International Journal of Clinical Medicine
Vol.5 No.7(2014), Article ID:44728,17 pages DOI:10.4236/ijcm.2014.57053
Approach to Epidemiological Mechanism of Infection or Colonization of Egg-Laying Chicken Farms by Salmonella enterica Serovar Enteritidis (SE) Becoming the Main Source of Contamination in Food Poisoning (Review)
Yukiko Toyota-Hanatani1*, Yushi Nakagawa2, Toshimitsu Hatabu2, Yuko Miyao3, Hiroaki Ohta4
1The Laboratory of Veterinary Internal Medicine, School of Veterinary Science, Osaka Prefectural University, Izumisano, Japan
2Department of Animal Science, Graduate School of Natural Science and Technology, Okayama University, Okayama, Japan
3Department of Biotechnology, Faculty of Life Science and Biotechnology, Fukuyama University, Hiroshima, Japan
4CAF Laboratories, Fukuyama, Japan
Email: *hanatani@sakamoto-egg.co.jp
Copyright © 2014 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/


Received 13 January 2014; revised 10 February 2014; accepted 8 March 2014
ABSTRACT
Salmonella enterica serovar Enteritidis (SE)-induced diarrhea in humans is the typical non-typhoid diarrhea. It develops acutely or subacutely and may be fatal. This SE infectious disease suddenly became a major public health issue worldwide in the 1980s. The main causative food material of SE food poisoning is chicken eggs, and many outbreaks of food poisoning caused by chicken eggs occurred throughout the world. SE epidemics occurred in layer farms, and this was the main cause of SE-induced food poisoning in humans. The major subject of our epidemiological study described in this report is why SE-contaminated eggs became the main causative food. In this study, we focused on difference of molecular expression for farm-isolated SEs. That is because recent studies have demonstrated that O-antigen enlargement may be related to pathogenicity in mice as well as 22-kDa polypeptide-expression (SEp22). We have discovered that many SE strains isolated from chicken farms do not express SEp22, and a deficiency or decreased level of cellular antigen 0 - 12 in SE strains isolated from chicken farms was clarified in a report. Additionally, SEp22 was deficient in SE strains passaged through chickens, whereas SEp22 was expressed at a high level in SE strains passaged through mice. These findings suggest that SE infection and retention more effectively occur in layer farms than in other animal maintenance environments, which may be a basis of the epidemiological hypothesis to explain the high-levelproduction of SE-contaminated eggs (the presence of mice may be the basis of the retention of SE infection in layer farms, and this may also be the mechanism causing the high-level production of SE-contaminated eggs).
Keywords:Salmonella enterica Serovar Enteritidis (SE), Epidemiological Role, Mouse, Outbreaks

1. Introduction
The course of SE infection varies with patients. Its predominant symptom is generally diarrhea at the early stage of infection. Some high-risk patients present with bacteremia and die. However, the pathogenic mechanism of diarrhea due to SE infection remains unclear. Previous studies have described the pathogenic mechanism of diarrhea due to Salmonella infection in detail; therefore, a brief summary was provided in this review. Both SE and its hosts have been investigated and mouse models have been analyzed to reveal the pathogenic mechanism of diarrhea due to SE infection [1] . Antigens, such as pathogenic island gene (e.g., InvA gene) products and outer membrane proteins, have been investigated as SE factors. In mice, a 22-kDa polypeptide (SEp22) and flagellum components are known to play important roles as SE virulence factors [2] . However, their roles in diarrhea in humans remain unclear. In addition, the epidemiological roles of mice have not been investigated. Infants, elderly people, and patients are at a clinically high risk of SE infection (a population with a high incidence of SE infection) [3] . This finding suggests a relationship between immunological development and susceptibility to SE infection in humans. However, because we are not specializing in this field, please refer to previous studies for more information. SE food poisoning is caused by ingesting SE-contaminated food. SE-contaminated eggs have been recognized as a major cause of SE food poisoning. We specialize in this field of research and have investigated SE infection from SE-contaminated eggs.
Recent studies on chickens and SE have focused on the mechanism of egg contamination through the oral intake of SE. Many studies have been published on the interaction between the innate immune system and SE [4] -[7] . The roles of the O antigen in SE, in particular, have attracted attention. The elongated O chain in SE appears to allow avoidance from the attack of the host innate immune system, particularly complements [8] [9] . Chickens are generally infected with SE through oral ingestion. A part of SE incorporated into the body may reach the reproductive organs to contaminate eggs. However, our research has shown that not all SE strains reach the reproductive organs of laying chickens. Some strains repeatedly colonize and grow exclusively in the intestinal tract. Some strains invade solid organs, and occasionally form patchy necrotic foci in the liver [10] . The reasons why infection dynamics vary with SE strains and how SE strains reach the reproductive organs of chickens have not yet been examined. The properties of SE strains isolated from chicken farms were recently shown to differ from those of SE strains derived from patients with food poisoning. However, this has not yet been demonstrated experimentally.
Our studies on the infection dynamics of SE strains in chickens were introduced in this review. The developmental mechanisms of food poisoning, including their epidemiological roles in SE infection among chickens and mice in egg production farms, were also elucidated.
2. SE Food Poisoning in Japan
We are not specialists in the developmental mechanism of food poisoning after SE ingestion in humans; therefore, please refer to previous studies for more information. SE food poisoning has increased in Western countries since the mid-1980s. Various retrospective studies have been conducted on causative foods. SE-contaminated eggs have been recognized as a causative food since 1990. Fully fledged SE measures have been taken in chicken farms since the mid-1990s. Because of the development of hygiene management, vaccines, and liveviral agents, SE food poisoning has decreased since the late 1990s. The number of food poisoning cases in Japan was 494 in 1999, with a peak in the number of patients with food poisoning of 12,212 being reported in 1996, both of which have gradually decreased [11] (Figure 1).
SE food poisoning affects many patients. For example, of 116 cases of Salmonella food poisoning in 1996, each of which affected 10 or more patients, 88 cases were caused by SE. More than 50% of subsequent cases were caused by SE [12] .
3. Egg Production Farms
As described above, SE-contaminated eggs are recognized as a major cause of SE food poisoning; therefore, egg production farms were described as follows. Large-scale egg production farms have recently been constructed. There are multiple poultry houses in most farms, in which several tens of thousands of chickens are reared. In these farms, a group of chickens to be reared in a single poultry house is introduced simultaneously, and culled chickens are shipped together. In Western countries, culled chickens are shipped at approximately 500 days old. Molting is induced at 400 - 500 days old in Japan, and culled chickens are shipped at approximately 700 days old. The sexual maturation of chickens following their introduction into a farm is controlled by light management, which stimulates them to start laying eggs at approximately 140 days old. Neonatal chicks are highly susceptible to SE, and show nearly a 100% fatality when infected with SE before feeding starts after birth. They rarely die after feeding is started because they become less susceptible to SE. SE is more likely to colonize in the intestines of chickens at approximately 120 days old when light management is started. Chickens are more susceptible to SE after they start laying eggs, but present with no clinical symptoms if they have no stress. However, in the presence of stress factors, such as the induction of molting, egg-laying chickens may present with various clinical symptoms.
4. SE Isolation in Egg Production Farms in Japan
Two to six SE-contaminated eggs were isolated from 10,000 eggs in Japan in 1995 [13] . According to a survey in 2010, the detection rate of SE-contaminated eggs was reduced to approximately one-tenth; one SE-contaminated egg is generally detected in every 30,000 eggs [14] . Inactivated SE vaccines only are approved in Japan, and the amount of vaccines produced each year is approximately a quarter of the number of chickens in Japan. However, SE vaccines are used to inoculate approximately 50% of chickens, which suggests that most chickens are inoculated with less than the specified amounts of vaccines. More than 100 SE isolates from egg production farms are currently preserved in our laboratory. The biochemical properties of these SE strains were found to be similar to those of SE strains derived from patients with food poisoning. No SEp22 was expressed in 14 of the 30 strains isolated between 1990 and 1993. All 14 strains harbored the SEp22 gene (unpublished data). Only one strain had a deletion in the Inv A gene [15] . Humphrey et al. examined the expression of the O-12 antigen in
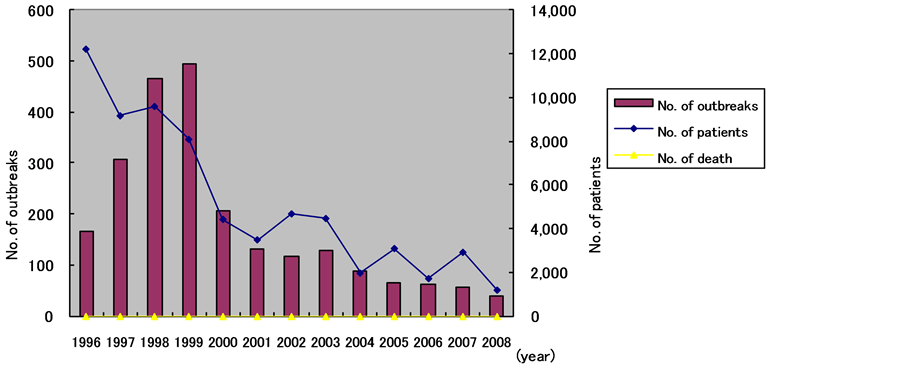
Figure 1. No. of outbreaks and patients caused by SE infections in Japan.
SE58 strains isolated from chicken farm environments, and demonstrated that no O-12 antigen was expressed in 15 strains [16] . Kauffmann et al. reported Salmonella O-antigen variations in the 1940s [17] -[20] . The O-12 antigen has three subclasses: O-121, -122, and -123. Deletions may occur randomly in some SE strains during theirpassage through chickens. However, whether such deletions occur at the genetic level remains unknown. In addition, the causes of such deletions remain unclear.
According to the findings of a recent study, the O chain may be elongated in SE strains isolated from chicken-rearing environments in order to avoid attacks from chicken complements. Some SE strains isolated from chicken-rearing environments may carry O antigens that have properties different from those of strains derived from patients with food poisoning. This needs to be further examined with reference to the results of other studies.
5. SE Infection and Immune Responses in Humans and Mice
5.1. SE Infection and Immune Responses in Humans
The kinetics of SE infection in humans has been investigated in other studies. Briefly, humans present with the characteristic localized symptoms of infection (e.g., diarrhea) for several hours or days after the ingestion of SE-contaminated food. Most gradually recover several hours or days after the onset. Humans with SE infections have infectious gastroenteritis with diarrhea as a predominant symptom with stomach pain, vomiting, and fever (38˚C - 40˚C) is characterized by loose or watery stools in most cases and mucous and bloody stools in severe cases. Micturition pain (urethritis), arthralgia (arthritis), ocular pain (conjunctivitis), and slight fever develop and persist for several months or years in a small number of cases. Infants and elderly are at high risk of bacteremia. Infants occasionally have acute encephalopathy with convulsions and impaired consciousness during the course of nontyphoidal Salmonella enteritis (i.e., Salmonella encephalopathy) [21] . At least 22 cases of SE infection (including one case caused by the O9 group) were reported between 1994 and 2003. Such diarrhea caused by SE infection in humans represents non-typhoid diarrhea, followed by an acute or subacute course.
Immunological reactions to SE and resistance to SE reinfection in humans have not been fully elucidated. Salmonella infections have generally been investigated in mouse models, with the relationship between innate immunity and Salmonella infection being examined in more detail. Highly toxic Salmonella infections impair dendritic cell functions [22] [23] . Salmonella infections cause diarrhea by changing the intestinal flora, due to the effects of Salmonella on the innate immune system. The relationship between the innate immune system and Salmonella infections is currently being investigated by many researchers (See other articles).
5.2. SE Infection and Immune Responses in Mice
SE infection dynamics and immune responses have been investigated in mice to provide a model of SE infection in humans. Only the efficacy of poultry live vaccines for Salmonella infections has been evaluated to epidemiologically investigate SE infections in mice [24] . Thus, most studies have been conducted using SE trains derived from patients with food poisoning. The mass mortality of mice in egg production farms after primary SE infection has not yet been reported, although most SE-infected mice die in laboratories.
6. Outline of SE Infection Dynamics in Chickens
SE infection in chickens is outlined below. Chickens, excluding neonatal chicks, typically remain asymptomatic following the ingestion of SE. SE excretion starts several hours after its ingestion and continues for several days. Chickens are highly resistant to SE infections during the rearing period. SE excretion has been shown to continue for as long as several weeks. The susceptibility of laying chickens to SE infections varies with age; it is highest in neonatal chicks and most chicks die after its ingestion. Susceptibility then markedly decreases. SE did not colonize the intestine in chickens that ingested 108 CFU/bird several days after birth, and no SE was isolated from the intestine. However, susceptibility increases again at the laying age, with an increase being observed in the colonization rate after infection. It then slightly decreases. However, chickens at the laying age are less resistant to SE infection than those in the rearing period, facilitating colonization. Susceptibility increases as chickens grow. Age-related changes in the susceptibility of chickens to SE infection are shown schematically [25] . Susceptibility is increased by various stress factors (e.g., induction of molting) [26] [27] . SE has the highest affinity for eggs from Salmonella bacteria.
SE is generally ingested orally by chickens, after which it colonizes the intestinal epithelium in the gastrointestinal tract. SE has flagella and is in an active phase. However, SE ingested orally loses its flagella and is in a stationary (colonial) phase when it colonizes the intestinal epithelia. SE expresses flagella again when it invades the solid organs through the intestinal tract wall in an active or stationary phase, and progresses to an invasive phase [28] -[30] . However, no study has been published on diarrhea in SE-infected chickens. Thus, unlike SEinfected humans and mice, SE in the intestinal epithelia of most SE-infected chickens is presumably in a stationary phase and does not damage the intestinal epithelia of chickens. The mechanism by which SE passes through the intestinal tract wall in laying chickens remains a matter of speculation. The mechanism underlying the phase transition from the active to stationary phase in SE in the intestinal tract of chickens has not yet been elucidated in detail. Macrophage cells have been shown to extend filopodia to SE in the intestines of SE-infected mice. However, such a phenomenon has not been reported in chickens.
7. Immune Responses in SE-Infected Chickens
Many studies have been conducted on immune responses in SE-infected chickens in order to develop SE vaccines. Although the antibody response to bacterial antigens is commonly observed during SE infections, no such response to the flagella antigen has been observed in 4-week-old or older chickens [31] . However, SE infections around the time of molting promote the production of flagellar antibodies [32] , and this has been observed when SE expressing a large amount of the flagella antigen grows in the solid organs of chickens. Chicken lymphocytes were shown to be activated by flagella antigen, but not by bacterial and ciliary antigens during cell-mediated immune responses to SE components [33] . In the present study, sufficient immune competence as vaccines can only be induced with the g.m. site of the flagellar antigen [34] . However, the amino acid sequence of the g.m. antigenic site used in the present study was different from that of the previously reported amino acid sequence of the g.m. antigenic site. The effects of this difference on the immune induction capability of the vaccine remain unclear. Western blotting using the serum components of SE-infected field chickens revealed no characteristic patterns. However, a few 32-42-kDa bands were observed, suggesting the potential immune response to SE components during SE infection (unpublished data).
8. SE Contamination Routes in Egg Production Farms and SE Infection Routes to the Reproductive Organs of Chickens
Two routes are possible for SE to infect the reproductive organs (e.g., ovaries). One route is that SE attached to the cloaca migrates upward to reach the top of the fallopian tube and ovaries. The other route is that SE passes through the intestinal tract wall and reaches the reproductive organs through the lymph or blood. An intravaginal SE inoculation test showed that SE migrated upward in the fallopian tube [35] . On the other hand, an SE infection experiment in chickens revealed a higher isolation rate for reproductive organs than solid organs. Recent studies have been conducted on the assumption that SE passes through the intestinal tract wall and reaches the reproductive organs.
9. Roles of Mice in SE-Contaminated Egg Production as a Cause of Food Poisoning in Egg Production Farms
Elucidating the roles of mice in SE contamination in egg production farms is the most important issue in this study. We initially understood that SE strains isolated from egg production farms had the same properties as those of SE strains derived from patients with food poisoning, and considered that all SE strains isolated in egg production farms cause food poisoning. However, SE was also detected in egg production farms that we managed as veterinary clinics. Thus, why no food poisoning occurred through SE-contaminated eggs produced there and also why the incidence of food poisoning was relatively low in spite of the large number of SE strains isolated from chicken farms remained unclear. The reason for the absence of mass mortality in spite of the large number of mice in chicken farms with SE primary infections was also unknown. Thus, we identified a slight difference in pathogenicity between SE strains isolated from egg production farms and those derived from patients with food poisoning in the early 1990s. However, the reason why SE-contaminated eggs become a major cause of SE food poisoning has yet to be established. Several studies have attempted to answer these questions. Approximately half of the SE strains isolated from egg production farms do not express SEp22 (Amano F., Osaka University of Pharmaceutical Sciences, unpublished data) whereas a relatively large number of strains isolated from egg production farms expressed the O-12 antigen [16] . Some strains isolated from egg production farms have elongated O-chains [36] -[38] .
An SE strain (SE#15h) isolated from egg production farms, which was pathogenic to mice, was intranasally administered to 20-day-old mice and laying chickens, followed by the isolation of SE strains (SE#15 m and SE#15c) to compare their properties. (Mouseand laying chicken-passaged SE strains are shown in Table 1). The expression ratio of the O-12 antigen to the O-9 antigen was determined by ELISA. SEp22 expression levels were compared by Western blotting (Figure 2). The pathogenicity of neonatal chicks and laying chickens was compared. As shown in the following Tables and Figures, the O-12/O-9 antigen ratio of mouse-passaged SE strains increased and became similar to those of the SE strains derived from patients with food poisoning.
Table 2 (SE administration to laying chickens: A larger number of chicken-passaged SE strains were isolated from the intestine).
Figure 3 (Results of the oral administration of mouse-passaged SE strains to laying chickens: Punctate lesions were noted).
Table 1. SE strains used in this study.
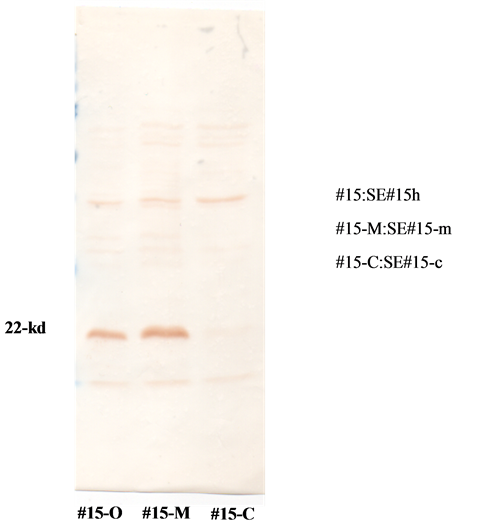
Figure 2. Expression of the 22 kDa-polypeptide (SEp22) by 3 SE strains: SE#15h, SE#15m, and SE#15c, was examined using Western blotting. Result; although the expression of SEp22 was observed by SE#15h and SE#15m, it was not by SE#15c.
Table 2. Aggregation in the mixed culture of the SE#15h strain in fresh chicken serum (FCS) under a microscope.
Aggregation was classified into − (no aggregation) to +++ (very strong aggregation). SE (108 CFU/0.1 ml) was mixed with various sera to observe aggregation under a microscope. SE aggregation was classified into − (no aggregation), + (weak aggregation), 2+ (strong aggregation), and 3+ (very strong aggregation). These results demonstrated that aggregation required magnesium ions and occurred even when the first complement pathway only was inactivated.
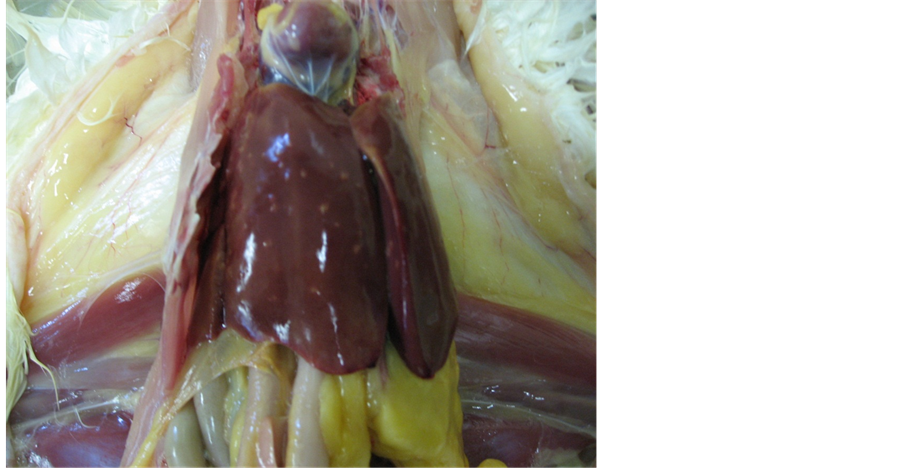
Figure 3. Postmortem appearance of liver abnormalities in chickens inoculated with the mouse-passaged SE strain. The strain (SE#15h-m) was passaged in mice and intra-orally inoculated into laying chickens (n = 8), which were necropsied 2 days post-inoculation. Abnormalities were observed in4 of 8 birds as white spotty lesions (this figure). Numerous SE could be isolated from these spotty lesionsusing a swab collection-smearing method on agar medium.
Figure 4 (Oral SE administration test in neonatal chicks: The survival time of chicks that were orally administered mouse-passaged SE strains was short, while that of chicks that were orally administered chicken-passaged SE strains was long).
Figure 5 (Comparison of the O-12/O-9 antigen ratios of various SE strains: The amount of O-12 antigen in the SE strains derived from patients with food poisoning was large, while that in the SE strains that we preserved was small).
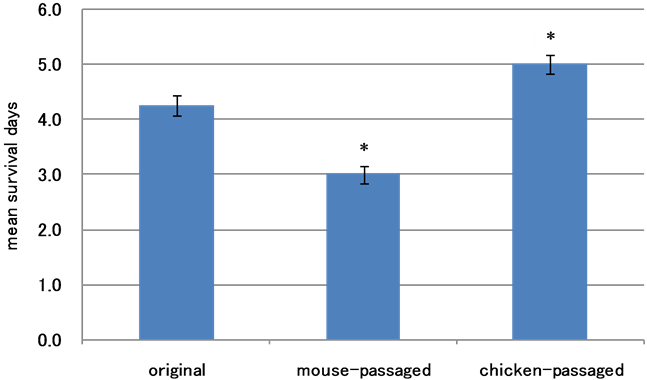
Figure 4. Survival of chickens inoculated with SE strains. The SE#15-c (106.72 CFU/0.1 ml/bird), SE#15h-m (106.43 CFU/0.1 ml/bird), or parent (106.57 CFU/0.1 ml/bird) strain was administered orally to Day-0 chicks. The mean survival date and standard error (SE) was determined for each group. As shown in this figure, significant differences were observed in survival between the three groups (*p < 0.05) even though all birds inoculated with SE died.
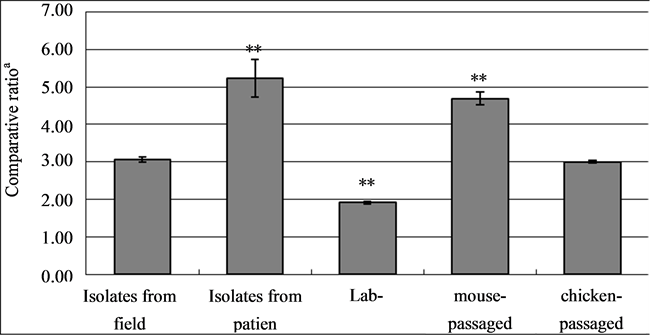
Figure 5. O antigen ratioa) (the amount of O-12 antigen/that of O-9 antigen) with various SE strains. Comparative O antigen ratios with the formalin-inactivated SE of various strains were examined by ELISA. Results; The SE strains used were 10 poultry field isolates (Isolates from the field), 5 patients (Isolates from patients), 4 labmaintained strains originating from poultry field isolates (4 colonies of the SE#15h strain were selected and used: lab-field), mouse-passaged strains (SE#15h was passaged in mice and 4 colonies were selected; mouse-passaged), and chicken-passaged strains (SE#15h was passaged in chickens and 4 colonies were selected; chickenpassaged) (a) comparative rate: O-12 antigen amount/O-9 antigen amount measured by ELISA). We compared the ratios of each SE groupbased on the O antigen ratio for field isolates (range shows the mean + standard error of the O antigen ratio). (**p < 0.01 based on that of isolates from the field).
The O-12/O-9 antigen ratios in the chicken-passaged SE strains were similar to those in SE strains isolated from farm environments. The O-12/O-9 antigen ratios in genetically identical SE strains were decreased through a passage in medium within a laboratory and increased by a passage in mice, and became similar to those in SE strains derived from patients with food poisoning.
We hypothesized the follow process:
1) SE-infected laying chickens excrete SE that is nonpathogenic to mice.
2) The excreted SE strains spread in farms, altering their properties to facilitate their entry into the solid organs of laying chickens. They subsequently reach the reproductive organs of chickens to contaminate eggs.
3) SE-contaminated eggs cause food poisoning.
Further studies are needed. We would like to receive advice, comments, and opposing views from many readers (the results will be reported in detail in scientific journals).
9.1. Materials and Methods
SE. Subculture of strains isolated from mice and chicken. The #15h strains isolated in layer farms were used in the present study (Table 1). The #15h strains belong to the PT1 type, i.e., field isolates which have undergone two subclonings (five subculturings from a colony). We have used these strains for various studies in our laboratory. The #15h strains are associated with a high mortality when orally administered to chicks immediately after hatching and develops no symptoms when orally administered to ≥3-week-old chickens [31] . Desoxycholate-hydrogen sulfide-lactose agar medium (DHL, CTL No. 1.11435.0500, Merck, Darmstadt, Germany) was used for subculture. HI agar medium (HI, Code No. 05505, Nissui Tokyo, Japan) was used for cloning. The #15h strains were cultured in DHL agar medium in 6-cm diameter dishes for 18 - 24 hours, after which 5 ml of Brain heart infusion medium (BHI, Nissui Pharmaceuticals, Tokyo, Japan) was added to the culture. Colonies were collected, mixed thoroughly, and transferred into 25 ml of BHI medium. After 2-hour culture at 37˚C, the culture was centrifuged at 2000 g for 15 minutes. To the pellet, 30 ml of BHI was added to be administered to chickens and mice. The biochemical properties of the strains were investigated using an identification kit for chemical characteristics with Enterobacteriaceae (ID kit/EB-20, Code No. 06626, Nissui) according to the manufacturer’s instructions.
The SE #15h strain (about 109 CFU/ml/bird; CFU: colony forming unit) was administered to 150-day-old SPF chickens. Two days later, the chickens were anesthetized to remove ceca. The ceca were weighed, and 9 volumes of PBS were added to homogenize the tissues. The homogenates were cultured in DHL agar medium containing the strain at various concentrations. Eighteen hours later, four colonies were selected from 10 - 50 colonies that grew in the dish, and were used as SE#15h-c strains.
To subculture the SE#15h strain in mice, the SE#15h strain (about 108 CFU/0.1 ml/bird) was orally administered to five BALB/c mice. Two days later, the mice were anesthetized for laparotomy to remove the digestive tracts. The digestive tracts were weighed. Nine volumes of PBS were added to conduct procedures similar to those for chicken samples. Four colonies were picked up from mouse samples to be used as SE#15h-m strains in this experiment.
SE strains used to compare O-antigen ratios. The parent strains, strains subcultured in chickens or mice, 10 strains isolated in layer farms, and six strains isolated from patients which were kindly given from Dr. H. Izumiya in National In statute of Infectious Disease Japan, were used.
Animals.
Animal experiments.
O-antigen ratio (O-9/O-12 antigen ratio). As described in 3.1, formalin was added to a bacterial suspension in BHI to a final concentration of 0.2%, followed by inactivation at 37˚C for 16 hours. After inactivation, the bacteria were washed three times by centrifugation to remove formalin. Finally, the bacteria were suspended in BHI and stored in a freezer at −80˚C for the subsequent experiments. The frozen bacterial solution was thawed, followed by centrifugation at 3000 rpm for 20 minutes. The supernatant was discarded, and distilled water was added to resuspend the bacterial pellet. Distilled water was added to the suspension to adjust the absorbance (OD value; OD: optimum density) at 440 nm to 0.6. Coating buffer was added to 1 ml of the bacterial suspension. The bacterial suspension was dispensed into plate wells. Subsequently, the plate was swirled and coated at 4˚C overnight. All the bacterial suspensions to be tested were coated onto the same plate for enzyme reaction. Anti-Salmonella O-9 (Funakoshi, CTL No. FU82095309, Tokyo) and O-12 (Funakoshi, CTL No. FU82095349, Tokyo) monoclonal antibodies were 200-fold diluted to be used as primary antibodies in enzyme reaction. Anti-mouse IgG-specific antibody (Funakoshi, CTL No. 610-4302, Tokyo) was 3000-fold diluted to be used as a secondary antibody. Subsequently, 0.1 ml each of substrate (OPD: Sigma, CTL NO. P-9817) was added to the wells, followed by incubation at 25˚C for 10 minutes. Then, 0.1 ml of 3N sulfuric acid was added to the wells to stop the reaction. The absorbance (OD value) at 490 nm was determined for each combination of SE strain and O-antigen. The mean O-9/O-12 antigen ratio was determined (4 or more wells were used for one combination). O-antigen ratios were determined twice for each sample. The mean value of the two O-antigen ratios was taken as the O-antigen ratio of the sample.
9.2. Results
Survival time of chicks which received the SE#15h-m, SE#15h-c, or parent strain. The survival time of chicks which received the SE#15h strain (parent strain) (106.72 CFU/0.1 ml/ bird) or the strain subcultured in mice (106.43 CFU/0.1 ml/ bird) or chickens (106.57 CFU/0.1 ml/ bird) was examined. As shown in Figure 4, the chicks which received the parent strain survived for 4.25 ± 0.19 days (mean ± 2SE). The survival time of chicks which received the strain subcultured in mice was 3.00 ± 0.16 days. The survival time of chicks which received the strain subcultured in chickens was 5.00 ± 0.16 days.
Proliferative capacity of laying chickens which received each subcultured strain and lesions at necropsy. The SE#1 5h-c or SE#15h-m strain (about 109 CFU/ml/bird) was orally administered to laying chickens. On day 2, the chickens were necrotized to examine SE proliferation (Data not shown) The SE isolation rates were low for the spleens and livers of chickens which received the strain subcultured in chickens. SE was isolated from the ampulla of the uterine tube and vagina of chickens which received the strain subcultured in mice. However, SE was isolated from only one of eight chickens, thus precluding a comparison. In contrast, the SE isolates from the digestive tract showed high proliferative capacity. No abnormality was observed at necropsy for the strains subcultured in chickens. White spotty lesions were observed for four of the eight strains subcultured in mice (Figure 3). SE strains were isolated from the swabs of these lesions at high rates.
Antigen ratios (O-12/O-9 antigens) of the SE#15h-m and SE#15h-c strains, 10 strains isolated from laying chickens, and strains isolated from patients. The O-antigen ratios of 10 field SE isolates were 3.07 ± 0.07 (mean ± SE) (Figure 5), while those of the SE strains isolated from patients were 5.24 ± 0.50. The O-antigen ratios of four SE#15h-m strains, subcultured in mice, were 4.69 ± 0.17 (mean ± SE). Those of four SE#15h-c strains, subcultured in chickens, were 3.00 ± 0.04 (mean ± SE). The O-antigen ratios of the parent SE#15h strains (n = 4) were 1.91 ± 0.03 (mean ± SE). The O-antigen ratios of the strains isolated from patients and subcultured in our laboratory were 1.72 ± 0.10. As compared with the O-antigen ratios of 10 strains isolated from layer farms, those of the strains isolated from patients and the strains subcultured in mice were significantly high, while those of the strains subcultured in two laboratories (parent and Y-24 strains) were significantly low. The O-antigen ratios of the strains subcultured in chickens were comparable with those of the 10 strains isolated in layer farms.
10. Activation of Chicken Complements by SE
Interactions with the immune system of chickens, particularly the innate immune system, may play an important role in SE-contaminated egg production during the early phase of SE infection. In this section, two SE strains (SE#1 h and SE#15e strains) of the same origin were used to examine the relationship between chicken complements, which play a major role in the innate immune system, and SE. Unfortunately, the chicken innate immune system has not been examined in detail. The activation of chicken complements (alternative pathway) was examined using a classical method that we established in the 1980s [39] . The antibody-independent bacteriolytic effects of chicken complements were examined. As shown in Figure 6, the number of SE#15h strains decreased to approximately one-thousandth after a 3-hour mixed culture in fresh chicken serum (FCS), which indicated bacteriolytic effects. The strain started growing 6 hours after the start of the mixed culture. At 18 hours, its number increased that observed after the mixed culture in heat-inactivated chicken serum. Such a phenomenon was not observed for the SE#15e strain with the same origin as that of SE#15h (Figure 7). To examine whether the bacteriolytic effects of FCS on the SE#15h strain were caused by activation of the second complement pathway, FCS was treated with heat, inulin, and carrageenan. Bacteriolytic effects were only maintained with the carrageenan treatment, suggesting that bacteriolytic effects on the SE#15h strain were caused by the second complement pathway. However, the SE#15e strain had identical properties, such as drug resistance, biochemical properties, serological properties, morphological properties of colonies (S-T-R, O-antigen, and M-N changes), relative amount of the Vi antigen (Vi antigen change), and relative amount of the flagellar antigen (H-O and phase changes), which suggested the lack of bacteriolytic effects by FCS on the SE#15e strain. This mechanism for this remains unknown. SE#15h and SE#15e strains were cultured in FCS to determine changes in the relative amounts of flagella (H-G antigen). The amount of the H-G antigen decreased immediately after mixing when the SE#15h strain was cultured in FCS, suggesting that flagella may be detached from the bacterial body. No decrease in the H-G antigen was observed in the SE#15e strain, with flagella attached to the bacterial body. The SE#15h and SE#15e strains were incubated in FCS or its heat-treated serum at 37˚C for 30 minutes to observe aggregation under a microscope. Both strains showed bacterial aggregation when cultured in FCS. However, no aggregation was observed when cultured in heat-treated serum. The SE#15e strain caused a smaller amount of aggregates in FCS than the SE#15h strain. The aggregation that occurred through the reaction between the SE#15h strain and FCS was not observed for heat, inulin, or FCS-treated mammalian erythrocytes(inactivation of the first and second complement pathways), but was observed for carrageenan-treated serum (inactivation of the first complement pathway) (Table 2). Aggregation required magnesium ions, but not calcium ions. Thus, the reactivity of chicken complements was markedly different between SE strains of the same origin. These phenomena preclude elucidation of the interaction between the chicken innate immune system and SE.
The pathogenicity of the #15h strain that reacted with FCS (SE surviving bacteriolysis) was subsequently examined. The SE#15h strain that reacted with FCS or heat-inactivated serum was administered orally to neonatal chicks. As shown in Table 3, the strain was isolated from chicks receiving the SE#15h strain that reacted with FCS; no deaths were observed when a small number (102) of the strain was administered to these chicks. Deaths were observed at a low rate among neonatal chicks receiving a small number (101) of the SE#15h
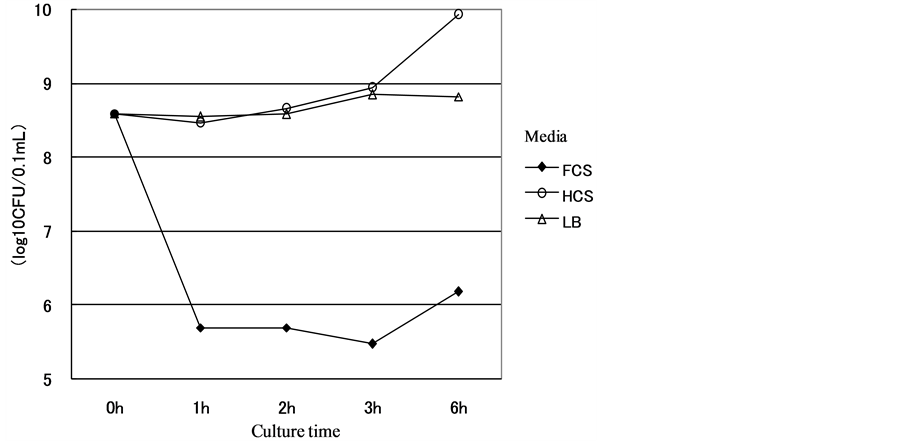
Figure 6. Growth curve of the SE#15h strain in FCS.

Figure 7. Growth curve of the SE#15e strain in FCS. Results: The numbers of SE#15h and SE#15e strains cultured in FCS or HCS were examined. The results are shown in the figures. The number of the SE#15h strain before the start of the culture was 3.9 × 108 (colony forming unit: CFU/ml), while that after the 1 - 6-hour cultures decreased to 3.0 - 15 × 105 CFU in FCS and 2.9 - 8.5 × 108 CFU in HCS. The number of the SE#15h strain cultured in non-serum (LB) medium was 3.8 - 7.0 × 108 CFU. Thus, the number of the SE#15h strain cultured in FCS was significantly lower than that in LB. On the other hand, the number of the SE#15h strain cultured in FCS medium was 5.5 × 108 CFU at the start and 4.5 - 60 × 108 CFU after 1 - 6 hours, while that in HCS was 5.7 - 15 × 107 CFU. The number of the SE#15h strain after the 6-hour culture in LB medium was similar to that in FCS medium (5.0 - 6.1 × 108 CFU). The experiments were repeated twice, which ensured that the results were reproducible.
Table 3. Pathogenicity of the SE#15h strain in FCS or HCS administered orally to 2-day-old chicks.
Number of bacteria administered (CFU/bird); The SE#15h strain cultured in FCS or HCS was administered orally to neonatal chicks to determine mortality. Chicks that received 3.3 × 102 CFU or below of the SE#15h strain in FCS did not die, while some chicks that received the same amount of the SE#15h strain in HCS died within 3 - 5 days after its administration. Thus, complement-treated SE could not invade the solid organs of neonatal chicks.
strain that reacted with heat-inactivated serum, and the strain was isolated from the chicks. Thus, the reaction with FCS may have effects on at least the pathogenicity of some SE strains. (The results described in this review will be published in more detail in anotherscientific journal.)
10.1. Materials and Methods
SE. The SE#15 strain of SE was isolated from an open-house-chicken farm (Table 1). One phage-type strain cloned at the National Institute of Infectious Diseases was passaged at Osaka University of Pharmaceutical Sciences and designated as the SE#15e strain. After identification by the National Institute of Infectious Diseases (Shinjuku, Tokyo), the #15e strain was supplied to our laboratory, and passaged as the SE#15h strain.
Chickens and preparation of chicken serum. Embryonating chicken eggs were purchased from SPAFAS Co. (Preston CT) or Nippon Institute for Biological Science (NIBS), Oume, Tokyo), and hatched and grown to chickens in our laboratory. Blood was collected from the birds under an anesthetic at 5 - 7 weeks of age, and sera were individually separated. The sera were individually heated at 56˚C for 30 minutes, and stored at −70˚C until use. Untreated serum was designated as fresh chicken serum (FCS). Hemolytic activity of FCS [39] was detected at 1:16 - 32 in the classical pathway and 1:8 - 16 in the alternative pathway.
SE growth test in media containing FCS and heat-treated chicken serum (HCS). Five ml of phosphate buffer (PBS) was added to each of the #15h and #15e strains passaged on deoxycholate agar (DHL agar medium, Nissui Pharmaceutical, Tokyo). The bacteria were collected and adjusted with PBS to a total volume of 25 ml. These bacterial suspensions were centrifuged at 3000 rpm for 5 minutes, and each precipitate was combined and mixed well with 25 ml, from which 2.5 ml was centrifuged at 3000 rpm for 20 seconds. The precipitate was combined and mixed well with 2.5 ml of FCS or HCS prepared from chickens purchased from NIBS, followed by incubation at 37˚C. From each bacterial suspension, 0.3 ml was sampled 1, 2, 3, and 6 hours after the initiation of incubation, and the bacteria were counted. As a serum-free control culture, each strain was incubated with LB medium (Luria-Bertani, Becton Dickinson, MD). For counting SE after incubation, each dilution was spread on 4 dishes, and the counts were statistically analyzed.
Observation of aggregation after incubation of SE with various serum preparations. Each SE strain was precultured in brain heart infusion (BHI) broth (Becton Dickinson, MD) at 37˚C for 3 hours. The precultured bacteria were centrifuged at 3000 rpm for 5 minutes, and suspended with trypticase soy (TS) broth (Becton Dickinson, MD). The bacterial count in the suspension was about 109 CFU/ml in all tests. A portion (0.1 ml) of the SE suspension in TS broth was combined with an equivalent volume of FCS or HCS prepared from SPF chickens supplied by NIBS, incubated at 37˚C for 30 minutes, and centrifuged at 1000 rpm for 1 minute. The precipitate (SE) was suspended with 10 volumes of PBS, and this suspension was distributed onto a microplate at 0.1 ml/well and observed under a microscope. SE aggregation was classified into: no (−), weak (+), strong (2+), and strongest (3+) aggregations.
To investigate the SE component that causes aggregation, bacteria were incubated with FCS or HCS purchased from NIBS on microplates coated with #15h-derived deflagellated SE somatic antigen, the g.m. region [31] was purified by genetic engineering, and aggregates were observed under a microscope. SE aggregation was classified into 4 levels as abovementioned.
Inactivation of serum complement and chelation of Ca and Mg ions. Both complement pathways were inactivated by treatment with inulin (Wako, Osaka), zymosan A (Sigma, St. Louis, MO), carrageenan (Sigma), and commercial mammalian red blood cells (sensitized sheep or horse RBC: 50% packed volume RBC in PBS, horse RBC: 50% packed volume RBC in PBS). Specifically, inulin, zymosan, and carrageenan were individually-added to FCS purchased from NIBS at 2 mg/ml and reacted at 37˚C for 30 minutes, followed by centrifugation at 3000 rpm for 15 minutes. The supernatants were passed through 0.2 mm filters. Horse and sensitized sheep RBC were reacted with FCS at 37˚C for 30 minutes, and similarly filtered, respectively.
Ca and Mg ions were chelated by adding 0.2 ml of 10 mM ethylenediamine-tetraacetate (EDTA) (Wako Pure Chemicals, Tokyo, in PBS) to 1 ml of FCS. The classical complement pathway was inactivated with carrageenan, and Ca ions were chelated by adding 1 ml of 10 mM ethylene-glycol-bis-aminoethylether-N-N’-tetraacetate (EGTA) plus MgSO4, (Wako, in PBS) [39] . 1984).
10.2. Results
SE cultured in media containing FCS and HCS. The #15h and #15e strain bacteria were counted after incubation with FCS and HCS for 1 - 6 hours. The results are shown in Figure 6, Figure 7. The preincubation count of the #15h strain was 3.9 × 108 CFU/ml. The counts after incubation with FCS for 1 - 6 hours were 3.0 - 15.0 × 105 CFU, which were significantly fewer than those after incubation with HCS (2.9 - 8.5 × 108 CFU). The counts in serum-free (LB) medium were 3.8 - 7.0 × 108 CFU, showing that the counts after incubation with FCS were also significantly lower than this. When the #15e strain was incubated with FCS, the counts at the time of initiating incubation and after incubation for 1 - 6 hours were 5.5 × 108 and 4.5 - 6.0 × 108 CFU, respectively, and that after incubation with HCS was 5.7 - 15 × 107 CFU, showing no significant difference. The #15e count after incubation in LB medium for 6 hours was not measured, but it was similar to that after incubation with FCS (5.0 - 6.1 × 108 CFU). This test was repeated two more times, and similar results were obtained (data not shown).
Aggregation of the #15h and #15h strains in incubation with FCS. When the #15h strain was incubated with 4 sera each prepared from SPF chickens purchased from SPAFAS and NIBS, 8 sera in total, aggregation occurred in all groups, but not in any group incubated with HCS. Thus, FCS prepared from chickens supplied by NIBS was used in the tests below. When the #15e strain was incubated with FCS, weak aggregation was noted, but not after incubation with HCS. The complement activity of FCS was inactivated, and reacted with SE to investigate whether inactivated FCS aggregates SE. Sera were treated with inulin, carrageenan, zymosan, sensitized sheep RBC, and horse RBC to inactivate complement, and then reacted with the #15h strain. Only serum treated with carrageenan caused aggregation (Table 2). The dependence on the FCS factor to induce #15h aggregation in the presence of bivalent ions was investigated. No bacterial aggregation was caused by serum in which Ca and Mg ions were chelated; aggregation occurred when only Ca ions were chelated.
On both microplates coated with deflagellated bacterial somatic and g.m. antigens, aggregates were noted under a microscope when incubated with FCS, but not when incubated with HCS.
11. Discussion of Sections 10 and 11, Studies Unpublished in This Review
The properties of SE strains isolated from chicken-rearing environments were examined, and indicated the existence of two kinds of O antigens. O-9 and -12 antigenmeasurements demonstrated that strains isolated from chicken-rearing environments could be divided into two groups: strains expressing these antigens at the same level and those expressing the O-12 antigen at a higher level than that of the O-9 antigen. O-12 antigen levels in the former group were the same as those in chicken-passaged strains, while those in the latter group were the same as those in mouse-passaged strains and similar to those in SE strains derived from patients with food poisoning. Thus, the SE strains of both the former and latter groups have been identified in chicken-rearing environments. As described above, some SE strains undergo the bacteriolytic effects of complements contained in FCS, while others do not. The bacteriolytic effects of complements contained in FCS on mouseor chickenpassaged strains should be examined to demonstrate the epidemiological significance of mice and chickens in SE food poisoning. The mouse-passaged SE strain (SE#15m) that invaded the solid organs of chickens may not undergo the bacteriolytic effects of complements contained in FCS. Further studies are needed to clarify this.
The properties of SE strains that survived bacteriolysis caused by complements contained in FCS should also be examined. Differences in the properties of mouseand chicken-passaged SE strains may be of epidemiological significance. The results described in this review should provide clues to our questions. Most working hypotheses can generally not be demonstrated. The properties of SE strains isolated in egg production farms are largely divided into two kinds based on the presence or absence of SEp22. This should be important in order to demonstrate our working hypothesis. SE strains with the properties (SEp22+) of mouse-passaged strains are pathogenic to mice and humans, while those with the properties (SEp22-) of chicken-passaged strains are infectious, but not pathogenic to mice, and this facilitates the spread of SE in chicken farms. Thus, SE strains colonize egg production farms through alternate infections between mice and chickens because mice cannot be completely eliminated from chicken farms. In other words, large-scale egg production farms that allow alternate infections between mice and chickens may cause food poisoning through contaminated eggs. SE infections in chickens have been described in many studies. Please refer to them for more information [40] -[42] . We expect more studies to be conducted on this epidemiological issue.
Acknowledgements
We published this review in response to an invitation mail from the International Journal of Clinical Medicine (IJCM). Even though the contents of this review remain controversial, its publication was strongly recommended. We thank the IJCM for giving us the opportunity to publish this review.
We also thank the following people for their support and advice regarding the studies described in this review.
1) Shizunobu Igimi, Director of the National Institute of Health Sciences.
2) Fumio Amano, Professor of Osaka University of Pharmaceutical Sciences.
3) Hidemasa Izumiya, Director of the National Institute of Infectious Diseases.
References
- Koupal, L.R. and Deibel, R.H. (1975) Assay, Characterization, and Localization of an Enterotoxin Produced by Salmonella. Infection and Immunity, 11, 14-22.
- Tamura, A., Yamasaki, M., Okutani, A., Igimi, S., Saitoh, N., Ekawa, T., Ohta, H., Katayama, Y. and Amano, F. (2009) Dry-Resistance of Salmonella enterica Subsp. Enterica Serovar Enteritidis Is Regulated by Both SEp22, a Novel Pathogenicity-Related Factor of Salmonella, and Nutrients. Microbes and Environments, 24, 121-127. http://dx.doi.org/10.1264/jsme2.ME09111
- Varga, C., Pearl, D.L., McEwen, S.A., Sargeant, J.M., Pollari, F. and Guerin, M.T. (2013) Incidence, Distribution, Seasonality, and Demographic Risk Factors of Salmonella Enteritidis Human Infections in Ontario, Canada, 2007-2009. BMC Infectious Diseases, 13, 212. http://dx.doi.org/10.1186/1471-2334-13-212
- Babu, U.S., Sommers, K., Harrison, L.M. and Balan, K.V. (2012) Effects of Fructooligosaccharide-Inulin on Salmonella-Killing and Inflammatory Gene Expression in Chicken Macrophages. Veterinary Immunology and Immunopathology, 149, 92-96. http://dx.doi.org/10.1016/j.vetimm.2012.05.003
- Johnston, C.E., Hartley, C., Salisbury, A.M. and Wigley, P. (2012) Immunological Changes at Point-of-Lay Increase Susceptibility to Salmonella enterica Serovar Enteritidis Infection in Vaccinated Chickens. PloS ONE, 7, e48195. http://dx.doi.org/10.1371/journal.pone.0048195
- Kogut, M.H., Genovese, K.J., He, H., Swaggerty, C.L. and Jiang, Y. (2013) Modulation of Chicken Intestinal Immune Gene Expression by Small Cationic Peptides as Feed Additives during the First Week Posthatch. Clinical and Vaccine Immunology, 9, 1440-1448. http://dx.doi.org/10.1128/CVI.00322-13
- Matulova, M., Varmuzova, K., Sisak, F., Havlickova, H., Babak, V., Stejskal, K., Zdrahal, Z. and Rychlik, I. (2013) Chicken Innateimmune Response to Oral Infection with Salmonella enterica Serovar Enteritidis. Veterinary Research, 44, 37. http://dx.doi.org/10.1186/1297-9716-44-37
- Kogut, M.H., Rothwell, L. and Kaiser, P. (2003) Differential Regulation of Cytokine Gene Expression by Avian Heterophils during Receptor-Mediated Phagocytosis of Opsonized and Nonopsonized Salmonella Enteritidis. Journal of Interferon & Cytokine Research, 23, 319-327. http://dx.doi.org/10.1089/107999003766628160
- Sebkova, A., Karasova, D., Crhanova, M., Budinska, E. and Rychlik, I. (2008) Aro Mutations in Salmonella enterica Cause Defects in Cell Wall and Outer Membrane Integrity. Journal of Bacteriology, 9, 3155-3160. http://dx.doi.org/10.1128/JB.00053-08
- He, G.Z., Tian, W.Y., Qian, N., Cheng, A.C. and Deng, S.X. (2010) Quantitative Studies of the Distribution Pattern for Salmonella Enteritidis in the Internal Organs of Chicken after Oral Challenge by a Real-Time PCR. Veterinary Research Communications, 34, 669-676. http://dx.doi.org/10.1007/s11259-010-9438-6
- Toyofuku, H. (2008) Epidemiological Data on Food Poisonings in Japan Focused on Salmonella, 1998-2004. Food Additives & Contaminants, Part A, Chemistry, Analysis, Control, Exposure & Risk Assessment, 25, 1058-1066.
- Yoshida, I., Hayashi, Y., Katayama, K. and Yamada, S. (1998) Bacteriological and Virological Studies on the Cause of Sporadic Acute Gastroenteritis in Tama, Tokyo (1991-1996). Kansenshogaku Zasshi, 72, 599-608.
- Murase, M., Kurokawa, M., Nukina, M., Nakanishi, H. and Haruta, T. (2001) Surveillance of Various Enteropathogenic Bacteria from Diarrheal Cases during 1989-1999 in Kobe City. Kansenshogaku Zasshi, 75, 883-893.
- Esaki, H., Shimura, K., Yamazaki, Y., Eguchi, M. and Nakamura, M. (2013) National Surveillance of Salmonella Enteritidis in Commercial Eggs in Japan. Epidemiology and Infection, 141, 941-943. http://dx.doi.org/10.1017/S0950268812001355
- Ekawa, T., Terai, S., Amano, F., Hanatani, Y. and Ohta, H. (2009) Diverse Pathogenicity of Salmonella Enteritidis Clones Isolated from Poultry Farms in Chicks and BALB/c Mice. Japanese Journal of Poultry Science, 46, 370-376. http://dx.doi.org/10.2141/jpsa.46.370
- Chart, H., Rowe, B., Baskerville, A. and Humphrey, T.J. (1990) Serological Response of Chickens to Salmonella Enteritidis Infection. Epidemiology and Infection, 104, 63-71. http://dx.doi.org/10.1017/S0950268800054534
- Guard-Petter, J. (1998) Variants of Smooth Salmonella enterica Serovar Enteritidis That Grow to Higher Cell Density than the Wild Type Are More Virulent. Applied and Environmental Microbiology, 64, 2166-2172.
- Guard-Petter, J., Keller, L.H., Rahman, M.M., Carlson, R.W. and Silvers, S. (1996) A Novel Relationship between O-Antigen Variation, Matrix Formation, and Invasiveness of Salmonella Enteritidis. Epidemiology and Infection, 117, 219-231. http://dx.doi.org/10.1017/S0950268800001394
- Parker, C.T., Liebana, E., Henzler, D.J. and Guard-Petter, J. (2001) Lipopolysaccharide O-Chain Microheterogeneity of Salmonella Serotypes Enteritidis and Typhimurium. Environmental Microbiology, 3, 332-342. http://dx.doi.org/10.1046/j.1462-2920.2001.00200.x
- Ross, I.L. and Heuzenroeder, M.W. (2009) A Comparison of Two PCR-Based Typing Methods with Pulsed-Field Gel Electrophoresis in Salmonella enterica Serovar Enteritidis. International Journal of Medical Microbiology, 299, 410- 420. http://dx.doi.org/10.1016/j.ijmm.2008.12.002
- Arii, J., Tanabe, Y., Miyake, M., Mukai, T., Matsuzaki, M., Niinomi, N., Watanabe, H., Yokota, Y., Kohno, Y. and Noda, M. (2002) Clinical and Pathologic Characteristics of Nontyphoidal Salmonella Encephalopathy. Neurology, 58, 1641-1645. http://dx.doi.org/10.1212/WNL.58.11.1641
- Crhanova, M., Hradecka, H., Faldynova, M., Matulova, M., Havlickova, H., Sisak, F. and Rychlik, I. (2011) Immune Response of Chicken Gut to Natural Colonization by Gut Microflora and to Salmonella enterica Serovar Enteritidis Infection. Infection and Immunity, 79, 2755-2763. http://dx.doi.org/10.1128/IAI.01375-10
- Matiasovic, J., Stepanova, H., Volf, J., Kubala, L., Ovesna, P., Rychlik, I. and Faldyna, M. (2011) Influence of the Lipopolysaccharide Structure of Salmonella enterica Serovar Enteritidis on Interactions with Pig Neutrophils. Veterinary Microbiology, 150, 167-172. http://dx.doi.org/10.1016/j.vetmic.2011.01.007
- Mitra, A., Loh, A., Gonzales, A., Laniewski, P., Willingham, C., Curtiss III, R. and Roland, K.L. (2013) Safety and Protective Efficacy of Live Attenuated Salmonella Gallinarum Mutants in Rhode Island Red Chickens. Vaccine, 31, 1094-1099. http://dx.doi.org/10.1016/j.vaccine.2012.12.021
- Ohta, H. and Toyota-Hanatani, Y. (2012) Salmonella enterica Serovar Enteritidis (SE) Infection in Chickens and Its Public-Health-Risk Control Using an SE Vaccine in Layer Flocks. In: Kumar, Y., Ed., Salmonella, Intech, 279-308. http://dx.doi.org/10.5772/29068
- Burkholder, K.M., Thompson, K.L., Einstein, M.E., Applegate, T.J. and Patterson, J.A. (2008) Influence of Stressors on Normal Intestinal Microbiota, Intestinal Morphology, and Susceptibility to Salmonella enteritidis Colonization in Broilers. Poultry Science, 87, 1734-1741. http://dx.doi.org/10.3382/ps.2008-00107
- Coward, C., Sait, L., Williams, L., Humphrey, T.J., Cogan, T. and Maskell, D.J. (2012) Investigation into the Role of Five Salmonella enterica Serovar Enteritidis Genomic Islands in Colonization of the Chicken Reproductive Tract and Other Organs Following Oral Challenge. FEMS Microbiology Letters, 336, 73-78. http://dx.doi.org/10.1111/j.1574-6968.2012.02652.x
- Allen-Vercoe, E., Dibb-Fuller, M., Thorns, C.J. and Woodward, M.J. (1997) SEF17 Fimbriae Are Essential for the Convoluted Colonial Morphology of Salmonella enteritidis. FEMS Microbiology Letters, 153, 33-42. http://dx.doi.org/10.1111/j.1574-6968.1997.tb10460.x
- Altekruse, S., Koehler, J., Hickman-Brenner, F., Tauxe, R.V. and Ferris, K. (1993) A Comparison of Salmonella enteritidis Phage Types from Egg-Associated Outbreaks and Implicated Laying Flocks. Epidemiology and Infection, 110, 17-22. http://dx.doi.org/10.1017/S0950268800050639
- Bravo, D., Carter, J.A., Hoare, A., Alvarez, S.A., Blondel, C.J., Zaldiveno, M.A., Valvono, M.A. and Contreras, I. (2008) Growth-Phase Regulation of Lipopolysaccharide O-Antigen Chain Length Influences Serum Resistance in Serovars of Salmonella. Journal of Medical Microbiology, 57, 938-946. http://dx.doi.org/10.1099/jmm.0.47848-0
- Mizumoto, N., Toyota-Hanatani, Y., Sasai, K., Tani, H., Ekawa, T., Ohta, H. and Baba, E. (2004) Detection of Specific Antibodies against Deflagellated Salmonella Enteritidis and S. Enteritidis Fli C-Specific 9 kDa Polypeptide. Veterinary Microbiology, 99, 113-120. http://dx.doi.org/10.1016/j.vetmic.2003.11.009
- Piao, Z., Toyota-Hanatani, Y., Ohta, H., Sasai, K., Tani, H. and Baba, E. (2007) Effects of Salmonella enterica subsp. enterica Serovar Enteritidis Vaccination in Layer Hens Subjected to S. Enteritidis Challenge and Various Feed Withdrawal Regimens. Veterinary Microbiology, 125, 111-119. http://dx.doi.org/10.1016/j.vetmic.2007.05.008
- Okamura, M., Lillehoj, H.S., Raybourne, R.B., Babu, U. and Heckert, R. (2003) Antigen-Specific Lymphocyte Proliferation and Interleukin Production in Chickens Immunized with Killed Salmonella Enteritidis Vaccine or Experimental Subunit Vaccines. Avian Diseases, 47, 1331-1338. http://dx.doi.org/10.1637/6096
- Toyota-Hanatani, Y., Kyoumoto, Y., Baba, E., Ekawa, T., Ohta, H., Tani, H. and Sasai, K. (2009) Importance of Subunit Vaccine Antigen of Major Fli C Antigenic Site of Salmonella Enteritidis II: A Challenge Trial. Vaccine, 27, 1680-1684. http://dx.doi.org/10.1016/j.vaccine.2009.01.024
- Miyamoto, T., Horie, T., Fukata, T., Sasai, K. and Baba, E. (1998) Changes in Microflora of the Cloaca and Oviduct of Hens after Intracloacal or Intravaginal Inoculation with Salmonella Enteritidis. Avian Diseases, 42, 536-544. http://dx.doi.org/10.2307/1592680
- Coward, C., Sait, L., Cogan, T., Humphrey, T.J. and Maskell, D.J. (2013) O-Antigen Repeat Number in Salmonella enterica Serovar Enteritidis Is Important for Egg Contamination, Colonization of Chicken Reproductive Tract and Survival in Egg Albumen. FEMS Microbiology Letters, 343, 169-176. http://dx.doi.org/10.1111/1574-6968.12143
- Guard-Petter, J., Parker, C.T., Asokan, K. and Carlson, R.W. (1999) Clinical and Veterinary Isolates of Salmonella enterica Serovar Enteritidis Defective in Lipopolysaccharide O-Chain Polymerization. Applied and Environmental Microbiology, 65, 2195-2201.
- Kawahara, K., Hamaoka, T., Suzuki, S., Nakamura, M., Murayama, S.Y., Arai, T., Terakado, N. and Danbara, H. (1989) Lipopolysaccharide Alteration Mediated by the Virulence Plasmid of Salmonella. Microbial Pathogenesis, 7, 195-202. http://dx.doi.org/10.1016/0882-4010(89)90055-7
- Ohta, H., Yoshikawa, Y., Kai, C., Yamanouchi, K. and Okada, H. (1984) Lysis of Horse Red Blood Cells Mediated by Antibody-Independent Activation of the Alternative Pathway of Chicken Complement. Immunology, 52, 437-444.
- Cogan, T.A. and Humphrey, T.J. (2003) The Rise and Fall of Salmonella Enteritidis in the UK. Journal of Applied Microbiology, 94, 114-119. http://dx.doi.org/10.1046/j.1365-2672.94.s1.13.x
- Cowden, J.M., Chisholm, D., O’Manhony, M., Lynch, D., Mawer, S.L., Spain, G.E., Ward, L. and Rowe, B. (1989) Two Outbreaks of Salmonella enteritidis Phage Type 4 Infection Associated with the Consumption of Fresh Shell-Egg Products. Epidemiology and Infection, 103, 47-52. http://dx.doi.org/10.1017/S095026880003034X
- Guard-Petter, J. (2001) The Chicken, the Egg and Salmonella enteritidis. Environmental Microbiology, 3, 421-430. http://dx.doi.org/10.1046/j.1462-2920.2001.00213.x
NOTES

*Corresponding author.


