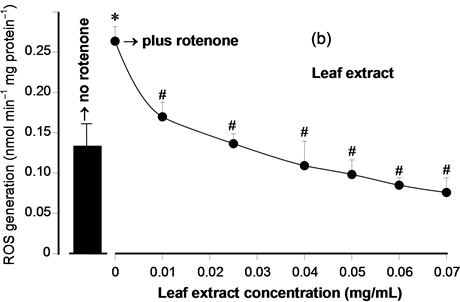Journal of Biosciences and Medicines
Vol.04 No.02(2016), Article ID:63578,13 pages
10.4236/jbm.2016.42003
The in Vitro Antioxidant Capacities of Hydroalcoholic Extracts from Roots and Leaves of Smallanthus sonchifolius (Yacon) Do Not Correlate with Their in Vivo Antioxidant Action in Diabetic Rats
Ana Carla Broetto Biazon1, Mariana Marques Nogueira Wendt2, Júlia Rosa Moreira2, Cristiane Vizioli Castro Ghizoni2, Andreia Assunção Soares2, Sandra da Silva Silveira2, Anacharis Babeto de Sá-Nakanishi2, Ciomar Aparecida Bersani Amado3, Rosane Marina Peralta2, Adelar Bracht2*, Jurandir Fernando Comar2
1Pharmacy Department, Faculdades Integrado, Campo Mourão, Brazil
2Biochemistry Department, University of Maringá, Maringá, Brazil
3Pharmacology and Therapeutics Department, University of Maringá, Maringá, Brazil

Copyright © 2016 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/



Received 6 January 2016; accepted 16 February 2016; published 19 February 2016
ABSTRACT
Leaf and root extracts of Smallanthus sonchifolius (yacon), have antihyper-glycemic activity and antioxidant properties. The present study aims to compare the in vivo hepatic antioxidant activity of hydroalcoholic extracts of yacon leaves and roots in rats with streptozotocin-induced diabetes in terms of their in vitro antioxidant capacity. Rats were treated during 14 days with 1060 mg∙Kg−1 root extract or 400 mg∙Kg−1 leaf extract. The latter was richer in phenolics and possessed a much higher in vitro antioxidant activity. Both extracts prevented hyperglycemia in diabetic rats. The liver of diabetic rats presented increased levels of protein carbonyls and ROS and decreased activities of antioxidant enzymes. Treatment with both root and leaf extracts restored the protein carbonyl levels to normality. The root extract also restored the ROS levels to normality, but the leaf extract was not effective. The root extract was also more effective in restoring the activity of at least two important antioxidant enzymes (glucose 6-phosphate dehydrogenase and glutathione peroxidase). In terms of the antioxidant load (which was 17 times lower in the root extract treatment), the in vivo action of the root extract was more effective than the leaf extract in reducing the hepatic oxidative stress that accompanies diabetes.
Keywords:
Smallanthus sonchifolius, Yacon, Streptozotocin-Induced Diabetes, Liver Oxidative Status

1. Introduction
Diabetes mellitus is a syndrome of multiple etiology that occurs due to the lack of insulin and/or to the inability of the cells to properly respond to it. The main feature of chronic diabetes is hyperglycemia, often accompanied by dyslipidemia and endothelial dysfunction [1] . These abnormalities are associated with the development of macro- and microvascular complications such as atherosclerosis, retinopathy, nephropathy and neuropathy. The exact mechanisms underlying the development and progression of diabetes mellitus and its complications are not clear. However, there is growing evidence that the excessive generation of reactive oxygen species (ROS), associated to a diminished capacity of the antioxidant system, causes oxidative stress in a variety of tissues, as already demonstrated for both patients and experimental diabetic animals [1] -[3] . Thus, one of the main goals of research in recent years has been to find therapies capable of attenuating the oxidative stress associated to diabetes [4] .
Smallanthus sonchifolius (Poepp. & Endl) H. Robinson is an herbaceous species from South America and popularly known as yacon. It is consumed as both food (tuberous roots) and medicinal preparation (roots and leaves). The tuberous roots are most commonly consumed fresh or as dried flakes and the leaves are primarily consumed as tea. The roots and leaves of yacon have been used in the popular medicine as an alternative for treatment of diabetes and dyslipidemias [5] [6] . Aqueous and hydroalcoholic extracts prepared with the roots improve hyperglycemia and serum triglyceride levels of rats with type I diabetes [7] [8] . The same effects were found when yacon root flour was administered as a diet supplement [9] . The consumption of yacon tuber syrup additionally produces beneficial effects against obesity and insulin resistance in humans [10] . Aqueous and hydroalcoholic extracts prepared with the leaves of yacon reduce hyperglycemia and the serum insulin levels of diabetic rats, although these effects are still controversial for aqueous extracts [11] -[13] . Organic (ethyl acetate and methanol) and aqueous extracts of yacon leaves are able to promote effects that were similar to those of insulin in the liver, such as the inhibition of gluconeogenesis and diminution of glucose release [14] -[16] and diminution of the glycogen levels [14] . The hypoglycemic effect of yacon extracts was attributed initially to the improvement of insulin secretion [13] , but subsequently to insulin-independent effects [14] . The main responsible for the hypoglycemic action of yacon leaves seems to be enhydrin, a sesquiterpene lactone isolated from the leaves [17] . However, caffeic, chlorogenic and several dicaffeoilquinic acids are possibly also contributing to the effect [17] .
Yacon can be helpful in the control of diabetes not only by affecting hyperglycemia or stimulating insulin secretion or action, but also by improving lipid metabolism, oxidative status or the endothelial vascular functions [18] . The treatment of diabetic rats with extracts with yacon protects against nephropathy, a complication that is mediated by free radicals [19] . Furthermore, extracts of yacon roots and leaves present a satisfactory radical scavenging activity when measured in vitro [20] [21] and both organic and aqueous extracts of yacon leaves protect rat hepatocytes in primary culture against the oxidative damage induced by tert-butyl hydroperoxide [14] [22] . The antioxidant properties of yacon have been attributed to the high levels of phenolic compounds in the tubers and leaves. L-tryptophan, chlorogenic acid and other derivatives of caffeic acid were identified as the main phenolic compounds of the yacon roots [7] [23] [24] . The leaves of yacon have even higher levels of phenolic compounds than the roots and the major components are chlorogenic acid and caffeic acid [22] .
As mentioned above, diabetes produces substantial changes in the hepatic carbohydrate metabolism, which have also been attributed, partly at least, to the increased oxidative stress that takes place in the diabetic liver [25] [26] . Given that the treatment with yacon has been described to reverse the hepatic metabolic changes in diabetic animals [16] [27] and that the yacon extracts are able to exert antioxidant effects in hepatocytes isolated from healthy rats, it is of interest to verify if similar effects occur in the liver of diabetic animals. This is precisely the scope of the present work in which the action of yacon extracts on the oxidative state of the liver from diabetic rats was extensively investigated. Taking into account that the action of aqueous extracts of yacon leaves against diabetes is controversial [12] , only hydroalcoholic extracts of yacon roots and leaves were used in the present work. Streptozotocin-induced diabetic rats were used as the experimental model, which should allow extrapolations to the liver of patients with type 1 diabetes.
2. Materials and Methods
2.1. Chemicals
Streptozotocin, Dinitrophenylhydrazine (DNPH), 2’-7’-dichloro-fluorescein-diacetate (DCFH-DA), oxidized di- chlorofluorescein (DCF), 1,1’,3,3’tetraethoxy-propane, Horse-radish peroxidase (HRP), o-phthalaldehyde (OPT), reduced glutathione (GSH), oxidized glutathione (GSSG), glutathione redutase and 2,6-bis(1,1-dimethylethyl)- 4-methylphenol (BHT) were purchased from Sigma Chemical Co (St. Louis, MO, USA). Commercial kits for albumin, AST and ALT assays were purchased from Gold Analisa Diagnóstica Ltda®, Belo Horizonte, Brazil. All other chemicals were of analytical.
2.2. Preparation of the Yacon Extracts
Dried yacon leaves and fresh yacon roots were acquired from Takashi Kakihara & Cia Ltda., Capão Bonito-São Paulo State, Brazil. A voucher specimen was deposited in the Herbarium of the State University of Maringá under the number HUEM 13021 (Identifier: Silmara Baroni). The roots in natura were firstly dehydrated as previously described [28] . The hydroalcoholic extracts of both leaves and roots were obtained from a 10% suspension (w:v) with 70% ethanol (10 g of dried leaves or dehydrated roots to a final volume of 100 ml 70% ethanol), under mechanical stirring for 5 hours. The extracts were then filtered (filter paper Whatman no. 1), slowly evaporated in a rotary evaporator (Technal, Piracicaba, SP, Brazil) at 600 mmHg to remove the solvent, lyophilized and stored at −20˚C. For the experiments the dried powders (hydroalcoholic extracts) of roots and leaves were suspended in water immediately before use. The lyophilized powder was a green solid and the total yields (w:w) of extraction were 58% for dried roots and 11% for dried leaves.
2.3. Animals and Induction of Diabetes
Male Wistar rats were fed ad libitum with a standard laboratory diet (Nuvilab®, Colombo, Brazil) and maintained on a regulated light-dark cycle. For the induction of diabetes, animals weighing 180 - 210 g were injected intraperitoneally with a single dose of streptozotocin (50 mg per Kg body weight), dissolved in 0.1 M citrate buffer (pH 4.6). Diabetes was confirmed by measuring blood glucose (tail incision) after 48 hours. Animals with glycemia above 250 mg∙dL−1 (14.0 mM) in the fed condition were included in the study. Control animals received a single intraperitoneal dose of 0.1 M citrate buffer. All experiments of the present work were done in accordance with the world-wide accepted ethical guidelines for animal experimentation and previously approved by the Ethics Committee for Animal Experimentation of the University of Maringá (Protocol 098/10-CEEA).
2.4. Experimental Design
Rats were randomly distributed into six groups: controls (C); controls treated with yacon root extract (C + RE); controls treated with yacon leaf extract (C + LE); diabetic (D); diabetic treated with yacon root extract (D + RE); and diabetic treated with yacon leaf extract (D + LE). The animals were treated daily by oral gavage with a single dose of the extracts (previously dissolved in water) during 14 days. Control (C) and diabetic (D) groups received saline in the same manner. The dose of the hydroalcoholic extracts of the leaves was 400 mg per Kg body weight as in a previous study [12] . The dose of the root extract was 1.06 mg per Kg, corresponding to 340 mg fructooligosaccharides per Kg, a value also based on previous investigations [9] [20] [29] .
2.5. Liver Homogenate Preparation and Mitochondria Isolation
At the end of the treatment period (14 days), 12 hours fasted rats were anesthetized by intraperitoneal injection of sodium pentobarbital (50 mg∙Kg−1). The criterion of anesthesia was the lack of body or limb movement in response to a standardized tail clamping stimulus. The abdominal cavity was exposed, the liver removed, freeze- clamped and stored in liquid nitrogen. The tissue was then homogenized in a Dounce homogenizer with 10 volumes of ice-cold 0.1 M potassium phosphate buffer (pH 7.4) and an aliquot was separated as the total homogenate. The remaining homogenate was centrifuged at 11,000 g during 20 min and the supernatant separated as the soluble fraction of the homogenate.
The mitochondria were isolated by differential centrifugation as previously described [30] . Briefly, the removed fresh liver was minced, washed and homogenized in the ice-cold buffer containing 230 mM mannitol, 70 mM sucrose, 1 mM ethylenediamine tetraacetic acid (EDTA) and 3 mM HEPES (pH 7.4). The homogenate was then centrifuged sequentially at 600 g (10 min) and 7000 g (10 min). The pellet with the intact mitochondria was washed twice and resuspended with the buffer without EDTA. The protein content in the homogenate and mitochondrial fraction was measured using the Folin phenol reagent [31] .
2.6. Total Phenolic Compounds and in Vitro Total Antioxidant Activity
Total phenolic compounds of the yacon extracts were measured by means of the Folin-Ciocalteu reagent and the results expressed as mg of catechin equivalents (g extract)−1 [32] . The antioxidant activity in vitro was measured spectrophotometrically at 515 nm as the ability of the extracts in scavenging the DPPH radicals (1,1-diphenyl-2- picrylhydrazyl) [33] . The antioxidant activity was calculated as the percentage of scavenging efficiency and the results expressed as the half-maximal effective concentration of the extract (EC50) in relation to the 0.2 mg∙ml−1 BHT, the reference compound. The EC50 was calculated by the Scientist software from MicroMath® (Salt Lake City, Utah) using Stineman’s interpolation formula [34] .
2.7. Protein Carbonyls Assay
The levels of protein carbonyl groups in the homogenate were used as oxidative injury marker. Protein carbonyl groups contents were measured spectrophotometrically using 2,4-dinitrophenylhydrazine (DNPH) (ε370 = 22 ×103 M−1∙cm−1) and the values expressed as nmol∙(mg∙protein)−1 [35] .
2.8. Reactive Oxygen Species (ROS) Assay
The total ROS content was quantified in the supernatant of the homogenate via the 2’-7’-dichlorofluorescein- diacetate (DCFH-DA) assay as described [36] . Briefly, the acetate groups are removed by cell esterases producing the reduced DCFH, which can be oxidized by peroxides to form the fluorescent oxidized dichlorofluorescein (DCF). The formation of DCF was measured spectrofluorimetrically and the excitation and emission wavelengths were set at 504 and 529 nm, respectively. A standard curve with oxidized dichlorofluorescein (DCF) was used to express the results as nmol・(mg∙protein)−1.
The rate of mitochondrial ROS production (real time ROS production), basically H2O2, was estimated by measuring the linear fluorescence increase (504 nm for excitation and 529 nm for emission) due to DCF formation from DCFH via oxidation by H2O2 in the presence of horseradish peroxidase [37] . Briefly, intact mitochondria (0.5 mg) were suspended in 2 ml of a mixture containing 250 mM mannitol, 1.36 mM DCFA-DA, 10 mM HEPES buffer (pH 7.2), and 10 mM succinate as respiratory substrate. The experiments were performed in the presence and absence of 10 μM rotenone, a mitochondrial electron transport blocker. The ROS generation is stimulated when isolated mitochondria are energized with succinate and it is further enhanced when the mitochondrial electron transport chain is blocked by rotenone [38] . Fluorescence was recorded during 10 min under agitation. The results were expressed as nmol∙min−1∙(mg∙protein)−1 and, alternatively, as the effective concentration of the extract that inhibits 50% (EC50) of the maximum ROS generation. The EC50 was calculated by numerical interpolation using Stineman’s interpolation formula [34] .
2.9. Glutathione Assay
Reduced glutathione (GSH) and oxidized glutathione (GSSG) were measured spectrofluorimetrically in the total homogenate (excitation 350 nm and emission 420 nm) by means of the o-phthalaldehyde (OPT) assay as described [39] . The fluorescence was estimated as GSH. For the GSSG assay, the sample was previously incubated with 10 mM N-ethylmaleimide (NEM) and subsequently with 1 M NaOH and 0.4 µM o-phthalaldehyde to detect the fluorescence. The results were calculated using a standard curve prepared with GSH or GSSG and the values expressed as nmol∙(mg∙protein)−1.
2.10. Enzyme Assays
Antioxidant enzymatic activities were assessed in the supernatant of the homogenate. The catalase activity was estimated by measuring changes in absorbance at 240 nm using H2O2 as substrate and expressed as µmol∙min−1∙(mg∙protein)−1 [37] . The glutathione reductase activity was estimated by measuring changes in absorbance at 340 nm using NADPH and GSSG as substrates and expressed as nmol∙min−1∙(mg∙protein)−1 [40] . The superoxide dismutase (SOD) activity was estimated by its capacity of inhibiting the pyrogallol autoxidation in alkaline medium. The latter was measured at 420 nm [41] . One SOD unit was considered the quantity of enzyme able to promote 50% inhibition and the results were expressed as U∙(mg∙protein)−1. The glucose 6-phos- phate dehydrogenase (G6PDH) activity was estimated by measuring the increase in absorbance at 340 nm due to NADP+-dependent glucose 6-phosphate transformation and expressed as nmol∙min−1∙(mg∙protein)−1 [40] . The glutathione peroxidase activity was estimated by measuring changes in absorbance at 340 nm due to NADPH consumption in the presence of H2O2, GSH and glutathione reductase and expressed as nmol∙min−1∙(mg∙protein)−1 [42] .
2.11. Plasma Analytical Assays
The glucose concentration and the activities of aspartate amino-transferase (AST) and alanine aminotransferase (ALT) were measured in the plasma. The peritoneal cavity of anesthetized rats was exposed and the blood was collected from the cava vein. After centrifugation at 3000 g for 10 min, plasma glucose, AST and ALT were measured spectrophotometrically using commercial Kits.
2.12. Statistical Analyses
The error parameters presented in graphs and tables are standard errors of the means. Statistical analysis was done by means of the GraphPad Prism Software (version 5.0). The statistical significance was analyzed by means of ANOVA with Newman-Keuls post-hoc testing. The significance level was 5% (p < 0.05).
3. Results and Discussion
3.1. Total Phenolic Content and in Vitro Antioxidant Activity
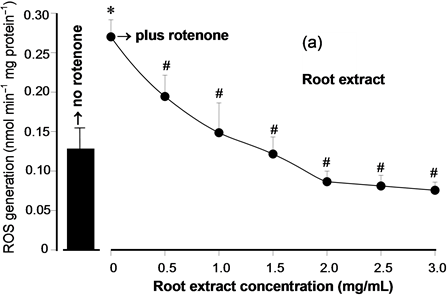
Total phenolic compounds and the antioxidant activity in vitro (DPPH assay) of the hydroalcoholic extracts were evaluated in order to compare the antioxidant potential of the root extracts with those of the leaf extracts and also to compare them with other types of yacon extracts. The results are shown in Table 1. The content in total phenolic compounds of the leaf extract was approximately twenty times higher than that of the root extract. The antioxidant activity via DPPH assay was nearly twenty times higher in the leaf extract. The antioxidant activity of the extracts was additionally explored by measuring their capacity of inhibiting ROS generation in rat liver mitochondria, which constitute a true biological system as opposed to the chemical DPPH assay. Figure 1 shows the concentration dependence of the inhibition of the mitochondrial ROS generation by the yacon extracts and Table 1 lists the EC50 values. Measurements in the presence of both extracts were done in the presence of rotenone, which enhances the production of ROS in isolated mitochondria. Both root and leaf extracts inhibited ROS generation in a well defined concentration-dependent manner. Here again, the leaf extract was much more effective as revealed by the lower concentrations that were required to inhibit ROS generation. Quantitatively this can be inferred from the concentrations producing 50% inhibition (EC50 values) given in Table 1 which reveal that the leaf extract is approximately 45 times more effective than the root extract.
3.2. Effects of the Yacon Extracts on Fasting Glycemia
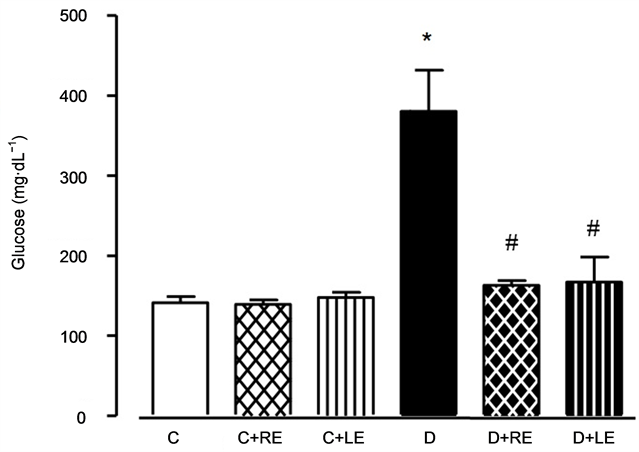
The effects of treating fasted rats with the hydroalcoholic yacon extracts on their blood glucose levels are shown in Figure 2. The antihyperglycemic effect of yacon extracts is well known [7] [8] [17] and these experiments were done in order to ascertain that this effect was preserved in both extracts that were used and to establish doses of both extracts capable of normalizing blood glucose concentration. Glycemia of the diabetic animals was 170% higher when compared to the controls. Treatment of the rats with 1060 mg∙Kg−1 root extract or 400 mg∙Kg−1 leaf extract during 14 days maintained the blood glucose concentration of diabetic rats close to normality. The treatment of control rats did not modify glycemia.
3.3. Plasma AST and ALT Activities
The plasma AST and ALT activities were measured to evaluate if the treatment with the extracts is hepatotoxic.
Table 1. Antioxidant in vitro activity and content of total phenolic compounds of the hydroalcoholic extracts of yacon roots and leaves. All assays were done as described in the Materials and Methods section. Values are mean ± mean standard error.
Figure 1. Concentration dependence of the inhibition of ROS generation in isolated rat liver mitochndria by the yacon extracts. (a) Mitochondrial ROS generation in the presence of leaf yacon extract; (b) Mitochondrial ROS generation in the presence of root yacon extract. The ROS generation was measured in isolated mitochondria, as described in the experimental section. The bar in the left of each panel indicates the ROS production in the absence of rotenone. All measurements in the presence of both extracts were done in the presence of 10 µM rotenone. Each datum point represents the mean ± standard error of the mean of 3 - 4 animals. *p < 0.05 for the enhanced ROS production in the presence of rotenone; #p < 0.05 for the inhibition of ROS generation in the presence of both extracts.
Figure 2. Effects of the hydroalcoholic extracts of yacon leaves and root on blood glucose levels in fasted non-diabetic and diabetic rats. The hydroalcoholic extracts of yacon roots and leaves were prepared and administered as described in the Experimental Section. The legends under each column represent: C, control rats; C + RE, controls treated with root extract; C + LE, controls treated with leaf extract; D, diabetic rats; D + RE, diabetic rats treated with root extract; D + LE, diabetic rats treated with leaf extract. Data are the mean ± standard error of the mean of 5 - 7 animals for each experimental condition. *p < 0.05 compared to the control group, #p < 0.05 compared to the diabetic group.
The results are shown in Table 2. The plasma AST activities in all groups were the same. The plasma ALT activity, however, was higher in diabetic rats when compared to the controls. The treatment of both diabetic and control rats with both extracts did not modify the plasma AST and ALT activities.
3.4. Tissue Oxidative Stress
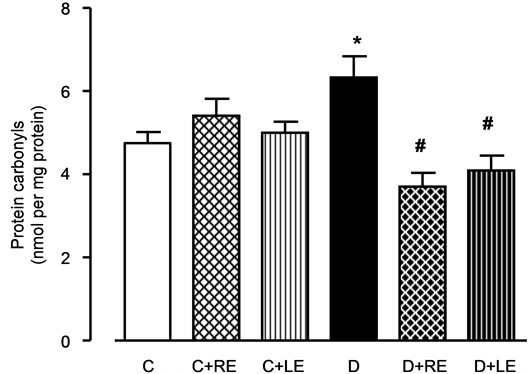
Oxidative injury of the liver was evaluated by the levels of protein carbonyl groups in the tissue homogenate and the results are shown in Figure 3. The protein carbonyl levels of non-treated diabetic animals were 40% higher when compared to the controls. It should be mentioned that there is a general agreement that protein carbonyls are a reliable indicator for oxidative damage to macromolecules, especially in the liver [37] . Treatment of the diabetic animals with the leaf and root extracts decreased these levels by 34% and 39%, respectively. The treatment of control rats did not modify the levels of protein carbonyl groups.
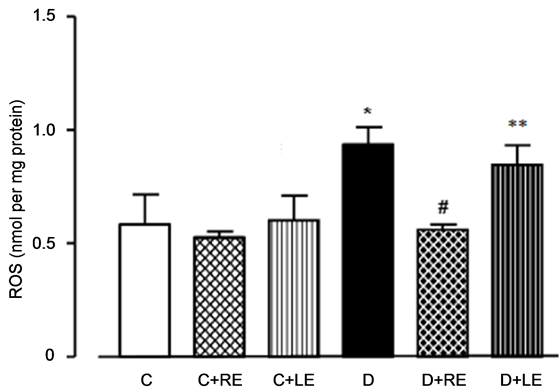
The tissue oxidative injury is normally caused by increases in the levels of reactive oxygen species (ROS) whose contents in the total homogenate are shown in Figure 4. The ROS content was 60% higher in the diabetic rats when compared to the controls. The treatment of the control rats with the yacon extracts did not modify the ROS content of the liver. The yacon treatment of diabetic rats, on the other hand, was effective in decreasing the ROS content only when the root extract was administered.
3.5. Glutathione Levels
Table 3 shows the levels of oxidized (GSSG) and reduced (GSH) glutathione in the liver. Diabetes did not modify the GSH or GSSG concentrations. Treatment of healthy rats with the leaf extract produced a significant increase of 48% in the GSH content. The root extract, on the contrary, tended to diminish the GSH content, the
Table 2. Effects of hydroalcoholic extracts of yacon leaves and roots on the plasma activity of AST and ALT. Control and diabetic animals were treated with the yacon leaf extract (400 mg∙kg−1) or with the yacon root extract (1.06 g∙kg−1) according to the experimental protocol described in the Experimental Section. The data represent the mean ± standard error of the mean from 4 to 9 animals. Values with superscript letters in the same line differ statistically (p < 0.05).
Figure 3. Effects of hydroalcoholic extracts of yacon leaves and roots on protein carbonylation in the liver. Protein carbonylation was measured in freeze-clamped livers as described in the Experimental Section. The legends under each column represent: C, control; C + RE, control treated with the root extract; C + LE, control treated with leaf extract; D, diabetic; D + RE, diabetic treated with root extract; D + LE, diabetic treated with leaf extract. Values represent mean ± standard error of 4 to 7 animals for each experimental condition. *p < 0.05 compared to the control group, #p < 0.05 compared to the diabetic group.
Figure 4. Effects of hydroalcoholic extracts of yacon leaves and roots on the ROS content in the liver. The ROS contents were measured as described in the Experimental Section. The legens under each column represent: C, control; C + RE, control treated with root extract; C + LE, control treated with leaf extract; D, diabetic; D + RE, diabetic treated with root extract; D + LE, diabetic treated with leaf extract. Values represent mean ± standard error of the mean from 5 to 8 animals for each experimental condition. *p < 0.05 compared to the control group, #p < 0.05 compared to the diabetic group, **p < 0.05 compared to the D + RE group.
Table 3. Effects of the hydroalcoholic extracts of yacon leaves and roots on the GSH and GSSG contents in liver homogenate. Control and diabetic animals were treated with the yacon leaf extract (400 mg∙Kg−1) or with the yacon root extract (1.06 g∙Kg−1) according to the experimental protocol described in the Experimental Section. The data represent the mean ± standard error of the mean from 4 to 6 animals. Values with the same superscript letters in the same line differ statistically (p < 0.05).
change, however, lacking statistical significance. Diabetes did not produce any change in the GSH or GSSG contents. Treatment of diabetic animals with both extracts produced significant increases in the GSH concentrations. The root extract in this case was somewhat more effective (+47%) than the leaf extract (+39%).
3.6. Antioxidant Enzymatic Activities
Five antioxidant enzymatic activities were measured and the results are shown in Table 4. Diabetes diminished the activities of catalase (30%), superoxide dismutase (50%), glutathione peroxidase (21%) and glucose 6- phosphate dehydrogenase (G6PDH; 34%). The glutathione reductase activity also tended to be smaller, a tendency lacking statistical significance. Treatment of healthy rats with both yacon extracts did not affect the enzymatic activities, although a strong tendency toward stimulation was apparent for the glutathione reductase activity upon the root extract treatment. Treatment of the diabetic rats with both the leaf and root extracts, on the other hand, had a positive effect on all the enzymatic activities listed in Table 4, but with several quantitative differences. The most remarkable difference was found for G6PDH, which was 77% increased by the root extract and only 26% by the leaf extract. The glutathione peroxidase activity was also considerably more increased by the root extract than by the leaf extract treatment, namely by 51% and 16%, respectively. The other enzymes were similarly increased by both extracts. In the case of superoxide dismutase this increase reached 100% or more.
Table 4. Effects of the hydroalcoholic extracts of yacon leaves and roots on the activity of antioxidant enzymes in the rat liver. Control and diabetic animals were treated with the extracts of yacon leaves (400 mg∙kg−1) or with the extracts of yacon roots (1.06 g∙kg−1) according to the experimental protocol described in the Experimental Section. Control and diabetic groups received saline for the same period. The data represent the mean ± standard errors from 4 to 6 animals. Values with the same superscript letters in the same line differ statistically from each other (p < 0.05).
4. Discussion
The levels of the total phenolic compounds in the hydroalcoholic extracts of the yacon roots in the present work are close to those reported previously for other organic or aqueous extracts [20] [43] [44] . The phenolic contents of the leaf extracts in the present work, however, are somewhat lower than those reported previously for aqueous extracts (107 and 118 mg∙g−1) and ethyl acetate extracts (200 mg∙g−1) [21] [22] . Consistently, the DPPH radical scavenging activity of the hydroalcoholic extracts used in the present work is lower than that reported for these extracts [21] [22] . This agrees with the general notion that the phenolics are the main responsible for the DPPH radical scavenging activity of the yacon leaf extracts. The DPPH free radical scavenging activity of the root extracts in the present study are also close to those reported for hydroalcoholic extracts [8] . For other extracts, comparisons are difficult because the data were not expressed as EC50, but as trolox equivalents [19] [43] [45] . A biologically more relevant way of evaluating the antioxidant activity is the inhibition of the mitochondrial ROS production. The EC50 value found in the present work (Table 1) for the hydroalcoholic leaf extract is very close to that reported for the inhibition of mitochondrial lipoperoxidation induced by t-butyl hydroperoxide by an ethyl acetate extract (EC50 = 0.022 mg∙mL−1, [22] ). Aqueous extracts obtained by decoction and infusion were much less effective with EC50 values of 0.21 and 0.40 mg∙mL−1, respectively [21] . Up to now there are no data in the literature concerning the antioxidant activity of yacon root extracts in isolated mitochondria or other organelles.
Complete prevention of fasting hyperglycemia in diabetic rats has already been demonstrated for hydroalcoholic extracts of yacon leaves at the same dosis (400 mg∙Kg−1) and treatment periods used in the present work (14 days) [9] . Doses of 200 mg∙Kg−1 were only partially effective [5] . Aqueous extracts are apparently ineffective in preventing hyperglycemia [9] . An aqueous extract of yacon roots, on the other hand, prevented only partially hyperglycemia of diabetic rats and solely when administered for 30 days at daily doses of 2500 mg∙Kg−1 [3] . Thus, the hydroalcoholic extract of yacon roots used in the present work (1.06 g∙Kg−1) was more effective in preventing fasting hyperglycemia than other extracts.
The results of our measurements of AST and ALT activities in the plasma confirm previous observations that hydroalcoholic leaf extracts do not cause liver or kidney toxicity when administered at doses of 400 mg∙Kg−1 during 14 days [12] . Similarly, no toxic effects were observed when a root extract was administered each day intragastrically at doses of 2000 mg∙Kg−1 during a 4-month period [46] . It should be mentioned, however, that a long treatment (90 days) with hydromethanolic extracts of yacon leaves induced substantial renal toxicity [47] .
Our experiments confirmed the higher ROS contents of the liver of diabetic rats reported by previous investigations [35] [48] [49] . Higher levels of protein carbonyl groups have also been reported previously for both the liver of rats with streptozotocin-induced diabetes and the plasma of diabetic rats and patients [4] [36] [50] . The fact that the yacon treatment of diabetic rats was effective in decreasing the ROS content only when the root extract was administered is somewhat surprising for at least two reasons. The first one is that the leaf extract was much more efficient than the root extract in inhibiting the ROS generation in mitochondria (45 times more efficient) and in scavenging the DPPH radicals (20 times more efficient). The second reason is linked to the content in phenolics, which is approximately ten times higher in the leaf extract. Phenolics are generally considered the main antioxidant compounds of the yacon extracts [8] [21] -[24] . It is true that the doses of the root extract that were given to the rats were higher by a factor of 2.65 when compared to the doses of the leaf extract. Even so, in terms of the potential antioxidant power, the administered leaf extracts were 17 times superior to the root extracts according to the antioxidant activity assay using isolated rat liver mitochondria (Table 1). Clearly, this is a situation in which the in vitro perspective does not match the in vivo reality, the compounds with antioxidant activity in the roots of yacon being apparently more active under the in vivo conditions than those present in the leaves.
A similar reasoning applies to the effects of both extracts on the recovery of enzyme activities affected by diabetes. Here again one should emphasize the disparity between the smaller load of antioxidant power represented by the root extract treatment and its more pronounced effects on the recovery of at least two enzymatic activities, namely G6PDH and glutathione peroxidase. The former is an important source of reducing power in the form of NADPH, which can be used in the reduction of GSSG to GSH (glutathione reductase). GSH, in turn, is the substrate of the glutathione peroxidase reaction in the removal of hydrogen peroxide (2GSH + H2O2 → GSSG + 2H2O). The latter is precisely the most important reactive oxygen species (ROS) that is detected when the antioxidant activity assays are carried out [49] . It is thus possible that the more pronounced effect of the root extract in diminishing the hepatic ROS levels (see Figure 4 and subsection 3.4) in diabetic rats may be connected in some way to the combined activities of enzymes that generate reducing power in the form of NADPH (e.g., G6PDH) and enzymes that remove H2O2, as glutathione peroxidase, for example. The reason why the root extract is more effective in the liver cannot be inferred with certainty from the available data. It could be that the antioxidant compounds in the root extract are more readily available to the whole organism than those of the leaf extract. This could be brought about, for example, by a more efficient gastric absorption. Another possibility is that the antioxidant compounds in the root extract are simply more effective in the living cell. A third possibility is that the actions of both root and leaf extract are, partly at least, connected to their antidiabetic effects. Favouring this interpretation, is the fact that both extracts affect only marginally the oxidative status in the liver of healthy animals. This suggests that the effects of both extracts are limited by internal factors and are exerted only after certain thresholds caused by diabetes are exceeded.
5. Conclusion
The results of the present work indicate that, both root and leaf extracts of yacon, administered at doses capable of normalizing the glycemic levels of diabetic rats, are effective in diminishing the pronounced hepatic oxidative stress that accompanies diabetes. In terms of the potential antioxidant power revealed by both a chemical (DPPH free radical scavenging activity) and a biological assay (mitochondrial ROS generation), the leaf extract load was between 8 and 17 times higher than the root extract. In vivo, however, the yacon root extract was more efficient in preventing oxidative stress and in restoring the antioxidant defenses in terms of the activities of antioxidant enzymes. Additional studies with both hydroalcoholic extracts should be encouraged for elucidating their mechanisms of action and to evaluate their effectiveness in diabetic humans.
Acknowledgements
The authors thank the financial support of the Fundação Araucária and the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq). Anacharis Babeto de Sá-Nakanishi is a post-doctoral fellow in the Food Science Post-Graduate Program of the University of Maringá.
Cite this paper
Ana Carla BroettoBiazon,Mariana Marques NogueiraWendt,Júlia RosaMoreira,Cristiane Vizioli CastroGhizoni,Andreia AssunçãoSoares,Sandrada Silva Silveira,Anacharis Babetode Sá-Nakanishi,Ciomar Aparecida BersaniAmado,Rosane MarinaPeralta,AdelarBracht,Jurandir FernandoComar, (2016) The in Vitro Antioxidant Capacities of Hydroalcoholic Extracts from Roots and Leaves of Smallanthus sonchifolius (Yacon) Do Not Correlate with Their in Vivo Antioxidant Action in Diabetic Rats. Journal of Biosciences and Medicines,04,15-27. doi: 10.4236/jbm.2016.42003
References
- 1. Giacco, F. and Brownlee, M. (2010) Oxidative Stress and Diabetic Complications. Circulation Research, 107, 1058-1070.
http://dx.doi.org/10.1161/CIRCRESAHA.110.223545 - 2. Winiarska, K., Szymanski, K., Gorniak, P., Dudziak, M. and Bryla, J. (2009) Hypoglycaemic, Antioxidative, and Nephroprotective Effects of Taurine in Alloxan Diabetic Rabbits. Biochimie, 91, 261-270.
http://dx.doi.org/10.1016/j.biochi.2008.09.006 - 3. Rochette, L., Zeller, M., Cottin, Y. and Vergelle, C. (2014) Diabetes, Oxidative Stress and Therapeutic Strategis. Biochimica et Biophysica Acta, 1840, 2709-2729.
http://dx.doi.org/10.1016/j.bbagen.2014.05.017 - 4. Pandey, K.B., Mishra, N. and Rizvi, S.I. (2011) Protein Oxidation Biomarkers in Plasma of Type 2 Diabetic Patients. Clinical Biochemistry, 43, 508-511.
http://dx.doi.org/10.1016/j.clinbiochem.2009.11.011 - 5. Valentová, K. and Ulrichová, J. (2003) Smallanthus sonchifolius and Lepidium Meyenii—Prospective Andean Crops for the Prevention of Chronic Diseases. Biomedical Papers, 147, 119-130.
http://dx.doi.org/10.5507/bp.2003.017 - 6. Lachman, J., Fernández, E.C. and Orsák, M. (2003) Yacon [Smallanthus sonchifolia (Poepp. et Endl.) H. Robinson] Chemical Composition and Use—A Review. Plant Soil and Environment, 49, 283-290.
- 7. Oliveira, G.O., Braga, C.P. and Fernandes, A.A. (2013) Improvement of Biochemical Parameters in Type 1 Diabetic Rats after the Roots Aqueous Extract of Yacon [Smallanthus sonchifolius (Poepp. & Endl.)] Treatment. Food and Chemical Toxicology, 59, 256-260.
http://dx.doi.org/10.1016/j.fct.2013.05.050 - 8. Park, J.S., Yang, J.S., Hwang, B.Y., Yoo, B.K. and Han, K. (2009) Hypoglycemic Effect of Yacon Tuber Extract and Its Constituent, Chlorogenic Acid, in Streptozotocin-Induced Diabetic Rats. Biomolecules & Therapeutics, 17, 256-262.
http://dx.doi.org/10.4062/biomolther.2009.17.3.256 - 9. Habib, N.C., Honoré, S.M., Genta, S.B. and Sánchez, S.S. (2011) Hypolipidemic Effect of Smallanthus sonchifolius (Yacon) Roots on Diabetic Rats: Biochemical Approach. Chemico-Biological Interactions, 194, 31-39.
http://dx.doi.org/10.1016/j.cbi.2011.08.009 - 10. Genta, S., Cabrera, W., Habib, N., Pons, J., Carillo, I.M., Grau, A. and Sánches, S. (2009) Yacon Syrup: Beneficial Effects on Obesity and Insulin Resistance in Humans. Clinical Nutrition, 28, 182-187.
http://dx.doi.org/10.1016/j.clnu.2009.01.013 - 11. Delgado, G.T.C., Tamashiro, W.M.S.C., Maróstica-Jr, M.R. and Pastore, G. (2013) Yacon (Smallanthus sonchifolius): A Functional Food. Plant Food for Human Nutrition, 68, 222-228.
http://dx.doi.org/10.1007/s11130-013-0362-0 - 12. Baroni, S., Suzuki-Kemmelmeier, F., Caparroz-Assef, S.M., Cuman, R.K.N. and Bersani-Amado, C.A. (2008) Effect of Crude Extracts of Leaves of Smallanthus sonchifolius (Yacon) on Glycemia in Diabetic Rats. Revista Brasileira de Ciências Farmacêuticas, 44, 521-530.
http://dx.doi.org/10.1590/S1516-93322008000300024 - 13. Aybar, M.J., Riera, A.N.S., Grau, A. and Sánchez, S.S. (2001) Hypoglycemic Effect of the Water Extract of Smallantus sonchifolius (Yacon) Leaves in Normal and Diabetic Rats. Journal of Ethnopharmacology, 74, 125-132.
http://dx.doi.org/10.1016/S0378-8741(00)00351-2 - 14. Valentová, K., Moncion, A., Waziers, I. and Ulrichová, J. (2004) The Effect of Smallantus sonchifolius Leaf Extracts on Rat Hepatic Metabolism. Cell Biology and Toxicology, 20, 109-120.
http://dx.doi.org/10.1023/B:CBTO.0000027931.88957.80 - 15. Levinthal, G.N. and Tavill, A.S. (1999) Liver Disease and Diabetes Mellitus. Clinical Diabetes, 17, 1-20.
- 16. Valentová, K., Truong, N.T., Moncion, A., Waziers, I. and Ulrichová, J. (2007) Induction of Glucokinase mRNA by Dietary Phenolic Compounds in Rat Liver Cells in Vitro. Journal of Agricultural and Food Chemistry, 55, 7726-7731.
http://dx.doi.org/10.1021/jf0712447 - 17. Genta, S.B., Cabrera, W.N., Mercado, M.I., Grau, A., Catalán, C.A. and Sánches, S.S. (2010) Hypoglycemic Activity of Leaf Organic Extracts of Smallanthus sonchifolius: Constituents of the Most Active Fractions. Chemico-Biological Interactions, 185, 143-152.
http://dx.doi.org/10.1016/j.cbi.2010.03.004 - 18. Tiwari, A.K. and Rao, J.M. (2002) Diabetes Mellitus and Multiple Therapeutic Approaches of Phytochemicals: Present Status and Future Prospects. Current Science, 83, 30-38.
- 19. Honoré, S.M., Cabrera, W.M., Genta, S.B. and Sanchez, S.S. (2012) Protective Effects of Yacon Leaves Decoction against Early Nephropathy in Experimental Diabetic Rats. Food and Chemical Toxicology, 50, 1704-1715.
http://dx.doi.org/10.1016/j.fct.2012.02.073 - 20. Campos, D., Bettaeluz-Pallardel, I., Chirinos, R., Aguilar-Galvez, A., Noratto, G. and Pedreschi, R. (2012) Prebiotic Effects of Yacon (Smallanthus Sonchifolius Poepp. & Endl), a Source of Oligosaccharides and Phenolic Compounds with Antioxidant Activity. Food Chemistry, 135, 1592-1599.
http://dx.doi.org/10.1016/j.foodchem.2012.05.088 - 21. Valentová, K., Sersen, F. and Ulrichová, J. (2005) Radical Scavenging and Anti-Lipoperoxidative Activities of Smallanthus sonchifolius Leaf Extracts. Journal of Agricultural and Food Chemistry, 53, 5577-5582.
http://dx.doi.org/10.1021/jf050403o - 22. Valentová, K., Cvak, L., Muck, A., Ulrichová, J. and Simánek, V. (2003) Antioxidant Activity of Extracts from the Leaves of Smallanthus sonchifolius. European Journal of Nutrition, 42, 61-66.
http://dx.doi.org/10.1007/s00394-003-0402-x - 23. Yan, X., Suzuki, M., Ohnishi-Kameyama, M., Sada, Y., Nakanishi, T. and Nagata, T. (1999) Extraction and Identification of Antioxidants in the Roots of Yacon (Smallanthus sonchifolius). Journal of Agricultural and Food Chemistry, 47, 4711-4713.
http://dx.doi.org/10.1021/jf981305o - 24. Takenaka, M., Yan, X., Ono, H., Yoshida, M., Nagata, T. and Nakanishi, T. (2003) Caffeic Acid Derivatives in Roots of Yacon (Smallanthus sonchifolius). Journal of Agricultural and Food Chemistry, 51, 793-796.
http://dx.doi.org/10.1021/jf020735i - 25. Schmatz, R., Perreira, L.B., Stefanello, N., Mazzanti, C., Spanevello, R., Gutierres, J., Bagatini, M., Martins, C.C., Abdalla, F.H., Serres, J.D.S., Zanini, D., Vieira, J.M., Cardoso, A.M., Schetinger, M.R. and Morsch, V.M. (2012) Effects of Resveratrol on Biomarkers of Oxidative Stress and on the Activity of Delta Aminolevulinic Acid Dehydratase in Liver and Kidney of Streptozotocin-Induced Diabetic Rats. Biochimie, 94, 374-383.
http://dx.doi.org/10.1016/j.biochi.2011.08.005 - 26. Ozcelik, D., Tuncdemir, M., Ozturk, M. and Uzun, H. (2011) Evaluation of Trace Elements and Oxidative Stress Levels in the Liver and Kidney of Streptozotocin-Induced Experimental Diabetic Rat Model. General Physiology and Biophysics, 30, 356-363.
http://dx.doi.org/10.4149/gpb_2011_04_356 - 27. Baroni, S., Comar, J.F., Suzuki-Kemmelmeier, F., Mito, M.S., Melo, J.O., Rocha, B.A. and Bersani-Amado, C.A. (2014) Beneficial Effects of Hydroethanolic Extracts of Smallanthus sonchifolius Leaves on the Metabolic Changes in Diabetic Rats. International Journal of Pharma and Bio Sciences, 5, 183-196.
- 28. Padilha, V.M., Rolim, P.M., Salgado, S.M., Livera, A.V.S. and Oliveira, M.G. (2009) Drying Evaluation Time and Yacon (Smallanthus sonchifolius) Enzymatic Activity Inhibition under Chemical Treatment. Ciência Rural, 39, 2178-2184.
http://dx.doi.org/10.1590/S0103-84782009005000142 - 29. Geyer, M., Manrique, I., Degen, L. and Beglinger, C. (2008) Effect of Yacon (Smallanthus sonchifolius) on Colonic Transit Time in Healthy Volunteers. Digestion, 78, 30-33.
http://dx.doi.org/10.1159/000155214 - 30. Bracht, A., Ishii-Iwamoto, E.L. and Salgueiro-Pagadigorria, C.L. (2003) O estudo do metabolismo energético em mitocondrias isoladas de tecido animal. In: Bracht, A. and Ishii-Iwamoto, E.L., Eds., Métodos de Laboratório em Bioquímica, Editora Manole, Sao Paulo, 227-247.
- 31. Lowry, O.H., Rosebrough, N.J., Farr, A.L. and Randall, R.J. (1951) Protein Measurement with the Folin Phenol Reagent. Journal of Biological Chemistry, 193, 265-275.
- 32. Singleton, V.L. and Rossi, J.A. (1965) Colorimetry of Total Phenolics with Phosphomolybdic-Phosphotungstic Acid Reagent. American Journal of Enology and Viticulture, 16, 144-155.
- 33. Choi, Y., Lee, S.M., Chun, J., Lee, H.B. and Lee, J. (2006) Influence of Heat Treatment on the Antioxidant Activities and Polyphenolic Compounds of Shiitake (Lentinus edodes) Mushroom. Food Chemistry, 99, 381-387.
http://dx.doi.org/10.1016/j.foodchem.2005.08.004 - 34. Stineman, R.W. (1980) A Consistently Well-Behaved Method for Interpolation. Creative Computing, 6, 54-57.
- 35. Levine, R.L., Garland, D., Oliver, C.N., Amici, A., Climent, I., Lenz, A.G., Ahn, B.W., Shaltiel, S. and Stadtman, E.R. (1990) Determination of Carbonyl Content in Oxidatively Modified Proteins. Methods in Enzymology, 186, 464-478.
http://dx.doi.org/10.1016/0076-6879(90)86141-H - 36. Manna, P., Das, J., Ghosh, J. and Sil, P.C. (2010) Contribution of Type 1 Diabetes to Rat Liver Dysfunction and Cellular Damage via Activation of NOS, PARP, IκBα, NF-κB, MAPKs, and Mitochondria-Dependent Pathways: Prophylactic Role of Arjunolic Acid. Free Radical Biology and Medicine, 48, 1465-1484.
http://dx.doi.org/10.1016/j.freeradbiomed.2010.02.025 - 37. Comar, J.F., Sá-Nakanishi, A.B., Oliveira, A.L., Wendt, M.M.N., Bersani-Amado, C.A., Ishii-Iwamoto, E.L., Peralta, R.M. and Bracht, A. (2013) Oxidative State of the Liver of Rats with Adjuvant-Induced Arthritis. Free Radical Biology and Medicine, 58, 144-153.
http://dx.doi.org/10.1016/j.freeradbiomed.2012.12.003 - 38. Zaccagnino, P., Saltarella, M., D’oria, S., Corcelli, A., Saponetti, M.S. and Lorusso, M. (2009) N-Arachidonylglycine Causes ROS Production and Cytochrome c Release in Liver Mitochondria. Free Radical Biology and Medicine, 47, 585-592.
http://dx.doi.org/10.1016/j.freeradbiomed.2009.05.038 - 39. Hissin, P.J. and Hilf, R. (1976) A Fluorometric Method for Determination of Oxidized and Reduced Glutathione in Tissues. Analytical Biochemistry, 74, 214-226.
http://dx.doi.org/10.1016/0003-2697(76)90326-2 - 40. Bergmeyer, H.U. (1974) Methods of Enzymatic Analysis. Verlag Chemie-Academic Press, London.
- 41. Marklund, S. and Marklund, G. (1974) Involvement of the Superoxide Anion Radical in the Oxidation of Pyrogallol and a Convenient Assay for Superoxide Dismutase. European Journal of Biochemistry, 47, 469-474.
http://dx.doi.org/10.1111/j.1432-1033.1974.tb03714.x - 42. Tappel, A.L. (1978) Glutathione Peroxidase and Hydroperoxides. Methods of Enzymology, 52, 506-513.
http://dx.doi.org/10.1016/S0076-6879(78)52055-7 - 43. Castro, A., Caballero, M., Herbas, A. and Carballo, S. (2012) Antioxidants in Yacon Products and Effects of Long Term Storage. Food Science and Technology, 32, 432-435.
http://dx.doi.org/10.1590/s0101-20612012005000064 - 44. Simonovska, B., Vovk, I., Andrensek, S., Valentová, K. and Ulrichová, J. (2003) Investigation of Phenolic Acids in Yacon (Smallanthus sonchifolius) Leaves and Tubers. Journal of Chromatography A, 1016, 89-98.
http://dx.doi.org/10.1016/S0021-9673(03)01183-X - 45. Mikami, I., Yamaguchi, M., Shinmoto, H. and Tsushida, T. (2009) Development and Validation of a Microplate-Based Beta-Carotene Bleaching Assay and Comparison of Antioxidant Activity (AOA) in Several Crops Measured by Beta-Carotene Bleaching, DPPH and ORAC Assays. Food Science and Technology Research, 15, 171-178.
http://dx.doi.org/10.3136/fstr.15.171 - 46. Genta, S.B., Cabrera, W.M., Grau, A. and Sánches, S.S. (2005) Subchronic 4-Month Oral Toxicity Study of Dried Smallanthus sonchifolius (Yacon) Roots as a Diet Supplement in Rats. Food and Chemical Toxicology, 43, 1657-1665.
http://dx.doi.org/10.1016/j.fct.2005.05.007 - 47. Oliveira, R.B., Paula, D.A.C., Rocha, B.A., Franco, J.J., Gobbo-Neto, L., Uyemura, S.A., Santos, W.F. and Costa, F.B. (2011) Renal Toxicity Caused by Oral Use of Medicinal Plants: The Yacon Example. Journal of Ethnopharmacology, 133, 434-441.
http://dx.doi.org/10.1016/j.jep.2010.10.019 - 48. Srinivasan, S. and Pari, L. (2012) Ameliorative Effect of Diosmin, a Citrus Flavonoid against Streptozotocin Nicotinamide Generated Oxidative Stress Induced Diabetic Rats. Chemico-Biological Interactions, 195, 43-51.
http://dx.doi.org/10.1016/j.cbi.2011.10.003 - 49. Siqueira, I.R., Fochesatto, C., Torres, I.L.S., Dalmaz, C. and Netto, C.A. (2005) Aging Affects Oxidative State in Hippocampus, Hypothalamus and Adrenal Glands of Wistar Rats. Life Sciences, 78, 271-278.
http://dx.doi.org/10.1016/j.lfs.2005.04.044 - 50. Parveen, K., Khan, M.R., Mujeeb, M. and Siddiqui, W.A. (2010) Protective Effects of Pycnogenol on Hyperglycemia-Induced Oxidative Damage in the Liver of Type 2 Diabetic Rats. Chemico-Biological Interactions, 186, 219-227.
http://dx.doi.org/10.1016/j.cbi.2010.04.023
NOTES

*Corresponding author.