International Journal of Clinical Medicine
Vol.1 No.2(2010), Article ID:3239,6 pages DOI:10.4236/ijcm.2010.12009
A Comparison of Polysomnographic Variables between Adolescents with Polycystic Ovarian Syndrome and Healthy Controls
![]()
Vestische Kinderund Jugendklinik, University of Witten/Herdecke, Datteln, Germany.
Email: GandM@web.de
Received August 13th, 2010; revised August 16th, 2010; accepted September 19th, 2010.
Keywords: PCOS, OSAS, adolescents, obesity, extreme obesity, polysomnography
ABSTRACT
Purpose: The prevalence of obstructive sleep apnea syndrome (OSAS) is clearly increased in adults with polycystic ovarian syndrome (PCOS). The symptoms of PCOS usually begin around menarche. However, data concerning polysomnographic variables in adolescents with PCOS are limited. As obesity is a well-known risk factor for OSAS, we aimed to analyze differences in polysomnographic variables between obese and extremely obese adolescents with PCOS and healthy, normal-weight, obese, and extremely obese controls. Methods: Sixteen obese and 17 extremely obese adolescents with PCOS, 18 normal-weight, 17 obese, and 13 extremely obese controls underwent polysomnography to compare mean transcutaneous arterial oxygen saturation (Sat O2), apnea-index (AI), hypopnea-index (HI), apnea-hypopnea index (AHI), the absolute number of obstructive apneas (NOA), percentage sleep stages 3 and 4 of non REM-sleep (stages 3 & 4), percentage of REM-sleep (% REM), sleep-onset latency, and sleep efficiency. Results: We found no significant differences between the groups concerning AI, HI, AHI, NOA, and stages 3 & 4. Significant differences between the groups were found regarding Sat O2, % REM, sleep-onset latency, and sleep efficiency. Conclusions: Concerning the respiratory variables, adolescents with PCOS do not seem to differ from healthy controls regardless of weight status, but there seem to be differences in sleep architecture.
1. Introduction
Polycystic ovarian syndrome (PCOS) is a common disorder affecting as many as 5-10% of women of reproduc-tive age [1]. According to the consensus workshop of the National Institutes of Health (NIH), PCOS can be diagnosed in a patient if she has chronic oligoanovulation and either biochemical or clinical signs of androgen excess, and other pathologies have been excluded [2]. Being a complex functional disorder, PCOS is furthermore associated with metabolic abnormalities such as insulin resistance and lipid abnormalities similar to those that belong to the metabolic syndrome [3], perhaps predisposing to cardiovascular disease [4].
In adults, studies have reported that the risk for and the prevalence of obstructive sleep apnea syndrome (OSAS) are increased in patients with PCOS [5-9]. Therefore, screening for OSAS has been recommended for PCOS patients [10]. Diagnosing and treating OSAS in patients with PCOS is of great importance, as OSAS is a risk factor for cardiovascular mortality and morbidity [11]. As the symptoms of PCOS usually begin around menarche [4], the question arises, whether polysomnographic variables in adolescents with PCOS differ from healthy controls.
In a previous study, we compared polysomnographic data between adolescents with PCOS and healthy lean and obese controls demonstrating no differences in respiratory variables between the groups [12]. However, this study was limited by the fact that the number of cases (n = 22) and controls (n = 29) was rather moderate. Furthermore, the study group was heterogeneous concerning their weight status ranging from normal weight (SDSBMI 0,38) to extremely obese (SDS-BMI 3,89), just as the control group of obese girls was heterogeneous concerning their weight status ranging from overweight (SDS-BMI 1,6) to extremely obese (SDS-BMI 4,25).
For a more detailed comparison, we enlarged the number of cases (n = 33) and controls (n = 48) and divided the participants according to their weight status, as obesity is well known to be a risk factor for OSAS in adolescents [13,14]. A further novel aspect of this study is the comparison of polysomnographic data between obese and extremely obese adolescents with PCOS.
2. Methods
2.1. Study population
We studied 16 obese adolescents aged 12 to 16 years with PCOS (Study group I, mean age 15.0 years ± 1.3, mean BMI 28.8 kg/m2 ± 2.3, mean SDS-BMI 2.1 ± 0.4) and 17 extremely obese adolescents aged 12 to 16 years with PCOS (Study group II, mean age 14.6 years ± 1.0, mean BMI 38.7 kg/m2 ± 4.8, mean SDS-BMI 3.2 ± 0.4) in the sleep laboratory and compared these findings to those of three control groups. Control group I consisted of 18 normal-weight adolescents without PCOS aged 13 to 16 years (mean age 15.0 years ± 0.9, mean BMI 20.6 kg/m2 ± 2.3, mean SDS-BMI 0.0 ± 0.8). Control group II consisted of 17 obese adolescents without PCOS aged 13 to 16 years (mean age 15.0 years ± 0.8, mean BMI 29.3 kg/m2 ± 1.6, mean SDS-BMI 2.2 ± 0.3). Control group III consisted of 13 extremely obese adolescents without PCOS aged 13 to 17 years (mean age 15.5 years ± 1.0, mean BMI 38.9 kg/m2 ± 5.7, mean SDS-BMI 3.2 ± 0.4).
All girls with PCOS were recruited from the outpatient Obesity and Endocrine Department of the Vestische Children’s Hospital, Datteln, Germany. The diagnosis of PCOS was based on the definition of the NIH [2]. Conditions such as non-classical adrenal 21-hydroxylase deficiency, androgen-secreting tumors, and Cushing’s syndrome were excluded by appropriate tests before the diagnosis of PCOS was made. All girls with PCOS were at least 2 years post menarche. Nineteen obese and extremely obese control patients were recruited from the outpatient Obesity and Endocrine Department of the Vestische Children’s Hospital, Datteln, Germany. The remaining 11 obese and extremely obese control patients and the 18 normal-weight controls were recruited from the sleep laboratory of our hospital. Polysomnography had ruled out sleep-related breathing disorders in these control patients. All control patients had normal menstrual cycles (28-35 days) and no clinical signs of androgen excess thereby excluding PCOS by the definition of the NIH [2]. All participants were without evidence of other diseases and were not currently taking any medications.
2.2. Subject evaluation
All participants underwent physical examination includeing the measurement of height and weight. Height was measured to the nearest centimeter using a rigid staidometer. Weight was measured unclothed to the nearest 0.1 kg using a calibrated balance scale. Body mass index (BMI) was calculated as weight in kilograms divided by height in meters square (kg/m2). Overweight was defined according to the International Obesity Task Force using population specific data [15,16]. We used the LMS method to calculate the standard deviation of BMI (SDS-BMI) as a measure for the degree of overweight due to the skewness of BMI distribution [17]. SDS-BMI was calculated according to German percentiles [16]: SDS-BMI > 1.28 defined overweight (> 90th percentile of BMI), SDS-BMI > 1.88 defined obesity (> 97th percentile of BMI), and SDS-BMI > 2.58 defined extreme obesity (>99.5th percentile of BMI). Menstrual history was obptained from all the patients. Disturbances of the menpstrual cycle in the patients with PCOS were divided into oligomenorrhea (less then 9 menstrual cycles/year), primary amenorrhea (no menstruation by the age of 16 years), and secondary amenorrhea (no menstruation for 3 or more months after menarche). The diagnosis of PCOS was confirmed by the measurement of serum androgens.
2.3. Polysomnography
All girls underwent overnight 12-channel polysomnography recording an electro-encephalogram (2 channels), an electrooculogram (2 channels), a submental electromyogram, an electrocardiogram, chest wall motion, abdominal wall motion, nasal-oral airflow, arterial oxygen saturation by pulse oximetry, transcutaneous pO2, and transcutaneous pCO2. All signals were recorded simultaneously and stored using the BRAINLAB 4 polysomnography system (DiaMedic-Schwarzer, Unna, Germany). A thermistor was used to measure nasal pressure (Sandman BreathSensor™, Nellcor Puritan Bennett (Melville) Ltd., Kanata, Canada). Bedtime was set between 21 and 22 h, and wake time occurred approximately at 5 h. Thus, we attempted to record sleep for at least 7 hours in each patient.
All polysomnographic records were evaluated by an experienced pediatric somnologist (BS). Sleep was staged according to standard criteria [18]. Arousals and respiratory events were defined according to the guidelines of the American Academy of Sleep Medicine [19,20]. Apnea was defined as a complete cessation in airflow of 5 or more seconds. They were classified as central if there was no associated effort (i.e. chest wall movement absent) and obstructive if respiratory effort (chest wall movement) was present. Hypopneas were scored as a clear reduction (50%) in amplitude in the oronasal flow signal for 5 or more seconds that was associated with either an oxygen desaturation of more than 3%, EEG arousal, or both. The apnea-index (AI) was calculated as the number of apneas divided by the number of hours of sleep, the hypopnea index (HI) as the number of hypopneas divided by the number of hours of sleep, and the apnea-hypopnea index (AHI) as the number of apneas plus hypopneas divided by the number of hours of sleep. As AI and AHI do not differentiate between central and obstructive apneas, we recorded the absolute number of obstructive apneas, as well. Sleep efficiency (%) was defined as the ratio of total sleep time in bed i.e. total sleep time/time in bed x 100. Sleep-onset latency (min.) was defined as the period of time measured from “lights out,” or bedtime, to the commencement of sleep.
2.4. Statistics
Comparisons between the five groups were performed with an ANOVA including post-hoc multiple pair wise comparisons. Data were presented as mean values and standard deviation. A p < 0.05 was set as significant. The clinical ethics committee of the Vestische Kinderund Jugendklinik, University of Witten/Herdecke approved this study. Written informed consent was obtained from all subjects and their parents.
3. Results
The girls in both study groups and in the three control groups did not differ in respect of age (p = 0.33). The mean BMI and the mean SDS-BMI of the obese girls with PCOS did not differ from the mean BMI and the mean SDS-BMI of the healthy obese girls, and the mean BMI and the mean SDS-BMI of the extremely obese girls with PCOS did not differ from the mean BMI and the mean SDS-BMI of the healthy extremely obese girls (table 1).
We found no significant differences between the five groups concerning the apnea-index (p = 0.57), the hypopnea-index (p = 0.36), the apnea-hypopnea index (p = 0.08), the absolute number of obstructive apneas (p = 0.48), and percentage sleep stages 3 and 4 of non REM-sleep (p = 0.36) (table 2).
Mean transcutaneous arterial oxygen saturation (Sat O2) in the obese girls with PCOS was significantly higher than in the normal-weight, obese, and extremely obese controls, and Sat O2 in the extremely obese girls with PCOS was significantly higher than in the extremely obese controls (p = 0.02). Post-hoc multiple pair wise comparisons showed that the percentage of REM-sleep in
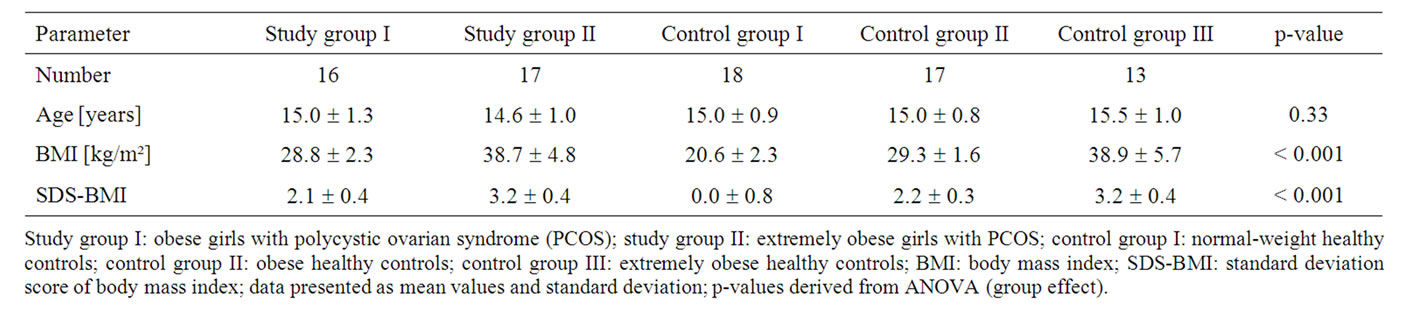
Table 1. Age and weight status of the girls in the study groups and control groups.
Study group I: obese girls with polycystic ovarian syndrome (PCOS); study group II: extremely obese girls with PCOS; control group I: normal-weight healthy controls; control group II: obese healthy controls; control group III: extremely obese healthy controls; BMI: body mass index; SDS-BMI: standard deviation score of body mass index; data presented as mean values and standard deviation; p-values derived from ANOVA (group effect).
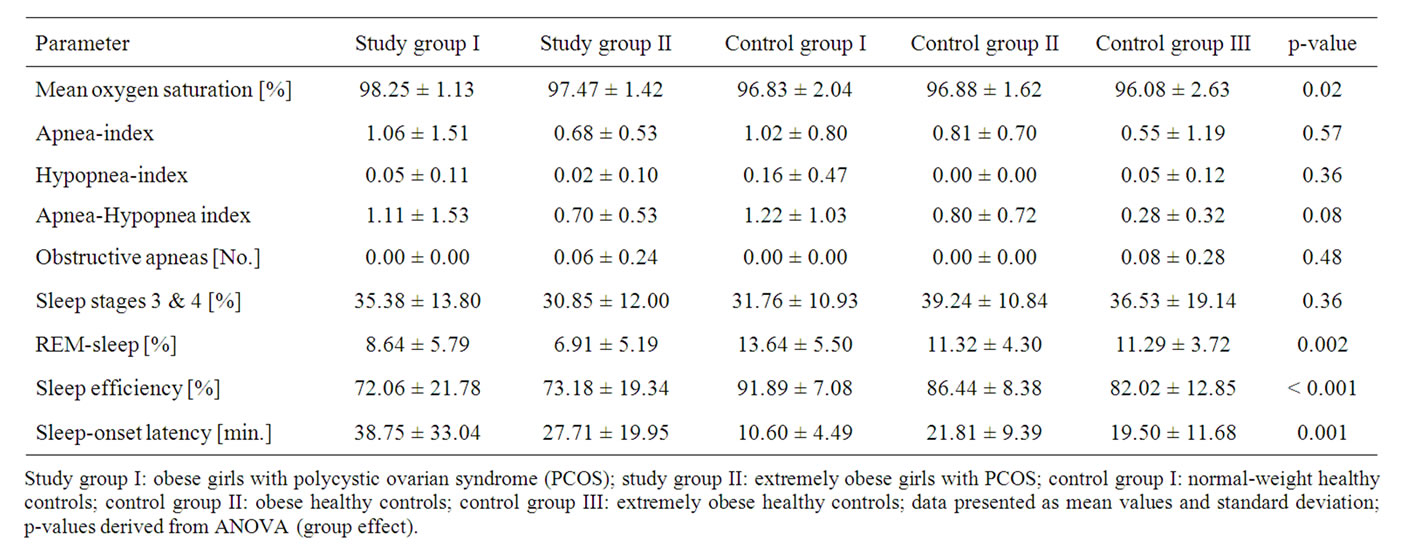
Table 2. Polysomnographic data of the girls in the study groups and control groups.
Study group I: obese girls with polycystic ovarian syndrome (PCOS); study group II: extremely obese girls with PCOS; control group I: normal-weight healthy controls; control group II: obese healthy controls; control group III: extremely obese healthy controls; data presented as mean values and standard deviation; p-values derived from ANOVA (group effect).
all control groups was significantly higher than in the extremely obese girls with PCOS, and the percentage of REM-sleep in the obese girls with PCOS was significantly normal-weight and obese controls in comparison to both study groups (p < 0.001). As demonstrated by post-hoc multiple pair wise comparisons, sleep-onset latency in the obese girls with PCOS was significantly higher than in all control groups, and sleep-onset latency in the extremely obese girls with PCOS was significantly higher than in the normal-weight controls (p = 0.001) (table 2).
4. Discussion
To the best of our knowledge, this is the first study comparing polysomnographic variables of obese and extremely obese adolescents with PCOS to those of healthy normal-weight, obese, and extremely obese controls. In this study, the obese and extremely obese adolescents with PCOS did not differ from their healthy, normal-weight, obese, and extremely obese peers concerning the apnea-index, the hypopnea-index, the apnea-hypopnea index, and the absolute number of obstructive apneas. These findings are in line with our previous study, in which we had compared polysomnographic data of adolescents with PCOS to healthy controls on a smaller scale [12].
A further novel aspect of this study is the comparison of polysomnographic data between obese and extremely obese adolescents with PCOS, as on the one hand the risk for and the prevalence of OSAS are clearly increased in adults with PCOS [5-9] and the symptoms of PCOS usually begin around menarche [4], and on the other hand obesity is a well-known risk factor for OSAS in adolescents [13,14]. In this study, we found no significant differences between the respiratory polysomnographic variables between the obese and extremely obese girls with PCOS. This finding can be seen in accordance with the results of Gopal et al. who had detected an increased prevalence of OSAS in patients with PCOS independently of obesity [7]. Accordingly, one study in adults with PCOS demonstrated that the apnea-hypopnea index of obese women with PCOS correlated with serum testosterone and unbound testosterone [5], and other studies in adults with PCOS demonstrated a correlation between the risk and severity of OSAS and parameters of glucose metabolism [6,8,9]. As disturbances of glucose metabolism in patients with PCOS are related to insulin resistance [21-23], and insulin resistance is more related to fat distribution/body composition than to obesity itself [24- 27], it seems probable that the development of OSAS in patients with PCOS is influenced more by the fat distribution/body composition of the patients than by obesity per se. Accordingly, studies in adults have shown that regional body fat distribution predicts the presence and degree of OSAS [28], that visceral fat accumulation is an important risk indicator for OSAS [29], and that the prevalence and severity of OSAS in overweight and obese patients is more dependent on the fat distribution than on the level of total fatness [30].
We found statistically significant differences between the five groups concerning mean transcutaneous arterial oxygen saturation (Sat O2), as mean Sat O2 of the obese girls with PCOS was significantly higher than in all three control groups, and mean Sat O2 of the extremely obese girls with PCOS was significantly lower than in the extremely obese controls. However, it has to be mentioned that these differences were not clinically relevant, as mean Sat O2 was between 96% and 98% in all groups and therefore within the normal range. Furthermore, we have to keep in mind that pulse oximetry has its physiological and technical limitations [31].
Regarding sleep architecture in adults with PCOS, Tasali et al. reported that in comparison to healthy nonobese controls, extremely obese women with PCOS (mean BMI 41.1 kg/m2 ± 2.9) had significantly lower sleep efficiency, longer sleep-onset latency, and less REM-sleep [9]. Our findings are in line with the data from Tasali et al. as in comparison to healthy normalweight controls, the extremely obese girls with PCOS demonstrated significantly lower sleep efficiency, longer sleep-onset latency, and less REM-sleep. Vgontzas et al. found obese PCOS women (mean BMI 38.7 kg/m2 ± 1.1) to have significantly longer sleep-onset latency than healthy controls, but found no difference in percentage of REM-sleep [6]. The healthy controls in the study by Vgontzas et al. had a mean BMI of 26.4 kg/m2 ± 0.3 and were therefore overweight, a weight status between normal-weight and obese. In accordance with Vgontzas et al. [6], the obese girls with PCOS in our study demonstrated significantly longer sleep-onset latency than healthy normal-weight and obese controls and did not differ significantly from the healthy obese girls regarding percentage of REM-sleep. However, we found a significant difference in percentage of REM-sleep between the obese girls with PCOS and the healthy normal weight controls. We can only speculate whether Vgontzas et al. would have found a significant difference in percentage of REM-sleep between the study group and healthy normal weight controls if they had divided their control group according to weight status, as the BMI of the women in their control group ranged from 16.1 to 59.9 kg/m2 [6].
As obese and extremely obese adolescents with PCOS do not seem to differ from their healthy normal-weight, obese, and extremely obese peers regarding respiratory polysomnographic variables, but studies have reported that the risk for and the prevalence of obstructive sleep apnea syndrome (OSAS) are clearly increased in adults with PCOS [5-9], it seems that some patients with PCOS go on to develop OSAS in the course of their disease. Further, longitudinally designed studies with a large cohort of cases and controls are necessary in order to identify the risk factors and pathomechanisms in the development of OSAS in patients with PCOS.
The strengths of this study are the fairly reasonable numbers of cases (n = 33) and controls (n = 48), the age-matched participants, and the division into groups according to weight-status. However, some important limitations of this study need to be mentioned: First, BMI percentiles were used to classify overweight. Although BMI is a good measure for overweight, one needs to be aware of its limitations as an indirect measure of fat mass. BMI and SDS-BMI do not reflect body composition, i.e. the distribution of fat or the relationship between lean body mass and adipose tissue. This could be of significance, as cardiovascular risk factors are closer related to fat distribution than to body weight [32]. Second, although the total number of cases and controls is fairly reasonable in this study, the numbers of girls within the groups are still rather moderate (n = 12 – n = 18). Surely, the numbers of cases and controls within the groups need to be much larger. However, polysomnography is a complex, costly, and time-consuming investigation requiring the overnight hospitalization of the patient, in our case of subjectively healthy adolescents. Furthermore, the numbers of patients with PCOS undergoing polysomnography in the adult studies were not higher. Gopal et al. assessed 23 women [7], Tasali et al. 8 women [9], and Fogel et al. 18 women [5]. Third, we did not assess subjective sleep quality using sleep surveys, such as the Pittsburgh Sleep Quality questionnaire (PSQ), the Berlin Questionnaire (BQ), or the Epworth Sleepiness Scale (ESS). However, in clinical practice such surveys are performed to screen for sleep-related breathing disorders, and the consequence of poor sleep quality (PSQ), a high risk for OSAS (BQ), or chronic daytime sleepiness (ESS) is to perform polysomnography. Fourth, the control patients from the sleep laboratory of our hospital (n = 29; 60.4% of the controls), but not the girls with PCOS, underwent a selection process, as girls with syndromes, girls with any kind of medical disorders, girls on any kind of medication, and girls with OSAS were excluded as controls. Therefore a selection bias of our analyses towards a positive finding in comparing girls with PCOS to controls cannot be ruled out for sure. However, some studies in adults had similar results concerning differences in sleep efficiency and sleep-onset latency [6,9] so that it seems improbable that these differences are caused by a selection bias alone.
In summary, we did not find differences concerning the apnea-index, the hypopnea-index, the apnea-hypopnea index, and the absolute number of obstructive apneas between obese and extremely adolescents with PCOS and healthy, normal-weight, obese, and extremely obese controls, but there seem to be differences concerning the sleep architecture. Furthermore, we found no significant differences between the respiratory polysomnographic variables between the obese and extremely obese girls with PCOS. Taking the data from adult studies into account, it seems that weight status/obesity might not the crucial pathogenic factor in the development of OSAS in patients with PCOS.
REFERENCES
- E. S. Knochenhauer, T. J. Key, M. Kahsar-Miller, W. Waggoner, L. R. Boots, R. Azziz, “Prevalence of the Polycystic Ovary Syndrome in Unselected Black and White Women of the Southeastern United States: a Prospective Study,” Journal of Clinical Endocrinology & Metabolis, Vol. 83, 1998, pp. 3082-3098.
- J. K. Zawadski and A. Dunaif, “Diagnostic Criteria for Polycystic Ovary Syndrome: Towards a Rational Approach,” In: A. Dunaif, J. R. Givens, F. P. Haseltine and G. R. Merriam, Ed., Polycystic Ovary Syndrome. Boston: Blackwell Scientific Publications, 1992, pp. 377-384.
- S. Sam and A. Dunaif, “Polycystic Ovary Syndrome: Syndrome XX?” Trends in Endocrinology and Metabolism, Vol. 14, 2003, pp. 365-370.
- D. A. Ehrmann, “Polycystic Ovary Syndrome,” The New England Journal Medicine, Vol. 352, 2005, pp. 1223- 1236.
- R. B. Fogel, A. Malhotra, G. Pillar, S. D. Pittman, A. Dunaif and D. P. White, “Increased Prevalence of Obstructive Sleep Apnea Syndrome in Obese Women with Polycystic Ovary Syndrome,” Journal of Clinical Endocrinology & Metabolis, Vol. 86, 2001, pp. 1175-1180.
- A. N. Vgontzas, R. S. Legro, E. O. Bixler, A. Grayev, A. Kales and G. P. Chrousos, “Polycystic Ovary Syndrome Is Associated with Obstructive Sleep Apnea and Daytime Sleepiness: Role of Insulin Resistance,” Journal of Clinical Endocrinology & Metabolis, Vol. 86, 2001, pp. 517- 520.
- M. Gopal, S. Duntley, M. Uhles and H. Attarian, “The Role of Obesity in the Increased Prevalence of Obstructive Sleep Apnea Syndrome in Patients with Polycystic Ovarian Syndrome,” Sleep Medicine, Vol. 3, 2002, pp. 401-404.
- E. Tasali, E. Van Cauter, L. Hoffman and D. A. Ehrmann, “Impact of Obstructive Sleep Apnea on Insulin Resistance and Glucose Tolerance in Women with Polycystic Ovary Syndrome,” Journal of Clinical Endocrinology &Metabolis, Vol. 93, 2008, pp. 3878-3884.
- E. Tasali, E. V. Cauter and D. A. Ehrmann, “Relationship between Sleep Disordered Breathing and Glucose Metabolism in Polycystic Ovary Syndrome,” Journal of Clinical Endocrinology & Metabolis, Vol. 91, 2006, pp. 36-42.
- S. Subramanian, A. Desai, M. Joshipura and S. Surani, “Practice Patterns of Screening for Sleep Apnea in Physicians Treating PCOS Patients,” Sleep and Breathing, Vol. 11, 2007, pp. 233-237.
- H. K. Yaggi, J. Concato, W. N. Kernan, J. H. Lichtman, L. M. Brass and V. Mohsenin, “Obstructive Sleep Apnea as a Risk Factor for Stroke and Deat,” The New England Journal Medicine, Vol. 353, 2005, pp. 2034-2041.
- G. D. Sousa, B. Schlüter, D. Buschatz, T. Menke, E. Trowitzsch, W. Andler and T. Reinehr, “A comparison of Polysomnographic Variables between Obese Adolescents with Polycystic Ovarian Syndrome and Healthy, Normal-Weight and Obese Adolescents,” Sleep and Breathing, Vol. 14, 2010, pp. 33-38.
- M. Kalra, T. Inge, V. Garcia, S. Daniels, L. Lawson, R. Curti, A. Cohen and R. Amin, “Obstructive sleep Apnea in Extremely Overweight Adolescents Undergoing Bariatric Surgery,” Obesity Research, Vol. 13, 2005, pp. 1175-1179.
- S. Redline, P. V. Tishler, M. Schluchter, J. Aylor, K. Clark and G. Graham, “Risk Factors for Sleep-disordered Breathing in Children Associations with Obesity, Race, and Respiratory Problems,” American Journal Respiratory Critical Care Medicine, Vol. 159, 1999, pp. 1527- 1532.
- T. J. Cole, M. C. Belizzi, K. M. Flegal and W. H. Dietz, “Establishing a Standard Definition for Child Overweight and Obesity Worldwide: International Survey,” British Medical Journal, Vol. 320, 2000, pp. 1240-1243.
- K. Kromeyer-Hauschild, M. Wabitsch, D. Kunze, F. Geller, C. Geib and V. Hesse, et al., “Percentiles of Body Mass Index in Children and Adolescents Evaluated from Different Regional German Studies,” Monatsschr Kinderheilkd, Vol. 149, 2001, pp. 807-818.
- T. J. Cole, “The LMS Method for Constructing Normalized Growth Standards,” European Journal Clinical Nutrition, Vol. 44, 1990, pp. 45-60.
- A. Rechtschaffen and A. Kales, “A Manual of Standardized Terminology, Techniques and Scoring System for Sleep Stages of Human Subjects,” Washington DC: U.S. Government Printing Office, Washington, 1968.
- AASM, “EEG Arousals: Scoring Rules and Examples,” A Preliminary Report from the Sleep Disorders Atlas Task Force of the American Sleep Disorders Association, Sleep, Vol. 15, 1992, pp. 174-184.
- AASM, “Sleep-related Breathing Disorders in Adults: Recommendations for Syndrome Definition and Measurement Techniques in Clinical Research,” The Report of an American Academy of Sleep Medicine Task Force. Sleep, Vol. 22, 1999, pp. 667-689.
- A. K Schröder, S. Tauchert, O. Ortmann, K. Diedrich and J. M. Weiss, “Insulin Resistance in Polycystic Ovary Syndrome,” Wien Klin Wochenschr, Vol. 115, 2003, pp. 812-821.
- A. K. Schröder, S. Tauchert, O. Ortmann, K. Diedrich and J. M. Weiss, “Insulin Resistance in Patients with Polycystic Ovary Syndrome,” Annals of Internal Medicine, Vol. 36, 2004, pp. 426-439.
- A. Galluzzo, M. C. Amato and C. Giordano, “Insulin Resistance and Polycystic Ovary Syndrome,” Nutrition Metabolism & Cardiovasc Diseases, Vol. 18, 2008, pp. 511-518.
- J. P. Després and I. Lemieux, “Abdominal Obesity and Metabolic Syndrome,” Nature, Vol. 444, 2006, pp. 881-887.
- B. L. Wajchenberg, “Subcutaneous and Visceral Adipose tissue: Their Relation to the Metabolic Syndrome,” Endocrine Reviews, Vol. 21, 2000, pp. 697-738.
- S. E. Kahn, R. L. Prigeon, R. S. Schwartz, W. Y. Fujimoto, R. H. Knopp, J. D. Brunzell, et al., “Obesity, Body Fat Distribution, Insulin Sensitivity and Islet beta-cell Function as Explanations for Metabolic Diversity,” Journal Nutrition, Vol. 131, 2001, pp. 354-360.
- P. Björntorp, “Metabolic Implications of Body Fat Distribution,” Diabetes Care, Vol. 14, 1991, pp. 1132-1143.
- H. Schäfer, D. Pauleit, T. Sudhop, I. Gouni-Berthold, S. Ewig and H. K. Berthold, “Body Fat Distribution, Serum lepton, and Cardiovascular Risk Factors in Men with Obstructive Sleep Apnea,” Chest, Vol. 122, 2002, pp. 829-839.
- E. Shinohara, S. Kihara, S. Yamashita, M. Yamane, M. Nishida and T. Arai, et al., “Visceral Fat Accumulation as an Important Risk Factor for Obstructive Sleep Apnoea Syndrome in Obese Dubjects,” Journal of Internal Medicine, Vol. 241, 1997, pp. 11-18.
- L. Busetto and G. Sergi, “Visceral Fat and Respiratory complications,” Diabetes Obesity Metabolism, Vol. 1, 2005, pp. 301-306.
- J. T. Moyle, “Uses and Abuses of Pulse Oximetry,” Archives Disease Childhood, Vol. 74, 1996, pp. 77-80.
- C. Maffeis, A. Pietrobelli, A. Grezzani, S. Provera and L.Tatò, “Waist Circumference and Cardiovascular Risk.
- Factors in Prepubertal Children,” Obesity Research, Vol. 9, 2001, pp. 179-187.

