International Journal of Clinical Medicine
Vol.3 No.1(2012), Article ID:16814,8 pages DOI:10.4236/ijcm.2012.31003
Ocular Manifestations in HIV Positive and AIDS Patients in Nepal
![]()
1Ophthalmolgy, Mechi Eye Hospital, Anarmani, Nepal; 2Ophthalmology, Institute of Medicine, Kathamandu, Nepal; 3Internal Medicine, Institute of Medicine, Kathamandu, Nepal.
Email: *purushottam_j@hotmail.com
Received October 7th, 2011; revised November 24th, 2011; accepted December 26th, 2011
Keywords: HIV; AIDS; Ocular Manifestations; Posterior Segment Involvement
ABSTRACT
Background: HIV has the capability to affect every organ system in the body. Ocular manifestations have been reported in up to 70% of individuals infected with HIV and the ocular manifestations reflect systemic disease and may be the first sign of disseminated infection. Aim: To identify different types of ocular involvement in the cases known to be infected with HIV. Methods: A cross sectional, descriptive study was undertaken during the period between January 2005 and July 2006. All the diagnosed cases of HIV infected individuals either coming to the hospital or collected from rehabilitation centres were included in the study. Results: 103 HIV infected cases were examined; of which 45 cases (43.6%) were AIDS cases. The mean age of presentation was 29.6 ± 9.8 years. The commonest systemic disease was pulmonary tuberculosis (65.9%). In the study group, 38.8% of the cases had ocular involvement. In the ocular findings, posterior segment lesions (32%) were most common. Ocular involvement among asymptomatic patient was 22.7% while it was 91.6% among symptomatic patients. HIV retinopathy (23.3%) was the most common HIV-associated ophthalmic lesions. Ocular involvement was the most common in cases that contracted the disease through sexual contact. A negative correlation was observed between CD4 level and ocular involvement. Conclusion: There needs to be awareness of ocular involvement among HIV infected individuals and an increased emphasis on regular ophthalmic examination in all HIV patients. Routine referral system for ocular evaluation from other medical departments seems mandatory for timely diagnosis of the vision threatening conditions.
1. Introduction
Human immunodeficiency virus (HIV) is a lentivirus (a member of the retrovirus family) that causes acquired immunodeficiency syndrome (AIDS), [1,2] a condition in humans in which progressive failure of the immune system allows life-threatening opportunistic infections and cancers to thrive. Infection with HIV occurs by the transfer of blood, semen, vaginal fluid, pre-ejaculate, or breast milk. Within these bodily fluids, HIV is present as both free virus particles and virus within infected immune cells. The four major routes of transmission are unsafe sex, contaminated needles, breast milk, and transmission from an infected mother to her baby at birth (vertical transmission). Screening of blood products for HIV has largely eliminated transmission through blood transfusions or infected blood products in the developed world. HIV-infected person is diagnosed with AIDS when his or her immune system is seriously compromised and manifestations of HIV infection are severe. The Centers for Disease Control (CDC) in the USA extended the definition of AIDS to include all those persons who are severely immunosuppressed (CD4+ T cell count < 200/cu mm) irrespective of the presence or the absence of the indicator of disease [3].
The total number of HIV patients in the world was estimated 33.4 million [31.1 - 35.8 million] in 2008. [4] In Nepal, as of November 2007, a total of 10,369 cases of HIV, 1578 AIDS cases and 423 AIDS deaths had been reported to the National Centre for AIDS and STD control (NCASC) [5]. But it is only the tip of the iceberg, the estimated number in Nepal as reported by UNAIDS is 75,000 [6].
HIV has the capability to affect every organ system in the body by direct damage by the virus or by rendering the host susceptible to opportunistic infections. Ocular manifestations have been reported in up to 70% of individuals infected with HIV and it has become apparent that the ocular manifestations almost invariably reflect systemic disease and may be the first sign of disseminated infection in many cases [5]. Several publications have described the ocular manifestations of the HIV/ AIDS [6-19]. Most of the studies on the prevalence of ocular complications in HIV/AIDS have been carried out in industrialized countries [20]. In contrast, more than 90% of all HIV sufferers live in the developing world [6]. Furthermore, there is strong evidence that both ocular complications and their prevalence differ substantially between developing and industrialized countries [20].
Ocular manifestations in HIV positive and AIDS patients range from simple Blepharitis to severe blinding conditions like CMV retinitis. As HIV is an illness that interferes with the immune system making people with AIDS much more likely to get infections, including opportunistic infections and tumors that do not affect people with working immune systems.
Ocular complications if detected at an early stage and managed properly, can be helpful to prevent or minimize potential visual damage.
Ocular complications are common, affecting 50% to 75% of all such patients at some point during the course of their illness. Cytomegalovirus retinitis is by far the most frequent cause of vision loss in patients with AIDS. Although the prevalence of cytomegalovirus retinitis is decreasing in industrialized countries because of the widespread availability of highly active antiretroviral therapy, between 10% and 20% of HIV-infected patients worldwide can be expected to lose vision in one or both eyes as a result of ocular cytomegalovirus infection. Less frequent but important causes of bilateral vision loss in patients with HIV/AIDS include varicella zoster virus and herpes simplex virus retinitis, HIV-related ischaemic microvasculopathy, ocular syphilis, ocular tuberculosis, cryptococcal meningitis, and ocular toxic or allergic drug reactions [21].
Till date, to our best of knowledge, there have been no published documents or studies indicating the ocular manifestation of HIV/AIDS from Nepal. Hence, this study has been undertaken to identify the ocular manifestations in HIV/AIDS in Nepalese population.
2. Material and Methods
A cross sectional study was carried out during the period of January 2008 to July 2009. All diagnosed cases of HIV infected individuals either coming to the Tribhuan University Teaching Hospital (the tertiary center) or collected from three rehabilitation centres in Kathmandu were included in the study. Written and verbal consent were obtained from the patients or their guardians. Detailed demographic profile of the patients including age, gender, ethnic group and duration of HIV infection diagnosed were undertaken. Detailed history was asked including history of receiving antiretroviral therapy, risk factor for the transmission of HIV.
Staging of disease was done according to revised WHO clinical staging of HIV as given in Table 1 [22].
Number of recent CD4 count was also noted. The severity of ocular manifestations was correlated with the number of CD4 count. Detailed ocular examination was carried out. Ocular examination included visual acuity assessment, ocular motility assessment, eyelid and adnexal examination, conjunctival and scleral examination, examination of cornea, anterior and posterior segment evaluation.
Visual acuity was noted by Snellen chart and was recorded in snellen fraction. Ocular motility was tested with torch light in cardinal gazes. Detailed orthoptics examination including Hess charting and diplopia charting were also carried out in suspected case of muscle
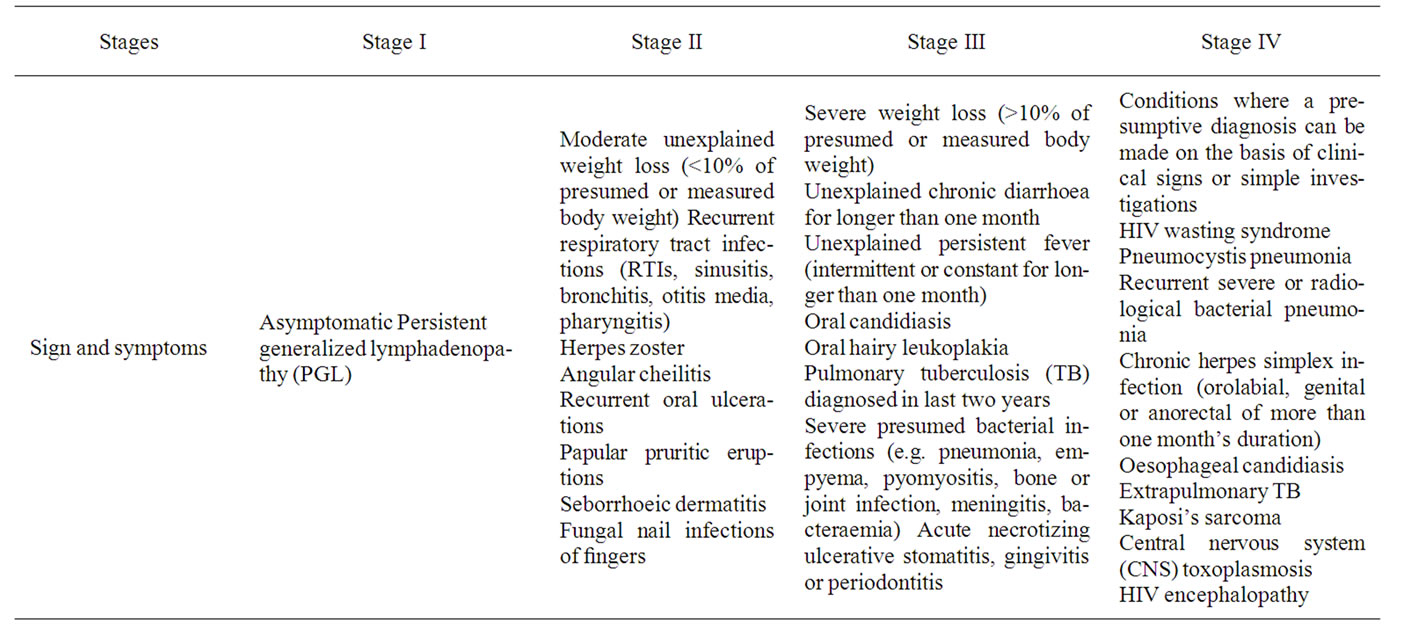
Table 1. Revised who clinical staging of HIV/AIDS for adults and adolescents.
palsy. Findings in eyelid, orbital and peri-orbital region was recorded and documented schematically. Conjunctiva, sclera and episclera were examined for any abnormalities and were documented. Cornea was examined for any defect, infiltrates and sensitivity. Anterior chamber finding was recorded. Cells, flare grading was done [13]. Posterior segment examination included vitreous and retinal examination. Vitreous cells and flares were also graded according to their severity [14]. Detail Fundus examinations under mydriasis were performed with Heine Beta 200 direct ophthalmoscope, binocular indirect ophthalmoscope with +20 D lens and Haag Streit 900 slit Lamp with +90 D Lens. Intra ocular pressure (IOP) was measured by non contact air Puff tonometer.
Data were collected in a proforma prepared for the study and the data were tabulated and analyzed in spss13. Mean, standard deviation, Pearson’s correlation coefficient were used as descriptive statistical tool and chisquare, ANOVA and paired t-tests were used as inferential statistical tool.
3. Results
A total of 103 patients were included in the study. 54.4% of patients were from hospital and 45.6% were from rehabilitation Centre. The mean age of the presentation was 29.6 ± 9.8 years. 81.5% of the total patients were in the age group 21 to 40 years (73.84% - 89.15%, CI-95%). 71 (68.9%) were male and 32 (31.1%) were female, with a ratio of 2.2:1.
Table 2 shows the patient distribution according to the visual acuity at presentation. 94.2% of the patients had visual acuity of 6/6 to 6/18 in each eye.
40 patients (38.8%) had ocular involvement. 35.2% of total male patients and 46.8% of the total female patients had ocular involvement. Out of 103 patients, 79 (76.7%) patients were asymptomatic. 87.5% the symptomatic group of patients had ocular involvement. Even in the asymptomatic group, 24% had ocular morbidity.
60 cases (58.3%) of the total patients had undergone the CD4 count investigation. 27 (45%) had count in the range 51 to 199. There were 13 patients (21.7%) with range 200 to 500 and 10 patients each in ≥500 and ≤50. There was a statistically significant, strong negative correlation between ocular involvement and CD4 count (r = –0.51, p = 0.00003).
Figure 1 shows that the ocular involvement in heterosexual mode was more than in intravenous drug users (IDU). There was no ocular involvement seen vertically transmitted cases. The association between mode of transmission and ocular involvement was statistically signifycant (p = 0.023).
Ocular involvement was correlated with the WHO clinical stage of the disease. 50% ocular involvement was observed in Stage IV followed by 12.5% in Stage III and 11.5% in Stage I. No ocular involvement was seen in Stage II. There was not statistically significant correlation between the clinical staging of the disease and the ocular involvement (p = 0.1, chi square test).
Table 2 shows the form of ocular disorder in symptomatic and asymptomatic patients. The table shows that even in those asymptomatic patients in 19 out of 79 patients accounting 22.7%, ocular lesions were seen. The most common finding was HIV retinopathy seen in 33 eyes (20.8%) of 17 patients. In the symptomatic group also, the most common lesion was HIV retinopathy seen in 10 eyes (20.8%) of 7 patients, followed by conjunctivitis seen in 8 eyes (16.6%) of 4 patients and CMV retinitis seen in 5 eyes (10.4%) of 4 patients.
7.76% of the cases had Neuro-ophthalmic Lesions. 2 cases (1.9%) had papilloedema, 1 case of retrobulbar optic neuritis, 2 cases of optic atrophy associated with retinal degeneration. 3 cases (2.9%) had cranial nerve palsy; one cases each of CN III with papillary sparing, CN VI and CN VII palsy.
Figure 2 shows among the total 48 ocular findings seen in 103 patients; HIV retinopathy was the most common accounting for 50% of the total ocular findings followed by CMV retinitis and Conjunctivitis each accounting for 8.3%.
4. Discussion
HIV infection in humans is considered pandemic by the World Health Organization (WHO). [23] HIV-related eye disease generally takes the form of opportunistic infections that can affect any of the ocular tissues, from the eyelids to the retina. In particular, those conditions affecting the retina may lead to chronic visual impairment or blindness.
In the current study, ocular involvement was seen in 38.8% cases. This was similar to the studies carried out in India by J. Biswas et al. [14] and Sriprakash K. S. et al. [21]. But the ocular involvement was higher in African and the US studies as compared in Table 3. The studies in the USA and Africa were carried out in the year 1995 and 1996 respectively and as the HAART was in its initial phase and not widely practiced, henceforth more ocular involvement were seen in those studies.
Even in the asymptomatic patients, 22.7% of the patients had some ocular findings and in the symptomatic patients, 91.6% had ocular findings. HIV retinopathy (20.8%) was the most common finding in asymptomatic patients, followed by a single case each of molluscum contagiosum and CN VII palsy. In the symptomatic group also, the most common lesion was HIV retinopathy seen in 10 eyes (20.8%) of 7 patients, followed by CMV retinitis seen in 5 eyes (10.4%) of 4 patients. In the
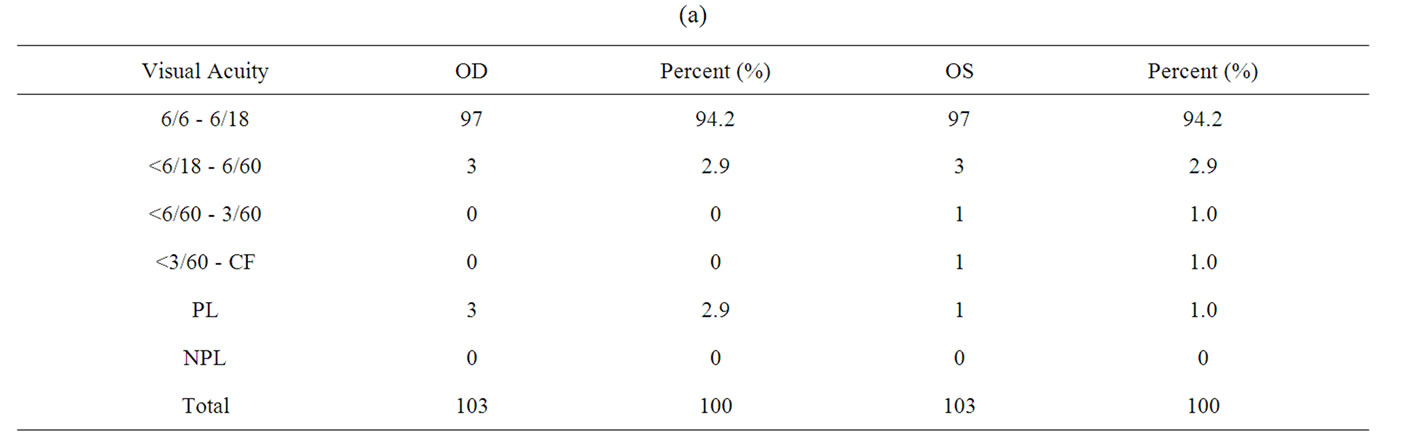
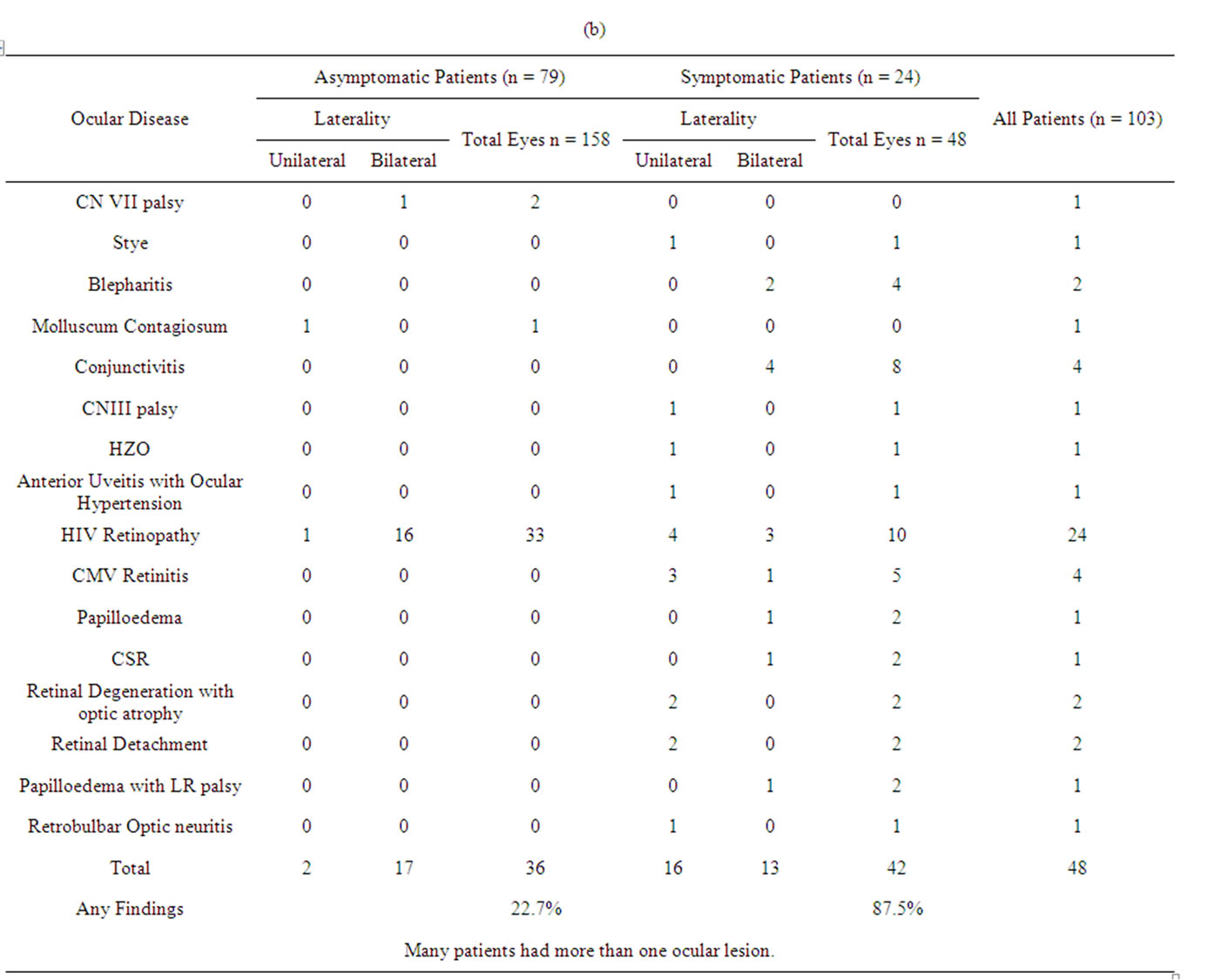
Table 2. (a) Visual acuity of the cases; (b) Patient distribution according to the ocular disease, laterality and the ocular symptoms.
study carried out by Jutalai Tanterdtam et al. [16], the commonest ocular lesion in asymptomatic group was HIV retinopathy as in our study, but it was CMV retinitis in the symptomatic group.
9.7% of the cases had anterior segment involvement. Table 4 shows the anterior segment findings from our study and other similar studies done elsewhere. The study findings were similar to the study done in India. HZO was present only in 1% of the cases in our study. J Biswas et al. [14] and in study by Jabs et al. [6] found 1%
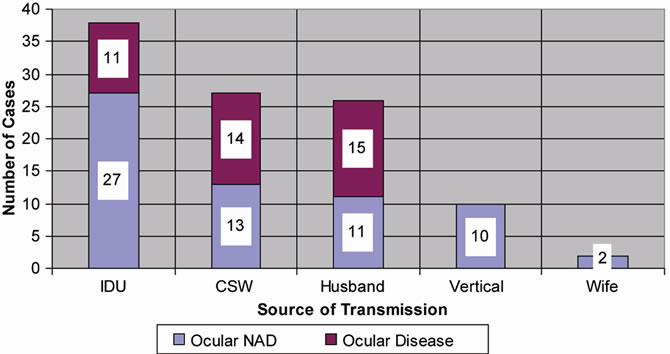
Figure 1. Bar diagram showing patient distribution according to the ocular involvement and source of transmission.
and 3% of HZO cases, but was present in 23% and 10.6% in the study done by Awan et al. [22] and Sriprakash et al. [21] The occurrence of blepharitis and molluscum contagiosum was similar to that in India. There were no cases of Kaposi’s sarcoma seen in our study and in Indian studies, but seen in the USA and Africa. The absence of Kaposi’s sarcoma in Indian study has been attributed to lower proportion of homosexual behavior and low prevalence of human herpes virus-8 in India. The same reason applies to the present study in Nepal, the neighboring country of India for the absence of Kaposi’s sarcoma.
32.03% of the cases had posterior segment lesions in our study, HIV retinopathy (23.3%) being the most common followed by CMV retinitis (3.9%). Similar trend was seen in the study done by Awan et al. [22] in Africa. In a study by N. A. V. Beare et al. [23] in Malawi, Africa only 0.5% of the cases had signs of CMV retinitis. This may be the result of mortality early in the disease course, or differences in race, HIV subtype, or comorbidity. But this was different from other studies done in USA and India as shown in Table 5. The occurrence of CMV retinitis was 37% in the study by Jabs et al. [7], 17% by J. Biswas [14] and 10.65% by Sriprakash K. S. et al. [21]. This may be because the study conducted in the USA was before the introduction of the HAART and in India [14,22] it was before the widespread use of HAART as mentioned in those studies. The occurrence of Retinal Detachment seen in our study was less than in other studies and this may be due to the use of HAART. In 2 cases (1.9%), the retinal degeneration with was seen. The retinal degeneration observed may be the sequelae of CMV retinitis. In our study we found one case having CSR in both eyes. Though not mentioned in any journals or textbooks as the posterior segment finding in HIV/ AIDS, it may be unrelated to HIV/AIDS and seen coincidentally.
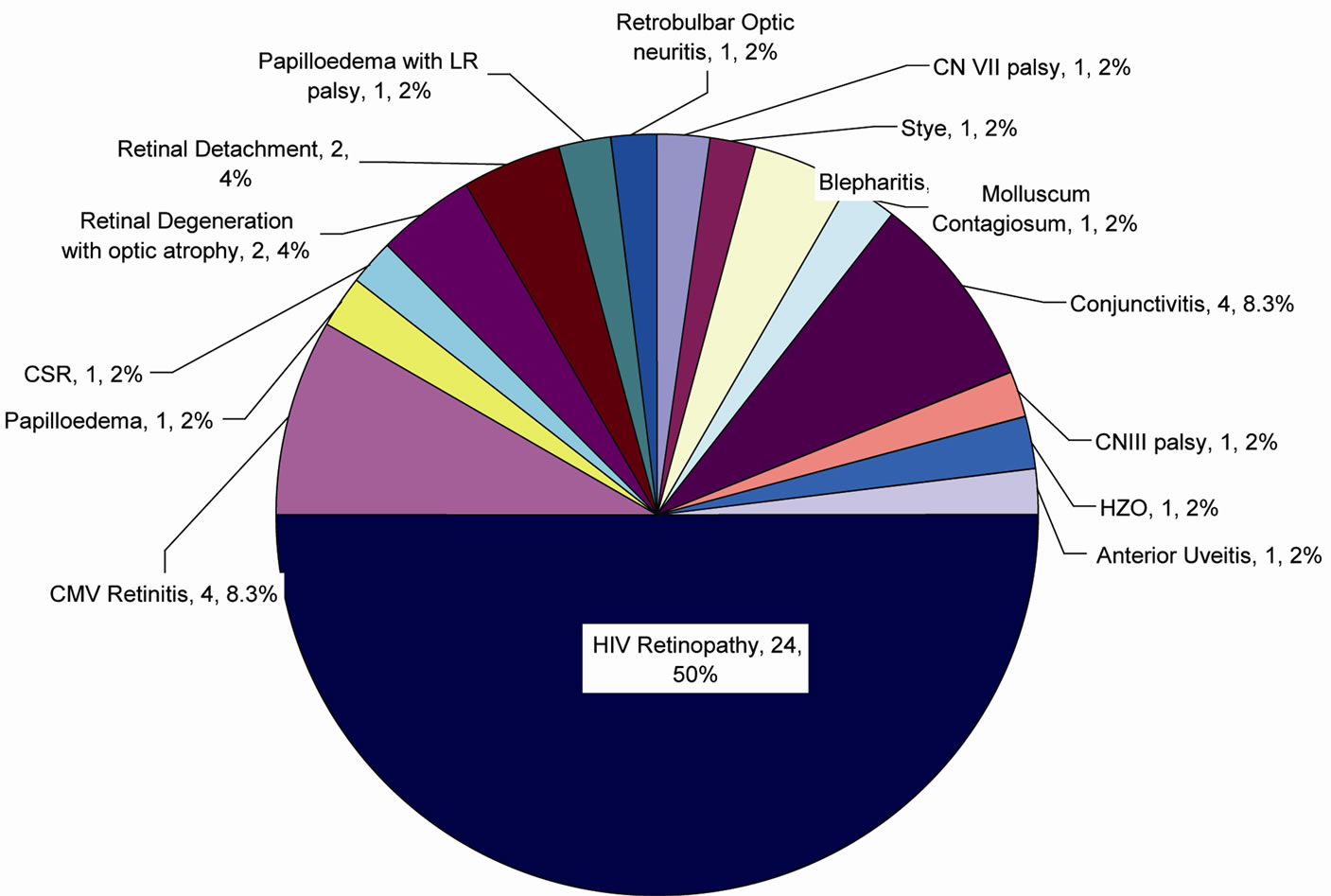
Figure 2. Pie diagram of different types of ocular manifestations in the cases having ocular involvement.
7.76% of the cases had Neuro-ophthalmic Lesions. The patterns of Neurophthalmic lesions are similar to Jabs et al. [7] and Sriprakash K. S. et al. [21] as shown in Table 6. 2 cases (1.9%) had papilloedema, 1 was due to raised ICP by Meningitis associated with CN VI palsy as well. The cause of papilloedema in other patient could not be found, but the patient responded to oral steroid and papilloedema subsided. There was one case of retrobulbar optic neuritis in our study that presented with HM vision and was prescribed oral steroid and the vision improved to 6/9.
5. Conclusions
Although prolonging the life of HIV infected patients is of prime importance, attention should also be paid to decrease the sufferings. Early detection and prompt management of ocular condition improve the visual prognosis.
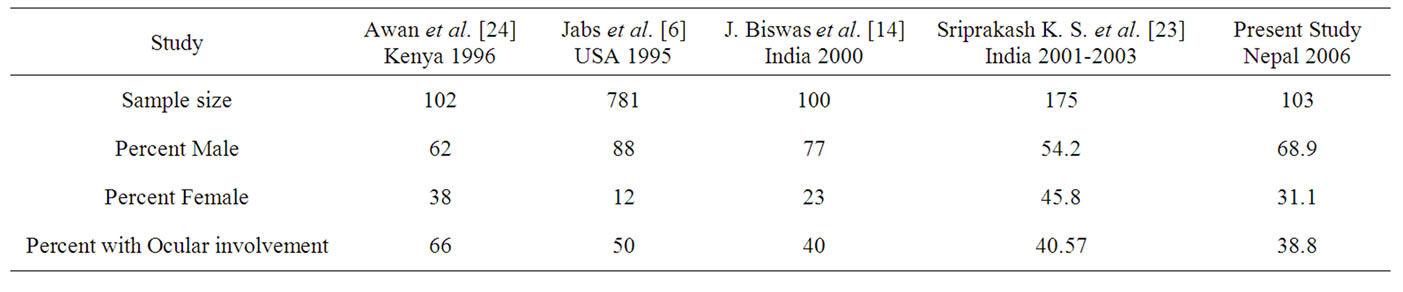
Table 3. Comparison with studies done in Africa, USA and India with the present study.
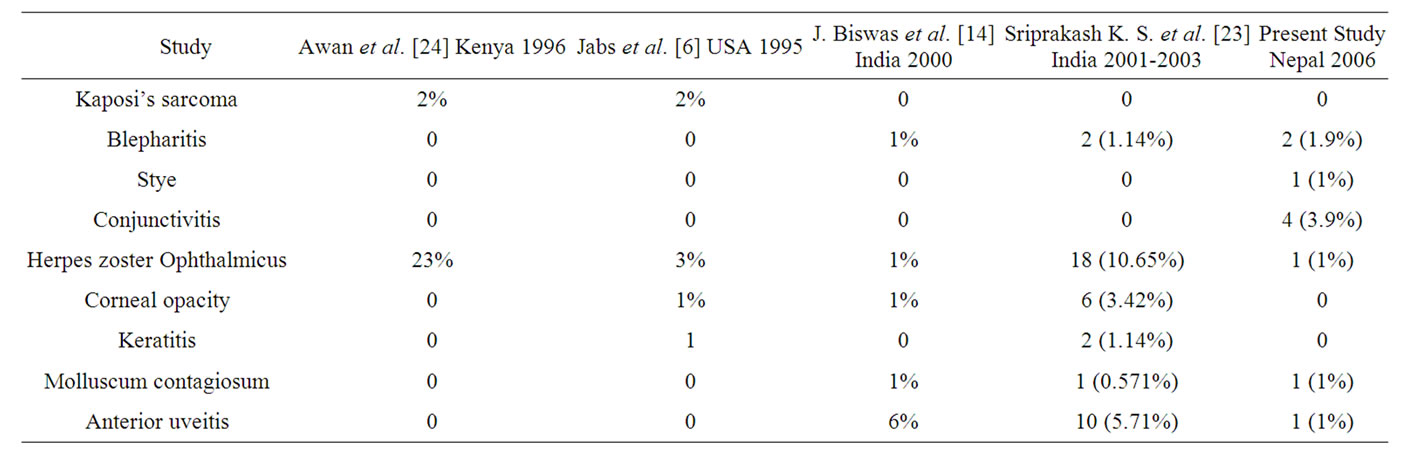
Table 4. Anterior segment findings in different studies.
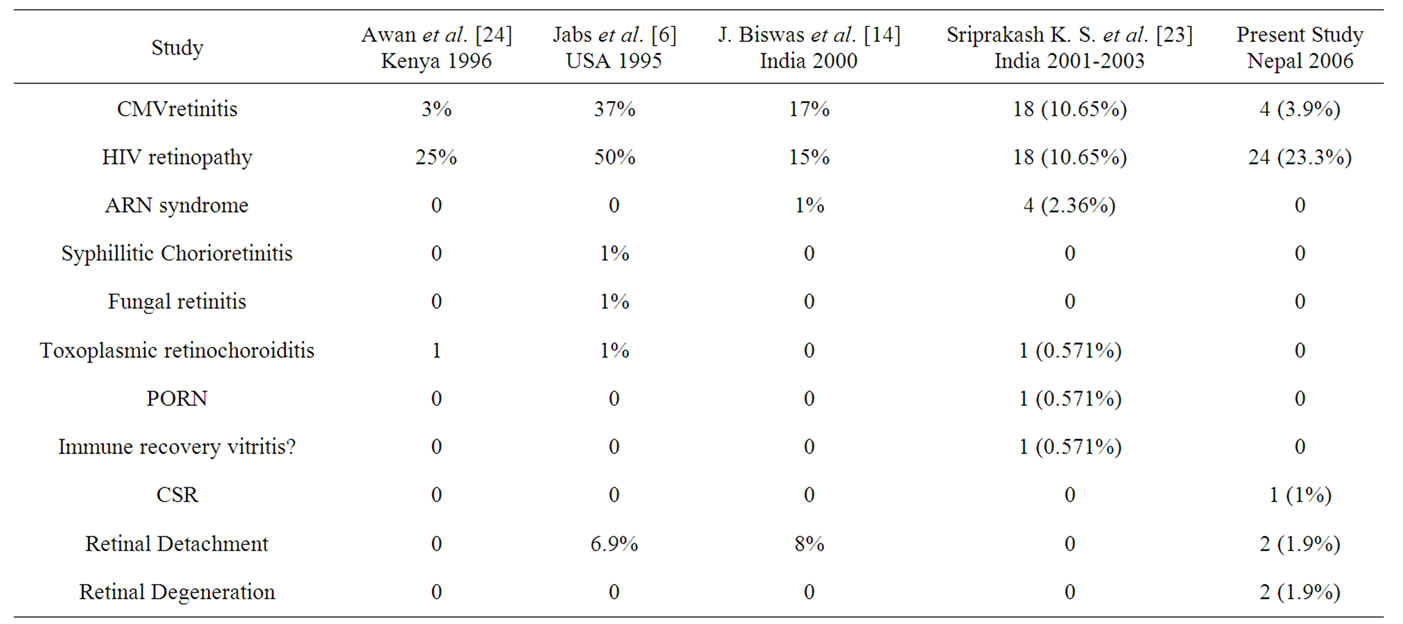
Table 5. Posterior segment lesions in different studies.
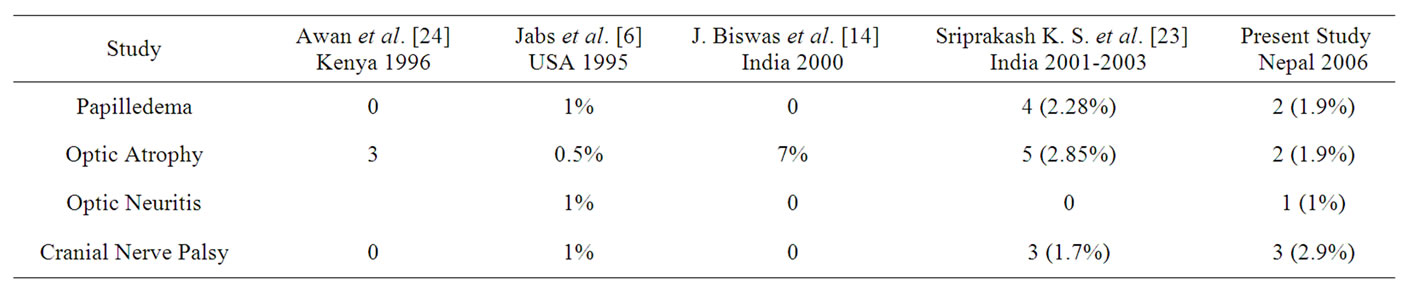
Table 6. Neuro-ophthalmic lesions seen in different studies.
Ocular examination is important not only from visual point of view, but also because ocular status is an important indicator of the disease process. So, when an HIV infected patient comes to an ophthalmologist, detail ocular examination should be undertaken. Proper evaluation of the fundus is a must.
Development of HAART has increased the longevity of HIV infected patients. But a blind patient may not be able to lead the life as his well seeing counterpart. So to have a better quality of life, it is necessary for the patients to have good vision. With timely detection of the ocular involvement, blindness can be prevented or minimized in these cases. Patients should be made aware of the potential ocular complications of the disease process through health education which they currently lack. Routine referral system of the HIV infected cases from other allied medical and surgical departments for ophthalmic check up is strongly recommended.
REFERENCES
- R. A. Weiss, “How Does HIV Cause AIDS?” Science, Vol. 260, No. 5112, 1993, pp. 1273-1279. doi:10.1126/science.8493571
- D. C. Douek, M. Roederer and R. A. Koup, “Emerging concepts in the immunopathogenesis of AIDS,” Annual Reviews, Vol. 60, No. 1, 2009, pp. 471-484. doi:10.1146/annurev.med.60.041807.123549
- CDC, “1993 Revised classification system for HIV infection and expanded surveillance case definition for AIDS among adolescents and adults,” MMWR, No. RR- 17, 1992, p. 41.
- UNAIDS and WHO, “AIDS Epidemic Update,” Joint United Nations Programme on HIV/AIDS and World Health Organization, Geneva, 2009.
- UNGASS, “Country Progress Report,” 2008.
- UNAIDS, “Joint United Nations Programme on HIV/AIDS (UNAIDS),” 2006 Report on the Global AIDS Epidemic, Geneva, 2006.
- American Academy of Ophthalmology, “Intraocular Inflammation and Uveitis Section 9. Basic and Clinical Science Course, 2001-2002,” San Francisco, 2001, pp. 216- 237
- D. A. Jabs, “Ocular manifestations of HIV infection,” Transactions on the American Ophthalmological Society, Vol. 93, 1995, pp. 623-683
- D. A. Jabs, J. T. Holbrook, M. L. Van Natta, R. Clark, M. A. Jacobson and R. L. Murphy, “Risk Factors for Mortality in Patients with AIDS in the Era of Highly Active Antiretroviral Therapy,” Ophthalmology, Vol. 112, No. 5, 2005, pp. 771-779
- I. Pecorrella, A. Ciardi, A. Garner, A. C. E. McCartney and S. lucas, “Postmortem histological survey of the Ocular lesion in a Brirish Population of AIDS Patients,” British Journal of Ophthalmology, Vol. 84, No. 11, 2000, pp. 1275- 1281. doi:10.1136/bjo.84.11.1275
- M. C. Lim, W. G. Cumberland, S. L. minassin, S. S. Ransome, M. J. Cornish, B. G. Terry and G. N. Holland, “Decreased Macular Leukocute Velocity in Human Immunodeficiency Virus-Infected Individuals,” American Journal of Ophthalmology, Vol. 132, No. 5, 2001, pp. 711-719.
- R. Belfort and C. Muccioli, “Experience of HIV/AIDS and the Eye in Brazil, South America,” Community Eye Health, Vol. 8, 1995, pp. 26-27.
- A. C. Wadood, B. Dhillon, G. McIlwaine and R. P. Brettle, “Delayed diagnosis of HIV infection in Ophthalmic practice,” Eye, Vol. 18, 2004, pp. 293-298. doi:10.1038/sj.eye.6700630
- K. G. A. Eong, S. Beatty and S. J. Charles, “Cytomegalovirus in patients with acquired immune deficiency syndrome,” Postgraduate Medical Journal, Vol. 75, 1999, pp. 585-590.
- P. A. Sample, D. J. Plummer, A. J. Mueller, K. I. Mastsubara, A. Sadun, I. Grant and W. R. Freeman, “Pattern of Early Visual Field Loss in HIV-Infected Patients,” Archives of Ophthalmology, Vol. 117, No. 6, 1999, pp. 755- 760
- J. Biswas, H. N. Madhwan, A. E. George, N. Kumarasamy and S. Solomon, “Ocular Lesion Associated with HIV Infection India: A Series of 100 Consecutive Patients Evaluated at Referral Center,” American Journal of Ophthalmology, Vol. 129, No. 1, 2000, pp. 9-15. doi:10.1016/S0002-9394(99)00415-8
- D. F. Rofsberger, M. H. Heineman, D. D. N. Friedberg and G. N. Holland, “Uveitis Associated with Human Immunodeficiency Virus Infection in India. American Journal of Ophthalmology, Vol. 125, No. 3, 1998, pp. 301-305
- J. Tanterdam, S. Suwannagool, C. Namatra and A. Singalavaniza, “A study of Ocular Manifestations in HIV patients,” Thai Journal of Ophthalmology, Vol. 10, No. 1, 1996, pp. 11-20.
- I. Cocherau, N. Milka-Cabanne, P. Godinaud, T. Niyangabo and B. Larouzea, “AIDS related eye disease in Burundi, Africa,” British Journal of Ophthalmology, Vol. 83, No. 3, 1998, pp. 374-382.
- R. B Nussenblatt and H. C. Lane, “Human Immunodeficiency Virus Disease: Changing Pattern of Intraocular Inflammation,” American Journal of Ophthalmology, Vol. 129, No. 3, 1998, pp. 373-382
- P. G. Kestelyn1 and E. T. Cunningham Jr., “HIV/AIDS and Blindness,” Bulletin of the World Health Organization, Vol. 79, No. 3, 2001, pp. 208-213.
- World Health Organization, “Interim who clinical staging of HIV/AIDS Case definitions for surveillance,” WHO, 2005. http://www.who.int/hiv/pub/guidelines/clinicalstaging.pdf
- UNAIDS, “Joint United Nations Programme on HIV/AIDS,” Report on the Global AIDS Epidemic, Geneva, 2006. http://www.unaids.org/en/hiv_data/2006globalreport/default.asp
- D. A. Jabs, “AIDS and Ophthalmology in 2004,” Archives of Ophthalmology, Vol. 122, No. 7, 2004, pp. 1040-1042. doi:10.1001/archopht.122.7.1040
- E. T. Cunningham Jr. and T. P. Margolis, “Ocular Manifestations of HIV Infection,” New England Journal of Medicine, Vol. 339, 1998, pp. 236-244. doi:10.1056/NEJM199807233390406
- K. S. Sriprakash, R. Babu, C. Kumar, et al., “Ocular Manifestations of HIV/AIDS. An experience at major eye hospital in South India,” 62nd Conference on All India Ophthalmologic Society, Varanasi, 8-11 January 2004.
- H. R. Awan and H. S. Adala, “Ophthalmic manifestations of acquired immunodeficiency syndrome in Kenya,” Ophthalmic Practice (Asian Edition), Vol. 1, 1996, pp. 92-102
- N. A. V. Beare, J. G. Kublin, D. K. Lewis, M. J. Schiffelen, R. P. H. Peters, G. Joaki, J. Kumwenda and E. E. Zijlstra, “Ocular disease in patients with tuberculosis and HIV presenting with fever in Africa,” British Journal of Ophthalmology, Vol. 86, No. 10, 2002, pp. 1076-1079. doi:10.1136/bjo.86.10.1076
- Department of Health Services, Ministry of Health, Nepal Government, “TB facts, figures and concepts 2003,” National Tuberculosis Programme, 2003.
NOTES
*Corresponding author.

