernatants were subjected to protein determination using BCA protein assay kit (Pierce). The cell lysatesas well as those of Golgi membrane preparations were then used either for immunoblots analysis, or proteins of interest were subjected to immunoprecipitation by incubation with the respective primary antibodies for 2 h at 4˚C, followed by overnight incubation with protein G-Sepharose beads. The immune complexes were precipitated by centrifugation, washed with lysis buffer, boiled in SDS sample buffer for 5 min, and subjected to SDS-PAGE using 40 µg protein/lane. The separated proteins were transferred onto nitrocellulose membranes, blocked for 1 h with 5% skim milk in Tris-buffered Tween (20 mM Tris-HCl, pH 7.4, 150 mM NaCl, 0.1% Tween-20), and probed with specific antibodies directed against PKD2, phospho-PKD2 (Ser876), Arf1, PKCδ, and phosphotyrosine (4G10) (EMD Millipore), phosphoserine PKC substrate (Cell Signaling), and cSrc, and α-tubulin (Sigma).
2.6. Data Analysis
All experiments were carried out using duplicate sampling, and the results are expressed as means ± SD. Analysis of variance (ANOVA) and nonparametric Kruskal-Wallis tests were used to determine significance. Any difference detected was evaluated by means of post hoc Bonferroni test, and the significance level was set at p < 0.05.
3. Results
MTs, the large filamentous cytoskeletal protein polymers, implicated in organelle positioning and vesicle transport, are increasingly gaining attention for their role in modulation of inflammatory signals in response to bacterial endotoxins [17] [23] . In this study, we investigated the nature of factors affecting MT stability dynamics and their influence on regulatory proteins implicated in the control of salivary gland acinar cell MMP-9 secretion in response to P. gingivalis stimulation. By following Arf1 activation and the levels of MMP-9 released into the medium by the acinar cells exposed to incubation with LPS of periodontopathic bacterium, P. gingivalis, in the presence of peptide hormone, ghrelin, in combination with pharmacological agents affecting MT stability, we found that the effect of the LPS, manifested in a substantial up-regulation in Arf1-GTP formation was associated with a marked increase in MMP-9 secretion (Figure 1). Moreover, we revealed that preincubation with ghrelin, recognized for its role in modulation of inflammatory responses, led to a significant reduction of the LPS effect. Further, we found that pretreatment with MT destabilizing agent, nocodazole, resulted in further amplification of the modulatory effect of ghrelin on the acinar cell MMP-9 secretion and Arf1 activation elicited by the LPS, while MT stabilizing agent, tubacin, exerted countering influence on the modulatory effect of ghrelin. Therefore, we concluded that the enhancement in MT stabilization by the LPS is intimately linked to the acinar cell Arf1 activation and up-regulation in MMP-9 secretion, and that the modulatory influence of ghrelin is associated with changes in MT stabilization dynamics.
Considering that Arf activation plays a critical role in the secretory pathway trafficking through Golgi by affecting the membrane recruitment and activation
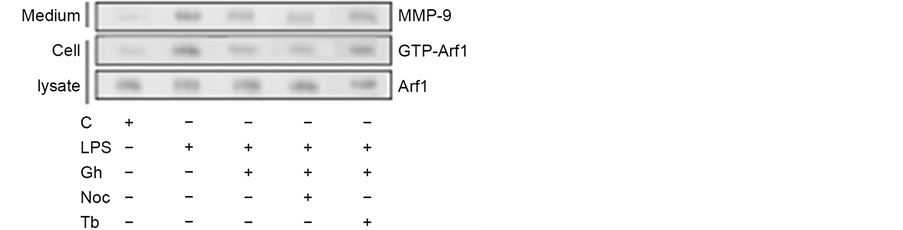
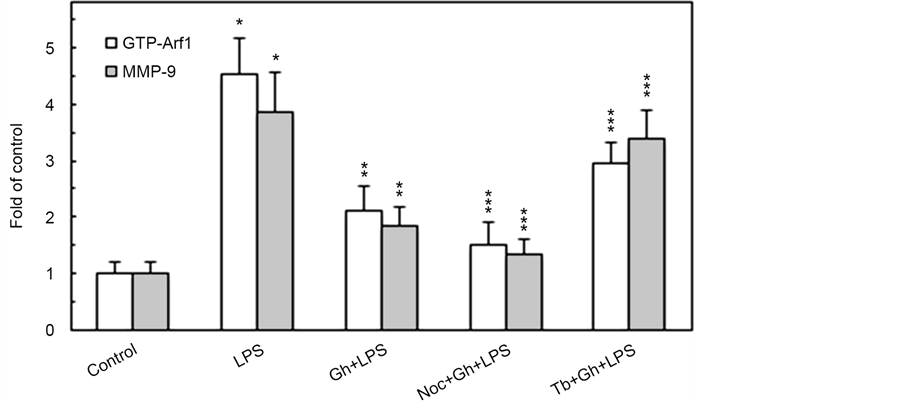
Figure 1. Impact of microtubule stabilizing agent, tubacin (Tb) and microtubule destabilizing agent, nocodazole (Noc) on the changes induced by P. gingivalis LPS and ghrelin (Gh) in Arf1 activation and MMP-9 secretion by sublingual salivary gland acinar cells. The cells, preincubated with 5 µM Tb or 10 µM Noc, were treated with 0.5 µg/ml Gh, and incubated with the LPS at 100 ng/ml. After 2 h of incubation the cell lysates were analyzed for Arf1 and Arf1-GTP, whereas the conditioned media following 16 h of incubation were subjected to immunoblotting for MMP-9 (a). The relative levels of protein are expressed as fold of control (b) and Arf1 was used as a lane loading check. The data represent the mean ± SD of four experiments. *P < 0.05 compared with that of control. **P < 0.05 compared with that of LPS. ***P < 0.05 compared with that of Gh + LPS.
of various coat and cargo proteins, including PKD2 required for phosphorylation of Golgi-localized substrates [15] , we next assessed the impact of MT stabilizing and destabilizing agents on the Golgi recruitment of Arf1 and PKD2. The analyses revealed that while the LPS-induced elevation in the Golgi membrane content of Ar1 and PKD2 was not affected by ghrelin and MT stabilizing agent, tubacin, the MT destabilizing agent, nocodazole, produced a marked inhibitory effect (Figure 2). Moreover, the LPS-induced Golgi membrane localization of Arf1 and PKD2 was inhibited by a well-known secretory cargo processing inhibitor, Brefeldin A. Further, we observed that preincubation of the acinar cells with Brefeldin A not only caused the inhibition in Arf1 activation, but also led also to a significant suppression in the LPS-induced MMP-9 secretion (Figure 3), thus suggesting that P. gingivalis exerts its effect on MMP-9 secretion at the level of the ER-to-Golgi trafficking that requires Arf1/PKD2 participation.
Consequently, as Arf1 activation occurs through the exchange of GDP for GTP that is catalyzed by guanine nucleotide exchange factors (GEFs), we have followed the leads as to role of SFK/PKC in Arf-GEF activation and MMP-9 secretion. For this, the acinar cells prior to incubation with P. gingivalis LPS were pretreated with a wide spectrum PKC inhibitor, GF109203X, or SFK-PTKs inhibitor, PP2, and subjected to Western blot analysis to assess the level of MMP-9 secretion and GTP-Arf1 formation. As shown in Figure 3, the LPS-induced up-regulation in MMP-9 secretion and GTP-Arf1 formation were susceptible to inhibition by ghrelin. Further, we found that while preincubation of the acinar
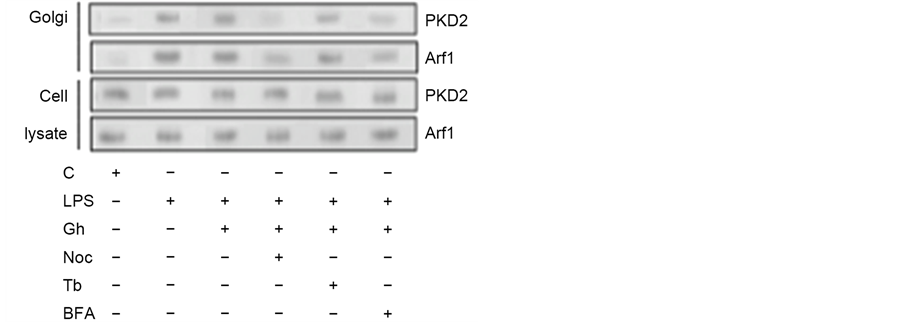
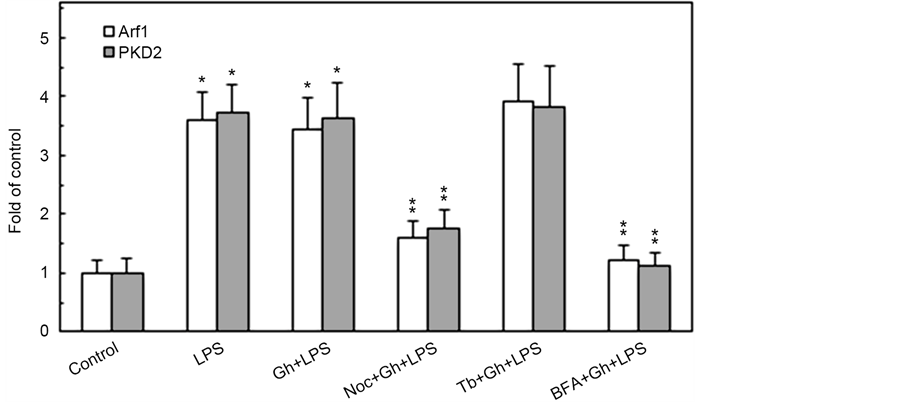
Figure 2. Influence Brefeldin A (BFA), tubacin (Tb) and nocodazole (Noc) on the changes induced in the acinar cells by P. gingivalis LPS and ghrelin (Gh) in Golgi membrane localization of Arf1 and PKD2. The cells, preincubated with 5 µg/ml BFA, 5 µM Tb, 0r 10 µM Noc, were treated with 0.5 µg/ml Gh, and incubated for 2 h in the presence of 100 ng/ml LPS. The lysates of whole cells and the corresponding Golgi membrane fractions were analyzed for Arf1 and PKD2 (a). The relative levels of protein are expressed as fold of control (b), and the total cell lysate of Arf1 and PKD2 were used as lane loading check. The data represent the mean ± SD of four experiments. *P < 0.05 compared with that of control. **P < 0.05 compared with that of Gh + LPS.
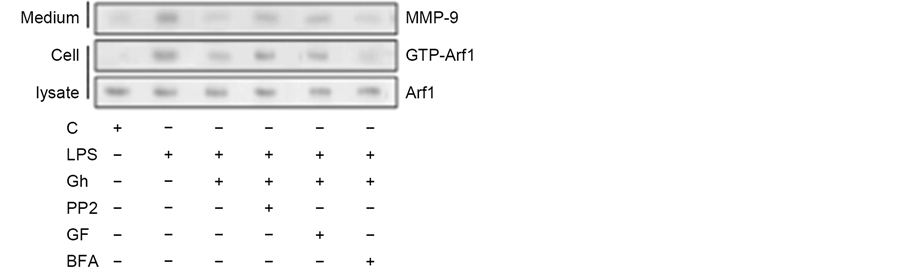
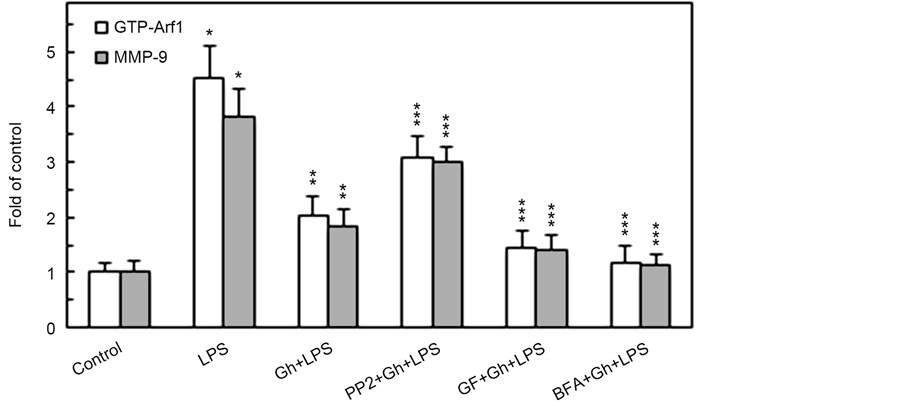
Figure 3. Impact of SFK-PTKs, PKC, and BFA inhibitors on the changes induced by P. gingivalis LPS and ghrelin (Gh) in Arf1 activation and MMP-9 secretion by the salivary gland acinar cells. The cells, preincubated with 30 µM of SFK-PTKs inhibitor, PP2, or 5 µM of wide spectrum PKC inhibitor, GF109203X (GF), or 5 µg/ml of ER-to-Golgi transport inhibitor, Brefeldin A (BFA), were treated with 0.5 µg/ml Gh, and incubated with the LPS at 100 ng/ml. After 2 h of incubation the cell lysates were analyzed for Arf1 and Arf1-GTP, whereas the conditioned media following 16 of incubation were subjected to Immunoblotting for MMP-9) (a). The relative levels of protein are expressed as fold of control (b) and Arf1 was used as a lane loading check. The data represent the mean ± SD of four experiments. *P < 0.05 compared with that of control. **P < 0.05 compared with that of LPS. ***P < 0.05 compared with that of Gh + LPS.
cells with GF109203X led to an enhancement in the inhibitory potential of ghrelin, the SFK-PTKs inhibitor, PP2, caused the suppression in ghrelin effect on the LPS-induced changes in MMP-9 secretion and GTP-Arf1 formation. These findings, thus support the involvement of SFK-PTKs and PKC, identified earlier as PKCδ [23] , in the modulation of salivary gland acinar cell MMP-9 secretion and Arf1 activation in response to P. gingivalis LPS.
As PKD along with PKC are the points of convergence and integration of stimuli triggered by Toll-like receptor 4 (TLR4) activation as well as G protein- coupled receptor (GPCR) stimulation, we next examined the impact of P. gingivalis LPS and ghrelin on PKD2 phosphorylation in the presence of PKC and SFK-PTKs inhibition. The results of analyses revealed that the effect of the LPS was manifested mainly by an elevation in PKD2 phosphorylation on Ser, while the impact of ghrelin was associated with a significant increase in PKD2 phosphorylation on Tyr and a decrease in the phosphorylation on Ser (Figure 4). Further, we observed that the LPS-induced phosphorylation of PKD2 on Ser was susceptible to inhibition by a wide spectrum PKC inhibitor, GF109203X, whereas the ghrelin-induced phosphorylation of PKD2 on Tyr was subject to suppression by SFK-PTKs inhibitor, PP2. These data, together with the results presented in Figure 2, suggest that while factors influencing MT dynamics affect the Golgi localization of PKD2, the PKCδ-mediated phosphorylation of the Golgi-localized PKD2 on Ser plays a major role in the LPS-induced up-regulation in
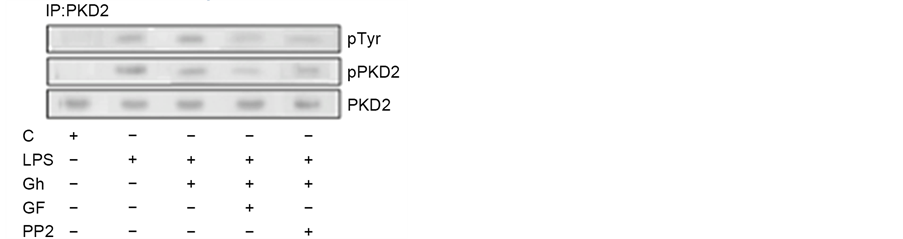
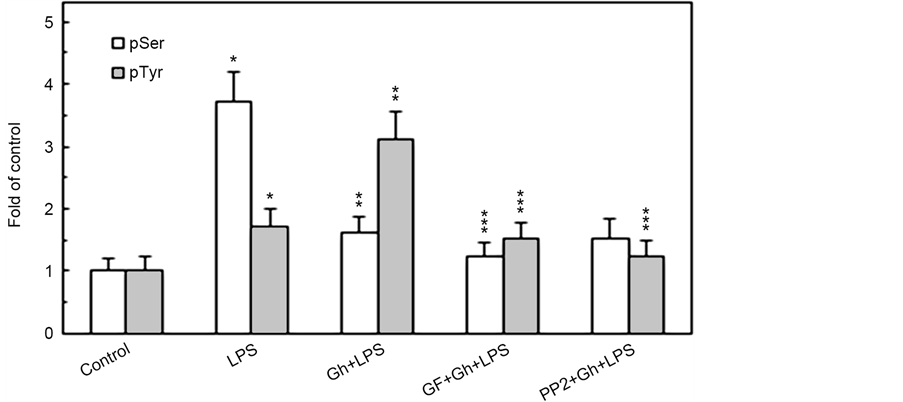
Figure 4. Impact of PKC and SFK-PTKs inhibition on the changes induced by P. gingivalis LPS and ghrelin (Gh) in the acinar cell PKD2 phosphorylation. The cells, preincubated with 5 µM of wide spectrum PKC inhibitor, GF109203X (GF) or 30 µM of SFK-PTKs inhibitor, PP2, were treated with 0.5 µg/ml Gh, and incubated for 2 h in the presence of 100 ng/ml LPS. Cell lysates were immunoprecipitated (IP) with anti-PKD2 antibody, immunoblotted (WB) with anti-PKD2, and reblotted with anti-pPKD2 (Ser) and anti-pTyr antibody (a). The relative densities of proteins are expressed as fold of control (b), and the total PKD2 was used as a lane loading check. The data represent the mean ± SD of four experiments. *P < 0.05 compared with that of control. *P < 0.05 compared with that of control. **P < 0.05 compared with that of LPS. ***P < 0.05 compared with that of Gh + LPS.
the acinar cell MMP-9 secretion, whereas the modulatory effect of ghrelin is reflected in SFK-PTKs-mediated phosphorylation of PKD2 on Tyr.
In further appraisal of the role of PKC and SFK-PTKs in P. gingivalis-induced up-regulation in MMP-9 secretion and the changes in the acinar cell MT stabilization dynamics, we have assessed the effect of the LPS and ghrelin on α-tubulin phosphorylation. The results revealed that the effect of the LPS was manifested in the elevation of α-tubulin phosphorylation on Ser, while the modulatory influence of ghrelin was reflected in a significant decrease in the phosphorylation on Ser and the increase in α-tubulin phosphorylation on Tyr (Figure 5). Moreover, the phosphorylation of α-tubulin on Ser by the LPS was susceptible to suppression by a wide spectrum PKC inhibitor, GF109203X, while the modulatory

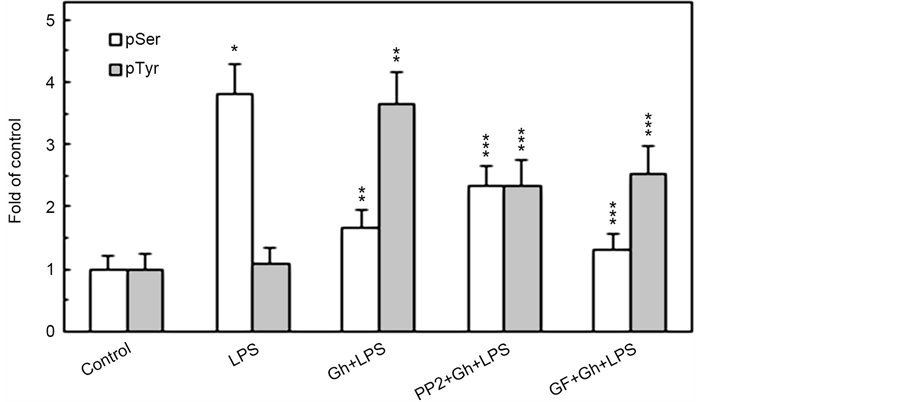
Figure 5. Impact of PKC and SFK-PTKs inhibition on the changes induced by P. gingivalis LPS and ghrelin (Gh) in the acinar cell α-tubulin phosphorylation. The cells, preincubated with 5 µM of wide spectrum PKC inhibitor, GF109203X (GF) or 30 µM of SFK- PTKs inhibitor, PP2, were treated with 0.5 µg/ml Gh, and incubated for 4 h in the presence of 100 ng/ml LPS. Cell lysates were immunoprecipitated (IP) with anti-α-tubulin antibody, immunoblotted (WB) with anti-α-tubulin, and reblotted with anti-pSer-PKC substrate and anti-pTyr (4G10) antibody (a). The relative densities of proteins are expressed as fold of control (b), and the total α-tubulin was used as a lane loading check. The data represent the mean ± SD of four experiments. *P < 0.05 compared with that of control. *P < 0.05 compared with that of control. **P < 0.05 compared with that of LPS. ***P < 0.05 compared with that of Gh + LPS.
potential of ghrelin was affected by both PKC inhibitor, GF109203X, and the inhibitor of SFK-PTKs, PP2. Thus, the findings affirm the involvement of SFK- PTKs and PKC, identified earlier as PKCδ [23] , in the regulation of MT stabilization through α-tubulin phosphorylation on Ser/Tyr.
Indeed, examination of the interaction between α-tubulin and PKCδ by co-immunoprecipitation indicated the existence of PKCδ in association with α-tubulin following the acinar cell stimulation with P. gingivalis LPS, but not in the presence of preincubation with ghrelin or PKC inhibitor, GF109203X (Fig- ure 6). Further, α-tubulin displayed little if any association with Src in the presence of the LPS, whereas the two proteins were found in association following preincubation with ghrelin. However, this association between the α-tubulin and Src was subject to interference by SFK-PTKs inhibitor, PP2. These results, thus,

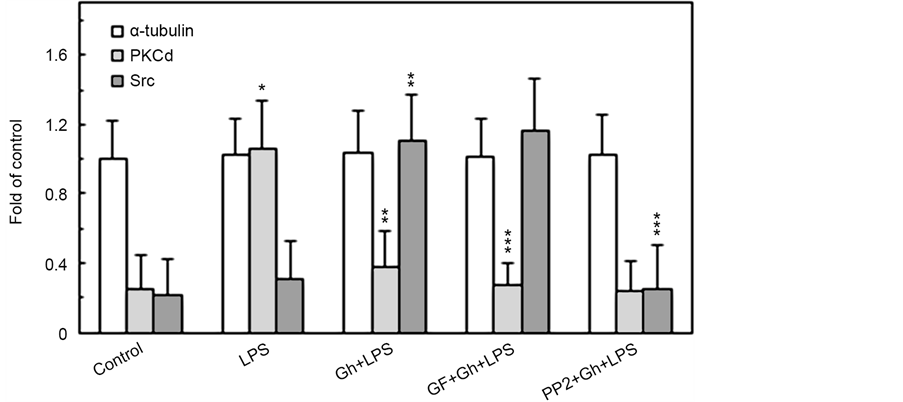
Figure 6. Impact of PKC and SFK-PTKs inhibition on the changes induced in the acinar cells by P. gingivalis LPS and ghrelin (Gh) in the association of α-tubulin with PKCδ and Src. The cells, preincubated with 5 µM GF109203X or 30 µM PP2, were treated with 0.5 µg/ml Gh, and incubated for 4 h in the presence of 100 ng/ml LPS. Cell lysates were immunoprecipitated (IP) with anti-α-tubulin antibody, immunoblotted (WB) with anti-α- tubulin, and reblotted with anti-PKCδ and anti-Src antibody (a). The relative densities of proteins are expressed as fold of α-tubulin control (b). The data represent the mean ± SD of four experiments. *P < 0.05 compared with that of control. **P < 0.05 compared with that of LPS. ***P < 0.05 compared with that of Gh + LPS.
underscore the role of α-tubulin phosphorylation on Ser/Tyr in the modulation of salivary gland acinar cell MT stabilization in response to P. gingivalis LPS as well as hormone, ghrelin.
4. Discussion
Rapid elevation in salivary gland acinar cell MMP-9 secretion in response to P. gingivalis proinflammatory challenge relays heavily on the factors that affect the protein processing along the ER, Golgi and TNG pathway, and occurs in concert with the changes in the stability dynamics of the major cytoskeleton polymeric structures, MTs [7] [9] [10] [11] [12] [23] . Moreover, the literature data indicate that the changes in MT stabilization play important role in the positioning of organelles and vesicles, and affect the membrane recruitment and activation of various coat and cargo proteins implicated in the secretory cargo processing, including Arfs and PKD [9] - [15] . Indeed, changes in MT stability dynamics elicited by LPS and associated with the increase in α-tubulin acetylation have been directly linked to the increase in MMP-9 secretion, and we reported recently on the role of α-tubulin phosphorylation in MT stabilization [17] [21] [23] [30] . Therefore, in the present study, we investigated the nature of factors affecting α-tubulin phosphorylation and MT stability dynamics in salivary gland acinar cells in response to stimulation by P. gingivalis LPS, and their influence on the recruitment and activation of the regulatory proteins involved in mediation of MMP-9 secretion, namely Arf1 and PKD2 [23] .
Relying on the earlier evidence as to the involvement of Arf1 and PKD2 in controlling the trafficking and sorting of secretory cargo through the Golgi-TGN [10] [11] [12] [13] [14] , we have demonstrated that the elevation in Arf1 activation (GTP-Arf1) upon P. gingivalis LPS stimulation paralleled that of the rise in MMP-9 secretion, while the impact of hormone, ghrelin, was associated with the reduction of the LPS effect. Furthermore, as the activation of Arf1 plays a critical role in the recruitment from the cytoplasm to the TGN of PKD2 that is required for phosphorylation of the Golgi-localized substrates [10] [14] [15] , we have examined the effect of P. gingivalis LPS and the pharmacological agents affecting the acinar cell MT stability on the Golgi localization of Arf1 and PKD2. Our findings revealed that while the LPS-induced elevation in the Golgi membrane content of Arf1 and PKD2 was not affected by ghrelin and MT stabilizing agent, tubacin, the MT destabilizing agent, nocodazole, produced an inhibitory effect. Moreover, the Golgi membrane localization of Arf1 and PKD2 was inhibited by Brefeldin A, a well-known ER-to-Golgi inhibitor of secretory cargo processing [31] , which also caused the inhibition in GTP-Arf1 formation and the suppression in the LPS-induced MMP-9 secretion. Considering the glycoprotein nature of MMP-9 and the fact that protein glycosylation occurs mainly in the Golgi- TGN [7] [31] , the above findings lend further support to the earlier data [23] , suggesting that P. gingivalis exerts its effect on the acinar cell MMP-9 secretion at the level of ER-to-Golgi trafficking that requires Arf1/PKD2 participation, and that the extent of MT stabilization plays a regulatory role in the process of Arf1 activation.
In further assessment of the role of changes in MT stabilization dynamics in the mediation of the acinar cell responses to P. gingivalis LPS, we have followed leads as to the role of SFK-PTK and PKC in mediation of GTP-Arf1 formation, PKD2 activation, and MMP-9 secretion [7] [9] [13] . Our findings revealed that the LPS-induced up-regulation in MMP-9 secretion as well as GTP-Arf1 formation were susceptible to inhibition by ghrelin, and that preincubation with PKC inhibitor, GF109203X, led to an enhancement in the inhibitory potential of ghrelin. The SFK-PTKs inhibitor, PP2, on the other hand, caused the suppression of the inhibitory effect of ghrelin on the LPS-induced GTP-Arf1 formation as well as MMP-9 secretion. Moreover, we demonstrated that the effect of the LPS associated with a marked elevation in the Golgi membrane content of PKD2 was manifested in the increase in phosphorylation on Ser, while preincubation with ghrelin led to a significant decrease in PKD2 phosphorylation on Ser and the increase in phosphorylation on Tyr. Further, we revealed that the LPS- induced phosphorylation of PKD2 on Ser was susceptible to inhibition by a wide spectrum PKC inhibitor, GF109203X, while the ghrelin-induced phosphorylation on Tyr was subject to suppression by SFK-PTKs inhibitor, PP2. Taking into account evidence as to the effect of pharmacological agents, tubacin and nocodazole, on the Golgi localization of GTP-Arf1 and PKD2 [15] [30] , the above data support the involvement of MT stabilization dynamics in modulation of Arf1 and PKD2 recruitment to the Golgi, and suggest that the PKCδ-mediated phosphorylation of the Golgi-localized PKD2 on Ser plays a major role in P. gingivalis LPS-induced upregulation in the acinar cell MMP-9 secretion, while the modulatory influence of ghrelin is reflected in SFK-PTKs-mediated phosphorylation of PKD2 on Tyr.
Further, taking into account recent reports indicating that PKC-mediated phosphorylation of α-tubulin on Ser prolongs the duration of MT growth (stabilization), while the phosphorylation o Tyr promotes the instability dynamic of MT [26] [27] , we have examined further, the impact of P. gingivalis LPS and ghrelin on the acinar cell tubulin phosphorylation. The results of immunoprecipitation analysis revealed that the effect of the LPS was manifested mainly with the elevation in α-tubulin phosphorylation on Ser, while the effect of ghrelin was reflected in a decrease in the phosphorylation on Ser and a marked increase in α-tubulin phosphorylation on Tyr. Moreover, we observed that the LPS-induced phosphorylation of α-tubulin on Ser was susceptible to suppression by a wide spectrum PKC inhibitor, GF109203X, while the modulatory potential of ghrelin was affected by both the PKC inhibitor, GF109203X, as well as the inhibitor of SFK-PTKs, PP2. Upon further analysis, we have established that the LPS- induced up-regulation in MMP-9 secretion is associated with PKCδ-mediated α-tubulin phosphorylation on Ser, while the modulatory influence of ghrelin is reflected in SFK-PTKs-dependent phosphorylation of α-tubulin on Tyr.
Thus, our findings underscore the role of signal-regulated changes in MT stability dynamic through α-tubulin phosphorylation in controlling the salivary gland acinar cell MMP-9 secretion in response to P. gingivalis LPS as well as the modulatory influence of ghrelin. The diagram depicting the influence of P. gingivalis LPS and ghrelin on MT dynamics and the acinar cell secretion of MMP-9 is depicted in Figure 7.
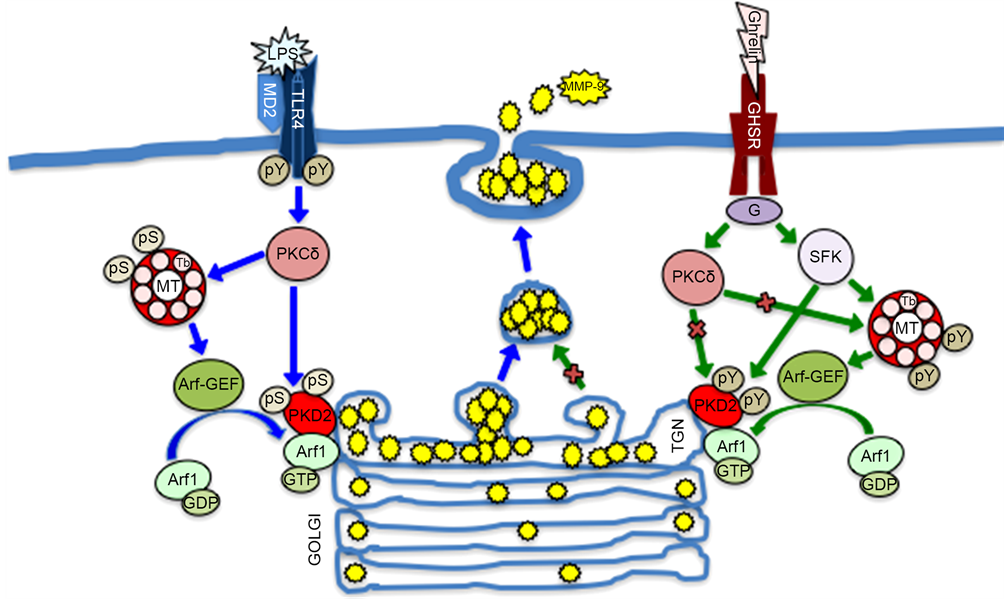
5. Conclusion
The data presented in this report show that the enhancement in salivary gland acinar cell MMP-9 secretion in response to P. gingivalis LPS challenge and the modulatory influence of peptide hormone, ghrelin, are the result of changes in MT dynamics occurring through signal-regulated α-tubulin phosphorylation on Ser/Tyr. The LPS-induced Toll-like receptor-4 (TLR4) activation and subsequent up-regulation in MMP-9 secretion is associated with PKCδ-mediated α-tubulin phosphorylation on Ser, while the modulatory influence of ghrelin, an endogenous ligand of the growth hormone secretagogue receptor type 1a (GHS-R1a), a G protein-coupled receptor (GPCR) is manifested in the SFK-PTKs-dependent phosphorylation of α-tubulin on Tyr. As the elevated levels of MMP-9 are found in gingival tissue of patients with periodontitis, our results suggest that factors
Figure 7. Schematic diagram depicting the role of α-tubulin phosphorylation and the changes in MT dynamics in modulation of salivary gland acinar cell MMP-9 secretion in response to P. gingivalis LPS and ghrelin. Engagement of TLR4 by the LPS triggers PKCδ-dependent α-tubulin phosphorylation on Ser and the enhancement in MT stabilization, that leads to the up-regulation in Arf-GEF-mediated translocation of Arf1-GTP to the Golgi, and promotes the recruitment and activation of PKD2. The activated PKD2, by acting on the TGN-localized substrates, affects the vesicle fission events and thus enhances the secretion of MMP-9. Ligation by ghrelin of GHS-R1a, on the other hand, leads to SFK-PTKs-mediated phosphorylation of α-tubulin on Tyr that maintains MT dynamics at its normal regulatory level. G heterotrimeric G protein, GEF guanine nucleotide exchange factor, MT microtubule, Tb tubulin, pS phosphoserine, pY phosphotyrosine.
affecting MT dynamics and MMP-9 secretion present a tempting target for therapeutic intervention in the treatment of chronic periodontitis.
Cite this paper
Slomiany, B.L. and Slomiany, A. (2017) Role of Signal-Regu- lated Changes in Microtubule Stability Dy- namics through α-Tubulin Phosphoryla- tion on Ser/Tyr in Modulation of Salivary Gland Matrix Metalloproteinase-9 (MMP- 9) Secretion in Response to Porphyromonas gingivalis and Ghrelin. Journal of Biosci- ences and Medicines, 5, 22-38. https://doi.org/10.4236/jbm.2017.52003
References
- 1. Colombo, A.P., Boches, S.K. and Cotton, S.L. (2009) Comparisons of Subgingival Microbial Profiles of Refractory Periodontitis, Severe Periodontitis, and Periodontal Health Using the Human Oral Microbe Identification Microarray. Journal of Periodontology, 80, 1421-1432.
https://doi.org/10.1902/jop.2009.090185 - 2. Slomiany, B.L. and Slomiany, A. (2010) Suppression by Ghrelin of Porphyromonas gingivalis-Induced Constitutive Nitric Oxide Synthase S-Nitrosylation and Apoptosis in Salivary Gland Acinar Cells. Journal of Signal Transduction, 2010, Article ID: 643642.
https://doi.org/10.1155/2010/643642 - 3. Mysak, J., Podzimek, S., Sommerova, P., et al. (2014) Porphyromonas gingivalis: Major Periodontopathic Pathogen Overview. Journal of Immunology Research, 2014, Article ID: 476068.
https://doi.org/10.1155/2014/476068 - 4. Slomiany, B.L. and Slomiany, A. (2011) Ghrelin-Induced cSrc Activation through Constitutive Nitric Oxide Synthase-Dependent S-Nitrosylation in Modulation of Salivary Gland Acinar Cell Inflammatory Responses to Porphyromonas gingivalis. American Journal of Molecular Biology, 2, 43-51.
https://doi.org/10.4236/ajmb.2011.12006 - 5. Slomiany, B.L. and Slomiany, A. (2015) Porphyromonas gingivalis-Stimulated TACE Activationfor TGF-α Ectodomian Shedding and EGFR Transactivation in Salivary Gland Cells Requires Rac1-Dependent p38 MAPK Membrane Localization. Journal of Biosciences and Medicines, 3, 42-53.
https://doi.org/10.4236/jbm.2015.311005 - 6. Ejeil, A.L., Igondio-Tchen, S., Ghomrasseni, S., Pellat, B., Godeau, G. and Gogly, B. (2003) Expression of Matrix Metalloproteinases (MMPs) and Tissue Inhibitors of Metalloproteinases (TIMPs) in Healthy and Diseased Human Gingiva. Journal of Periodontology, 74, 188-195.
https://doi.org/10.1902/jop.2003.74.2.188 - 7. Vandooren, J., Van den Steen, P.E. and Opdenakker, G. (2013) Biochemistry and Molecular Biology of Gelatinase B or Matrix Metalloproteinase-9 (MMP-9): The Next Decade. Critical Reviews in Biochemistry and Molecular Biology, 48, 222-272.
https://doi.org/10.3109/10409238.2013.770819 - 8. Slomiany, B.L. and Slomiany, A. (2016) Role of Rac1/p38 and ERK-Dependent Cytosolic Phospholipase A2 Activation in Porphyromonas gingivalis-Evoked Induction in Matrix Metalloproteinase-9 (MMP-9) Release by Salivary Gland Cells. Journal of Biosciences and Medicines, 4, 68-79.
https://doi.org/10.4236/jbm.2016.44010 - 9. Donaldson, J.G. and Jackson, C.L. (2011) ARF Family G Proteins and Their Regulators: Roles in Membrane Transport, Development and Disease. Nature Reviews Molecular Cell Biology, 12, 362-375.
https://doi.org/10.1038/nrm3117 - 10. Rozengurt, E. (2011) Protein Kinase D Signaling: Multiple Biological Functions in Health and Disease. Physiology, 26, 23-33.
https://doi.org/10.1152/physiol.00037.2010 - 11. Bonnemaison, M.L., Eipper, B.A. and Mains, R.E. (2013) Role of Adaptor Proteins in Secretory Granule Biogenesis and Maturation. Frontiers in Endocrinology, 4, 101.
https://doi.org/10.3389/fendo.2013.00101 - 12. Bourgoin, S.G. and El Azreq, M.A. (2012) Small Inhibitors of ADP-Ribosylation Factor Activation and Function in Mammalian Cells. World Journal of Pharmacology, 1, 55-64.
https://doi.org/10.5497/wjp.v1.i4.55 - 13. Cherfils, J. and Zeghouf, M. (2013) Regulation of Small GTPases by GEFs, GAPs, and GDIs. Physiological Reviews, 93, 269-309.
https://doi.org/10.1152/physrev.00003.2012 - 14. Eiseler, T., Wille, C., Koehler, C., Illing, A. and Seufferlein, T. (2016) Protein Kinase D2 Assembles a Multiprotein Complex at the Trans-Golgi Network to Regulate Matrix Metalloproteinase Secretion. Journal of Biological Chemistry, 291, 462-477.
https://doi.org/10.1074/jbc.M115.673582 - 15. Slomiany, B.L. and Slomiany, A. (2016) Role of Protein Kinase D2 Phosphorylation on Tyr in Modulation by Ghrelin of Helicobacter pylori-Induced Up-Regulation in Gastric Mucosal Matrix Metalloproteinase-9 (MMP-9) Secretion. Inflammopharmacology, 24, 119-126.
https://doi.org/10.1007/s10787-016-0267-2 - 16. Goode, B.L., Drubin, D.G. and Barnes, G. (2000) Functional Cooperation between the Microtubule and Actin in Cytoskeletons. Current Opinions in Cell Biology, 12, 63-71.
https://doi.org/10.1016/S0955-0674(99)00058-7 - 17. Hanania, R., Sun, H.S., Xu, K., et al. (2012) Classically Activated Macrophages Use Stable Microtubules for Matrix Metalloproteinase-9 (MMP-9) Secretion. Journal of Biological Chemistry, 287, 8468-8483.
https://doi.org/10.1074/jbc.M111.290676 - 18. Gu, S., Liu, Y., Zhu, B., et al. (2016) Loss of α-Tubulin Acetylation is Associated with TGF-β-induced Epithelial-Mesenchymal Transition. Journal of Biological Chemistry, 291, 5396-5405.
https://doi.org/10.1074/jbc.M115.713123 - 19. Howes, S.C., Alushin, G.M., Shida, T., Nachury, M.V. and Nogales, E. (2014) Effects of Tubulin Acetylation and Tubulin Acetyltransferase Binding on Microtubule Structure. Molecular Biology of the Cell, 25, 257-266.
https://doi.org/10.1091/mbc.E13-07-0387 - 20. Nirschl, J.J., Magiera, M.M., Lazarus, J.E., Janke, C. and Holzbaur, E.L.F. (2016) α-Tubulin Tyrosination and CLIP-170 Phosphorylation Regulate the Initiation of Dynein-Driven Transport in Neurons. Cell Reports, 14, 2637-2652.
https://doi.org/10.1016/j.celrep.2016.02.046 - 21. Jordan, M.A. and Wilson, L. (2004) Microtubules as a Target for Anticancer Drugs. Nature Reviews, 4, 253-265.
https://doi.org/10.1038/nrc1317 - 22. Fourest-Lieuvin, A., Peris, L., Gache, V., et al. (2006) Microtubule Regulation in Mitosis: Tubulin Phosphorylation by the Cyclin-dependent Kinase Cdk1. Molecular Biology of the Cell, 17, 1041-1050.
https://doi.org/10.1091/mbc.E05-07-0621 - 23. Slomiany, B.L. and Slomiany, A. (2016) Role of α-Tubulin Acetylation and Protein Kinase D2 Ser/Tyr Phosphorylation in Modulation by Ghrelin of Porphyromonas gingivalis-induced Enhancement in MMP-9 Secretion by Salivary Gland Cells. Journal of Biosciences and Medicines, 4, 82-94.
https://doi.org/10.4236/jbm.2016.47009 - 24. Laurent, C.E., Delfino, F.J., Cheng, H.Y. and Smithgall, T.E. (2004) The Human c-Fes Tyrosine Kinase Binds Tubulin and Microtubules Through Separate Domains and Promotes Microtubule Assembly. Molecular Cell Biology, 24, 9351-9358.
https://doi.org/10.1128/MCB.24.21.9351-9358.2004 - 25. Ma, X. and Sayeski, P.P. (2007) Identification of Tubulin as a Substrate of Jak2 Tyrosine Kinase and Its Role in Jak2-Dependent Signaling. Biochemistry, 46, 7153-7162.
https://doi.org/10.1021/bi700101n - 26. De, S., Tsimounis, A., Chen, X. and Rotenberg, S.A. (2014) Phosphorylation of α-Tubulin by Protein Kinase C Stimulates Microtubule Dynamics in Human Breast Cells. Cytoskeleton, 71, 252-272.
https://doi.org/10.1002/cm.21167 - 27. Yu, Y., Gaillard, S., Phillip, J.M., et al. (2015) Inhibition of Spleen Tyrosine Kinase Potentiates Paclitaxel-induced Cytotoxicity in Ovarian Cancer Cells by Stabilizing Microtubules. Cancer Cell, 28, 82-96.
https://doi.org/10.1016/j.ccell.2015.05.009 - 28. Slomiany, B.L. and Slomiany, A. (2015) Porphyromonas gingivalis-Induced GEF Dock180 Activation by Src/PKCδ-Dependent Phosphorylation Mediates PLCγ2 Amplification in Salivary Gland Acinar Cells: Effect of Ghrelin. Journal of Biosciences and Medicines, 3, 66-77.
https://doi.org/10.4236/jbm.2015.37008 - 29. Slomiany, A., Grabska, M., Slomiany, B.A., et al. (1993) Intracellular Transport, Organelle Biogenesis and Establishment of Golgi Identity: Impact of Brefeldin A on the Activity of Lipid Synthesizing Enzymes. International Journal of Biochemistry, 25, 891-901.
https://doi.org/10.1016/0020-711X(93)90245-A - 30. Slomiany, B.L. and Slomiany, A. (2016) Helicobacter pylori-Induced Changes in Microtubule Dynamics Conferred by α-Tubulin Phosphorylation on Ser/Tyr Mediate Gastric Mucosal Secretion of Matrix Metalloproteinase-9 (MMP-9) and Its Modulation by Ghrelin. Inflammopharmacology, 24, 197-205.
https://doi.org/10.1007/s10787-016-0278-z - 31. Visa, N.E., De Lemos-Chiarandini, C., Gravotta, D., Sabatini, D.D. and Kreibich, G. (1995) The Brefeldin A-Induced Retrograde Transport from the Golgi Apparatus to the Endoplasmic Reticulum Depends on Calcium Sequestered to Intracellular Stores. Journal of Biological Chemistry, 270, 25960-25967.
https://doi.org/10.1074/jbc.270.43.25960