Open Journal of Rheumatology and Autoimmune Diseases
Vol.3 No.1(2013), Article ID:27838,8 pages DOI:10.4236/ojra.2013.31003
Anti-Osteoporosis and Anti-Osteoarthritis Activity of Fresh Water Snail (Viviparous bengalensis) Flesh Extract in Experimental Animal Model*
![]()
1Laboratory of Toxinology & Experimental Pharmacodynamics, Department of Physiology, University of Calcutta, Kolkata, India; 2Indian Institute of Chemical Biology, Kolkata, India; 3Department of Zoology, Maulana Azad College, Kolkata, India.
Email: #agomescu@gmail.com
Received October 30th, 2012; revised December 5th, 2012; accepted December 27th, 2012
Keywords: Water Snail; Viviparous bengalensis; Traditional Medicine; Osteoporosis; Osteoarthritis
ABSTRACT
Aims: Aim was to evaluate the anti-osteoporotic and anti-osteoarthritic activity of fresh water snail (Viviparous bengalensis) (VB) flesh extract (VBE) in experimental model. Settings and Design: Experimental osteoporosis (OSP) was developed in female Wistar rats by bilateral overectomy and Osteoarthritis (OA) was developed in male Wistar rats by bacterial collagenase injection. Methods and Material: VB was collected locally and authenticated, then homogenized in 3500 rpm × 15 mins and supernatant was collected. Rats were divided into-Group-1: Sham control, Group-2: OSP/OA control, Group-3: Standard (vitD3 200 mg∙kg−1; p.o., calcium i.p. 1500 mg∙kg−1 × 15 days in OSP and indomethacin 0.25 mg∙kg−1, p.o. × 5 alternative days in OA), Group-4: VBE treated (1 gm∙kg−1; p.o. × 15 days), Group-5: VBE treated (2 mg∙kg−1; p.o. × 15 days). Anti-osteoporotic and anti-osteoarthritic activity of VBE was examined through physical, urinary and serum parameters. Statistical Use: Data were expressed in terms of mean ± SEM (n = 6). ANOVA was performed, p < 0.05 considered significance. Key Findings: It was observed that the body weight, ankle/ knee diameters, urinary markers hydroxyproline/glucosamine/calcium/phosphate/creatinine, serum ACP/ALP/TRAP, calcium/creatinine, cytokines (TNF-α/IL-1β/CINC-1) levels were changed significantly and restored after VBE treatment. Significance: Fresh water snail flesh extract possess anti-osteoporosis and anti-osteoarthritic activity in experimental animal models.
1. Introduction
Osteoporosis and Osteoarthritis are the two common bone-joint related diseases occur throughout the world especially among the elders population [1,2]. Osteoporosis affects both sexes and all races, albeit to different degrees [3]. These are the silent diseases of skeleton characterized by bone fragility due to a reduction in bone mass and possible alteration in bone microarchitecture which leads to a propensity to fracture with minimum trauma.
Osteoporosis is one of the (“porous bones”, in Greek: ortho means “bone” and poros meaning “pore”) common disease of bones that leads to an increased risk of fracture. Mostly it is seen after menopause, as the hormonal levels are altered at that time. Calcium, sodium absorption level is decreased. As a result bone mineral density (BMD) is reduced, bone microarchitecture deteriorates, and the amount and variety of proteins in bone is altered. Osteoporosis is defined by the World Health Organization (WHO) as a bone mineral density that is 2.5 standard deviations or more below the mean peak bone mass (average of young, healthy adults) as measured by DXA; the term “established osteoporosis” includes the presence of a fragility fracture. The disease may be classified as primary type 1, primary type 2, or secondary. The form of osteoporosis most common in women after menopause is referred to as primary type 1 or postmenopausal osteoporosis. Primary type 2 osteoporosis or senile osteoporosis occurs after age 75 and is seen in both females and males at a ratio of 2:1. Finally, secondary osteoporosis may arise at any age and affect men and women equally. This form of osteoporosis results from chronic predisposing medical problems or disease, or prolonged use of medications such as glucocorticoids, when the disease is called steroidor glucocorticoid-induced osteoporosis (SIOP or GIOP).
Osteoarthritis is the common inflammatory disorder of joint. This is characterized by inflammation of synovial membrane, pain and restricted joint movement. Osteoarthritis is derive from the Greek word osteo meaning the bone; artho meaning joint and itis meaning inflammation, although many suffers many have little or no inflammation. OA is the most common form of arthritis and one of the leading causes of chronic disability in the elderly person. Approximately 25% of persons of 55 yrs of age or older have knee pain and almost half of them having symptomatic osteoarthritis, that leads to physical disabilities or loss of functional capacity and reduce quality of life [4]. OA is classified into primary or idiopathic, when there is no obvious pre disposing cause and secondary when there is clearly defined pre disposing cause. Idiopathic osteoarthritis is the most common form of arthritis.
The factors which include the changes in the expression of collagen and other matrix molecules in OA had not been yet identified, but may include changes of the chondrocyte environment, mechanical loading, cytokines, growth factors and perhaps molecular fragments produced by the matrix metabolism [5]. It was accepted that chondrocyte is the target of cytokines action but the sources responsible for generating the cytokines are less understood in the context of OA pathogenesis. The degradation of cartilage and proteoglycans ion OA is due to the imbalance in the proteinases and the inhibitors synthesized by the chondrocyte [6]. Matrix metalloproteinases (MMPs) have been detected in the synovial fluids and cartilage of OA patients. It has been found that the increased levels of inhibitors of metalloproteinases in the synovial fluid of OA reflect an adaptive response to the increased levels of active MMPs. Genetic and hormonal factors also play an important role and established that the mutations in collagen genes (types II, IX, X) appear to contribute to the development of premature idiopathic OA [7].
In both cases, lots of medications are there. In osteoporosis, antiresorptive or bone anabolic agents are mainly used for treatment. Antiresorptive agents work primarily by reducing bone resorption, while bone anabolic agents build bone rather than inhibit resorption. Lifestyle changes are an important aspect of treatment. A major problem is gaining long-term adherence to therapy from patients with osteoporosis. Antiresorptive agents like Bisphosphonates, Estrogen analogs, Raloxifene, Calcitonin, Calcitonin; Bone anabolic agents like Teriparatide, Calcium salts, Sodium fluoride; other agents like strontium ranelate and nutritional therapy can be done for the treatment of osteoporosis. But these all agents either give side effect or work very slowly.
Physiotherapy, physical exercise and analgesics are often prescribed by the rheumatologists. Non-steroidal anti-inflammatory drugs (NSAIDS) like accelophenac, dichlophenac are the first line of defense against osteoarthritis. But NSAIDS work through cyclooxygenase (COX-1 and COX-2) inhibition that inhibit prostaglandins. COX-1 inhibition causes side effects like G.I tract irritation, platelet aggregation etc. Sometimes patients treated with COX-1b shows cardiovascular problems. The COX inhibition helps to protect against osteoarthritic inflammation, pain and arthritic fever. Glucocorticoid therapy also shows certain side effects. For this reason disease modifying anti-rheumatic drugs (DMARDS) are better advised. But DMARDS like methotrexate, cyclosporine A, anti-cytokine therapies all have certain side effects like sepsis, pulmonary and extra pulmonary tuberculosis etc. and also cost effective. So that natural product therapies are ventured.
Natural products such as honey, mussel, snake venom, curcumin, ginger, black tea [8] are used for the treatment of osteoporosis and arthritis. Fresh water snail (Viviparous bengalensis) an Indian gastropod which is consumed by the village people in several ailments. Indian and Chinese folk medicine it has mentioned that snail can increase the strength of bone and prevents joint disorders. Snail flesh also purify blood, boost immune system, snail water prevent conjunctivitis and snail flesh for liver problems [9]. Snail cream used against burn, wound and wrinkle. In the north Bihar, the flesh of Bellamia (Viviparous) bengalensis is used as a traditional medicine against arthritis [10]. Present study was an effort to explore the effect of fresh water snail extract in experimental osteoporosis and osteoarthritis.
2. Materials and Methods
2.1. Chemicals & Reagents
Amphicillin (Hetero Drug Limited, Hyderabad, India); Anesthetic ether (Narsons pharma, India); Arachitol (Solvay Pharma India Limited, India); Bovine serum albumin (Sigma, USA); Calcium sandoz (Novartis, India), Collagenase (Sigma, USA); Ehrlich reagent (SRL, India); CINC-1, IL-1β and TNF-α kit (R & D, USA); DPX (SRL, India); Eosin (Qualigen, India); Folin ciocalteu reagent (SRL, India); Hematoxylin (Qualigen, India); Indomethacin (SRL, India); Methyl prednisolone (Pfizer, India); Paradimethyl amino benzaldehyde (SRL, India); Osteomol (Merck, India); Sodium pentobarbitone (Loba, India); Sodium potassium tartarate (Merck, India); Xylene (Merck, India).
2.2. Preparation of Viviparous bengalensis Extract
Live adult fresh edible water snail (Viviparous bengalensis) were collected and foot pad portion was dissected out and 10% aqueous flesh homogenate (VBE) was prepared in PBS (0.01 M, pH 7.2) by homogenization. The concentration of Viviparous bengalensis extract (VBE) was expressed in terms of protein content [11].
2.3. Experimental Animals
Wistar female/male albino rats (120 ± 10 g) were procured from the approved animal breeders M/S. Reeta Ghosh, Kolkata, India and housed in standard polypropylene cages at controlled temperature (24˚C ± 2˚C), with light conditions (12 h light and dark cycle) and relative humidity (60% ± 5%). The animals were provided with pellet diet (Ashirbad & Company, Chandigarh, India), green vegetables, gram and water ad libitum. The experiments were conducted according to the departmental animal ethics committee for the purpose of control and supervision of experiments on animals. All animal experiments were approved by the animal ethics committee, Department of Physiology, University of Calcutta and were in accordance with the guideline of the committee for the purpose of control and supervision of experiments on animal (CPCSEA), Government of India (Animal Ethical Committee approval no. PHY/CU/IAEC/ 20/2008).
2.4. Development of Osteoporosis Model
OSP was developed in female albino rat by bilateral overectomy [12].
2.5. Develpoment of Osteoarthritis Model
OA was introduced by intra-articular junction of 20 ml bacterial collagenase (5 CDU) in the right knee joint. Same amount of 0.9% saline will be injected in the left knee joint [13].
Urinary Parameters
24 hr. urine was collected from each group on day 14 (in osteoarthritis) and day 25 (in osteoporosis) under light liquid paraffin. Urinary hydroxy-proline (OH-P) [14] and glucosamine [15] were measured spectophotometrically and Calcium (Ca2+), creatinine (CRE) and phosphate (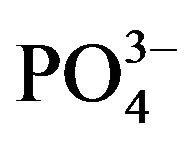 ) were also measured using biochemical kit (Merck, India).
) were also measured using biochemical kit (Merck, India).
2.6. Treatment Schedule
In both OSP/OA, all the rats were divided into five groups (n = 6) as follows—Group-1: Sham control, Group-2: OSP/OA control, Group-3: Standard, Group-4: VBE treated (1 g∙kg−1 body weight), Group-5: VBE treated (2 g∙kg−1 body weight). In case of OSP animals of Group-3 were received standard drug vitamin D3, arachitol (200 mg∙kg−1) and calcium (1500 mg∙kg−1) × 15 days, i.p.; in case of OA, standard group treated with standard antiarthritic drug Indomethacin (0.25 mg∙100 g−1 × 5 alternative days, p.o.). Animals of Group-4, Group-5 VBE treated (1 and 2 g∙kg−1, p.o. × 15 days). In case of OSP, at day 39, urine was collected & urinary parameters were performed. On day 41, body weight was recorded and the animals were anaesthetized with anaesthetic ether. Blood was collected from hepatic portal vein and serum was separated for analysis of biochemical markers. Tibias (long bones) were collected for histological studies.
In the same manner, in case of OA, urine was collected on day 14 & OA was confirmed through urinary hydroxy-proline and glucosamine measurement. On the day 16, blood was collected and serum parameters were done. Ankle/knee was collected for histological studies.
2.7. Biochemical Markers of Urine/Serum/Plasma
Urinary OH-P (Neuman and Logan, 1960) and glucosamine (Elson and Morgan, 1933) and minerals Ca2+, 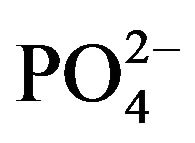 and CRE were measured. Serum enzymes alkaline and acid phosphatase (Karnovsky and Karnovsky, 1971) and minerals
and CRE were measured. Serum enzymes alkaline and acid phosphatase (Karnovsky and Karnovsky, 1971) and minerals 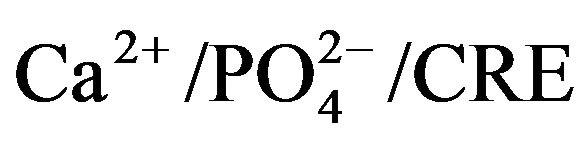 were measured by the previous methods. Serum interleukins CINC-1/TNFa/IL-1β were measured using ELISA kit with the help of ELISA Reader (Biotek, USA; Model No. EL × 800 MS).
were measured by the previous methods. Serum interleukins CINC-1/TNFa/IL-1β were measured using ELISA kit with the help of ELISA Reader (Biotek, USA; Model No. EL × 800 MS).
2.8. Bone Histology
Bones/Joints were fixed in 10% buffered formalin for 24 hours, decalcified in Osteomol for 4 - 5 days, dehydrated in graded alcohol (70%, 80%, 90%), cleared in xylol and embedded in paraffin wax (56˚C - 58˚C). Sections (6 mm) were cut with rotary microtome (Weswox Optik, India), stained with haematoxylin-eosin, and observed under bright field microscope (Motic BA 450, Germany) and photography were captured with Motic software (Motic Images Plus 2.0 software).
2.9. Statistical Analysis
Data were expressed as means ± SEM (n = 6). The repeated measure analysis of variance
(ANOVA) was used to determine significant differences between groups p < 0.05 was considered to be statistically significant.
3. Results
3.1. Anti-Osteoporosis Effect of VBE
VBE (1 & 2 g∙kg−1 body weight) treated groups showed 4.9% ± 0.7% and 8.1% ± 0.5% increase in body weight, whereas the standard drug treatment showed 5.1% ± 0.8% increase as compared with OSP control group. VBE (1 & 2 g∙kg−1) treated groups showed 23.05% ± 2.3% and 48.53% ± 3.9% decrease in urinary OH-P and 41.30% ± 3.6% and 53.90% ± 5.1% decrease in glucosamine level respectively; whereas the standard drug treatment showed 48.83% ± 2.7% decrease in OH-P and 54.32% ± 4.3% decrease in glucosamine level as compared with the OSP group (Table 1). It (1 & 2 g∙kg−1) showed decrease in urinary calcium (41.87% ± 8% and 54.52% ± 7%), phosphate (69.09% ± 4% and 81.36% ± 4%), creatinine (11.17% ± 6% and 11.71% ± 7%) level in OSP model, whereas standard drug treatment showed 48.49% ± 6%, 63.48% ± 7% and 13.15% ± 4% decrease respectively in urinary calcium, phosphorous and createnine as compared with the OSP control (Table 1). VBE (1 & 2 g∙kg−1) treated groups showed significant (p < 0.05) decrease in serum ACP (43.76% ± 3.6%, 51.77% ± 2.5%), ALP (15.80% ± 2.1%, 30.89% ± 4.1%) and TRAP (31.91% ± 1.9%, 49.11% ± 3.0%) level, whereas standard drug treatment showed 34.05 ± 3.0% decrease in ACP, 31.11% ± 2.0% decrease in ALP and 49.82% ± 1.8% decrease in TRAP levels as compared with OSP control (Figure 1). VBE (1 & 2 g∙kg−1) treated groups showed 28.30% ± 6.13%, 31.06% ± 4.51% decrease in serum calcium and 31.71% ± 5.08%, 48.56% ± 6.20% decrease in creatinine levels in OSP, whereas standard drug showed 39.11% ± 6.35% and 27.30% ± 4.11% decrease in both calcium and phosphorus level as compared with OSP control (Table 1).
3.2. Anti-Osteoarthritis Effect of VBE
In OA treated groups, ankle/knee swellings were significantly decreased after treatment with VBE (1 and 2 g∙kg−1, p.o. × 15 day). VBE (1 & 2 g∙kg−1) treated groups showed 53.64% ± 5.13% and 62.17% ± 6.09% decrease
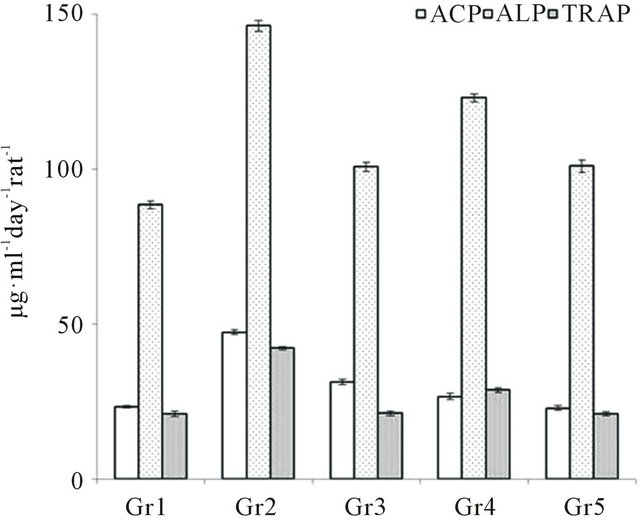
Figure 1. Effect of VBE and standard drug (Vit-D3 & arachitol) on serum ACP/ALP/TRAP levels of OSP in rats. Concentration of ACP/ALP/TRAP was expressed in terms of μmol of PNP released/minute. Data represent the mean ± SEM (n = 6) p < 0.05 when compared to OSP control group.
in urinary OH-P and 23.10% ± 0.77% and 56.65% ± 0.22% decrease in glucosamine; whereas standard drug (indomethacin) showed 56.66% ± 6.06% decrease in OH-P and 46.10% ± 4.56% decrease in glucosamine level as compared with OA control (Table 2). VBE (1 & 2 g∙kg−1) treated groups showed 14.38% ± 5.07%, 59.38% ± 4.14% decrease in urinary calcium, 71.09% ± 4.12%, 82.65% ± 4.07% decreased in phosphate and 13.17% ± 2.03% and 15.71% ± 2.04% decrease in creatinine level, whereas standard drug showed 52.19% ± 5.06%, 57.82% ± 5.11% and 13.87% ± 2.06% decrease respectively as compared with OA control (Table 2). VBE (1 & 2 g∙kg−1) treated groups showed 28.00% ± 2.72%, 41.49% ± 3.83% decrease in serum ACP and 13.68% ± 1.70%, 19.22% ± 1.98% decrease in ALP level, whereas standard drug treatment showed 49.28% ± 2.68% and 32.01% ± 1.32% decrease respectively as compared with OA control group (Table 2). VBE (1 & 2 g∙kg−1) treated groups showed 28.65% ± 0.42%, 32.11% ± 0.83% decrease in serum calcium and 39.31% ± 1.03%, 44.91% ± 3.02% decrease in creatinine levels, whereas standard drug treatment showed 30.56% ± 0.04% and 42.65% ± 3.02% decrease respectively as compared with OA control (Table 2). VBE (1 & 2 g∙kg−1) treatment signifycantly (p < 0.05) decreased in serum TNF-α (35.21% ± 2.12%, 45.40% ± 3.11%), IL-1β (29.65% ± 0.93%, 35.59% ± 1.45%) and CINC-1 (24.65% ± 4.98%, 35.61% ± 2.32%) level respectively in OA and standard drug decreased 30.23% ± 1.20%, 14.43% ± 2.72%, 32.41% ± 4.87% respectively on TNF-α, IL-1β and CINC-1 level as compared with OA control (Figure 2). VBE (1 & 2 g∙kg−1) treated group of rats showed sign of restoration of cartilage damage, decreased synovial space as compared with the arthritic control group of rats where cellular infiltra-
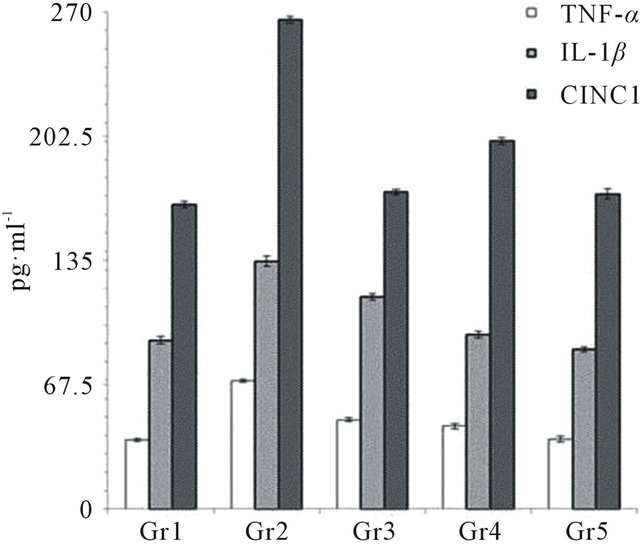
Figure 2. Effect of VBE and standard drug (Indomethacin) on serum TNF-α, IL-1β & CINC-1 levels of OA in rats. Concentration of TNF-α, IL-1β & CINC-1 was expressed in terms of pg/ml. Data represent the mean ± SEM (n = 6) p < 0.05 when compared to OA control group.
Table.1. Effect of VBE on urinary and serum parameters in OSP rats. Data represent the mean ± SEM (n = 6). p < 0.05 when compared to OSP control group.
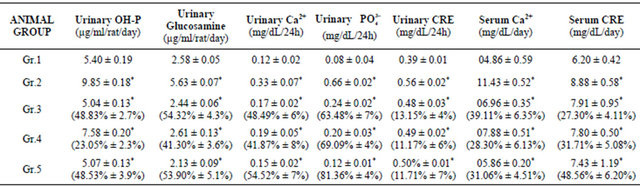
Data represent the mean ± SEM (n = 6). *p < 0.05 when compared to OSP control group. Data represent the mean ± SEM (n = 6). p < 0.05 when compared to OSP control group. Group 1: Sham control, Group 2: OSP control, Group 3: Standard drug (arachitol, calcium), Group 4: VBE (1 mg∙kg−1, p.o. × 15 day), Group. 5: VBE (2 mg∙kg−1, p.o. × 15 day).
Table 2. Effect of VBE on urinary and serum parameters in OA rats. Data represent the mean ± SEM (n = 6). p < 0.05 when compared to OA control group.
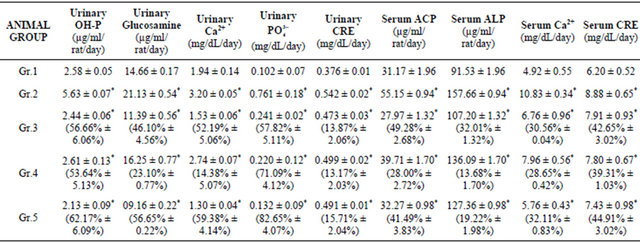
Data represent the mean ± SEM (n = 6). *p < 0.05 when compared to OA control group. Data represent the mean ± SEM (n = 6). p < 0.05 when compared to OA control group. Group 1: Sham control, Group 2: OA control, Group 3: Standard drug (indomethacin), Group 4: VBE (1 mg∙kg−1, p.o. × 15 day), Group 5: VBE (2 mg∙kg−1, p.o. × 15 day).
tion, cartilage damage, increased synovial space was observed.
4. Discussion
Present study showed that snail flesh extract (VBE-1 & 2 g∙kg−1) significantly reduced the ankle/knee swellings of the right hind leg of albino rats as compared with the arthritic group. Urinary hydroxyproline [16] and glucosamine [17,18] showed that the increased hydroxyproline level in albino rats of osteoporosis and osteoarthritic group significantly lower in treated group. Therefore, significant restoration of urinary hydroxyproline by VBE treatment indicated that it inhibits collagen break down and thereby preventing the cartilage damage caused by both overiectomy and collagenase induction. Glucosamine has been shown to exhibit preventive actions on osteoporosis and osteoarthritis in humans as well as in rats [19]. During both OSP/OA [20] bone resorption leads to release of calcium, phosphorous into extracellular fluid, then organic matrix resorbed and are excreted through urine. As a result, the serum and urinary level of calcium, phosphate and creatinine levels were altered. VBE treatment restored the levels of calcium, phosphate and creatinine. In OSP/OA, the ACP, ALP [21] and TRAP level increased in the serum [22]. VBE treatment significantly decreased the ACP and ALP levels which indicated that VBE could resist and restore the damages of lysosomal membrane integrity.
Due to T cell activation TNF-α, IL-1β, CINC1 levels were significantly increased in the arthritic group which was restored by VBE treatment. These results indicated that VBE treatment shifted the balance of the cytokine milieu in the joints away from the pro-inflammatory cytokines (CINC-1) and towards the production of antiinflammatory cytokines, such as TNF-α and IL-1β. The above results indicated that VBE is effective in experimental OSP/OA and also validated the traditional-folk use of snail flesh for the alleviation of bone and joint diseases, especially OSP/OA.
5. Conclusion
Fresh water snail Viviparous bengalensis flesh extract (VBE) significantly suppressed the development and progression of OSP/OA in experimental animals, which was evidenced from its effect on preventing ankle/knee swellings, decreasing the urinary markers, maintaining the lysosomal integrity by suppressing the serum enzymes and cytokines. Therefore, the findings suggested that VBE could be beneficial in the treatment of osteoporosis/ osteoarthritis but further studies are in progress to identify the mechanisms of action in bone-joint disorders.
6. Acknowledgement
This work was partly sponsored by Department of Science and Technology, New Delhi (Ref. No. SR/SO/HS- 58/2008).
REFERENCES
- J. A. Buckwalter, H. J. Mankin and A. J. Grodzinsky, “Articular Cartilage and Osteoarthritis,” Instructional Course Lectures, Vol. 54, 2005, pp. 465-480.
- J. E. Chrubasik, B. D. Roufogalis and S. Chrubasik, “Evidence of Effectiveness of Herbal Antiinflammatory Drugs in the Treatment of Painful Osteoarthritis and Chronic Low Back Pain,” Phytotherapy Research, Vol. 21, No. 7, 2007, pp. 675-683. doi:10.1002/ptr.2142
- L. J. Melton III, K. I. Alothman, S. Khosla, S. J. Achenbach, A. L. Oberg and H. Zincke, “Fracture Risk Following Bilateral Orchiectomy,” Journal of Urology, Vol. 169, No. 5, 2003, pp. 1747-1750. doi:10.1097/01.ju.0000059281.67667.97
- R. C. Lawrence, C. G. Helmick, F. C. Arnett, et al., “Estimates of the Prevalence of Arthritis and Selected Musculo Skeletal Disorders in the United States,” Arthritis & Rheumatism, Vol. 41, No. 5, 1998, pp. 778-799. doi:10.1002/1529-0131(199805)41:5<778::AID-ART4>3.0.CO;2-V
- M. B. Goldring, et al., “The Role of Cytokines as Inflammatory Mediators in Osteoarthritis: Lessons from Animal Models,” Connective Tissue Research, Vol. 40, No. 1, 1999, pp. 1-11. doi:10.3109/03008209909005273
- P. Garnero, et al., “Molecular Basis and Clinical Use of Biochemical Markers of Bone, Cartilage and Synovium in Joint Diseases,” Arthritis & Rheumatism, Vol. 43, No. 5, 2000, pp. 953-968. doi:10.1002/1529-0131(200005)43:5<953::AID-ANR1>3.0.CO;2-Q
- N. Deborah, “Role of NF-κβ in the Skeleton,” Cell Research, Vol. 21, No. 1, 2011, pp. 169-182. doi:10.1038/cr.2010.159
- P. Datta, A. Sarkar, A. K. Biswas and A. Gomes, “Anti Arthritic Activity of Aqueous Extract of Indian Black Tea in Experimental and Clinical Study,” Oriental Pharmacy and Experimental Medicine, Vol. 12, No. 4, 2012, pp. 265-271. doi:10.1007/s13596-012-0087-x
- A. Gomes, A. M. Alam, P. Datta, S. Bhattacharya and G. Aparna, “Hepatoprotective Activity of the Edible Snail (Bellamia bengalensis) Flesh Extract in Carbon Tetrachloride Induced Hepatotoxicity in Rats,” Journal of Ethnopharmacology, Vol. 138, No. 1, 2011, pp. 228-232. doi:10.1016/j.jep.2011.09.009
- A. K. Prabhakar and S. P. Roy, “Ethno-Medicinal Uses of Some Shell Fishes by People of Kosi River Basin of North-Bihar, India,” Ethnomedicine, Vol. 3, No. 1, 2009, pp. 1-4.
- O. H. Lowry, N. J. Rosebrough, A. L. Farr and R. J. Randall, “Protein Measurement with the Folin Phenol Reagent,” The Journal of Biological Chemistry, Vol. 193, No. 1, 1951, pp. 265-275.
- S. Halder, S. Das Gupta, G. Aparna, B. Giri, S. C. Das Gupta, A. Biswas, R. Mishra and A. Gomes, “A High Molecular Weight Protein Bengalin from the Indian Black Scorpion (Heterometrus bengalensis C.L. Koch) Venom Having Antiosteoporosis Activity in Female Albino Rats,” Toxicon, Vol. 55, No. 2-3, 2009, pp. 455-461. doi:10.1016/j.toxicon.2009.09.013
- P. M. Van der Kraan, E. L. Vitters, L. B. van de Putte and W. B. van den Berg, “Development of Osteoarthritic Lesions in Mice by ‘Metabolic’ and ‘Mechanical’ Alterations in the Knee Joints,” American Journal of Pathology, Vol. 135, No. 6, 1989, pp. 1001-1014.
- R. E. Neuman and M. A. Logan, “The Determination of Hydroxyproline,” The Journal of Biological Chemistry, Vol. 184, No. 1, 1950, pp. 299-306.
- L. A. Elson and W. T. Morgan, “A Colorimetric Method for the Determination of Glucosamine and Chondrosamine,” Biochemical Journal, Vol. 27, No. 6, 1933, pp. 1824-1828.
- T. A. Dull and P. H. Henneman, “Urinary Hydroxyproline as an Index of Collagen Turnover in Bone,” The New England Journal of Medicine, Vol. 268, No. 3, 1963, pp. 132-134.
- D. T. Felson, “Glucosamine and Chondroitin Sulfate in Knee Osteoarthritis: Where Now?” Nature Clinical Practice Rheumatology, Vol. 2, No. 7, 2006, pp. 356-357.
- L. Forchhammer, M. Thorn, O. Met, M. Gad, M. S. Weidner and M. H. Claesson, “Immunobiological Effects of Glucosamine in Vitro,” Scandinavian Journal of Immunology, Vol. 58, No. 4, 2003, pp. 404-411. doi:10.1046/j.1365-3083.2003.01313.x
- H. Nakamura, K. Masuko, K. Yudoh, T. Kato, T. Kamada and T. Kawahara, “Effects of Glucosamine Administration on Patients with Rheumatoid Arthritis,” Rheumatology International, Vol. 27, No. 3, 2007, pp. 213-218. doi:10.1007/s00296-006-0197-1
- L. G. Raisz and G. A. Rodan, “Pathogenesis of Osteoporosis,” Endocrinology and Metabolism Clinics of North America, Vol. 32, No. 1, 2003, pp. 15-24. doi:10.1016/S0889-8529(02)00055-5
- R. F. Mitchell, M. J. Karnovsky and M. L. Karnovsky, “The Distribution of Some Granule Associated Enzymes in Guineapig Polymorphonuclear Leucocytes,” Biochemical Journal, Vol. 116, No. 2, 1970, pp. 207-214.
- A. Gomes, S. Bhattacharya, M. Chakraborty, P. Bhattacharjee and R. Mishra, “Anti-Arthritic Activity of Indian Monocellate Cobra (Naja kaouthia) Venom on Adjuvant Induced Arthritis,” Toxicon, Vol. 55, No. 2-3, 2010, pp. 670-673. doi:10.1016/j.toxicon.2009.10.007
List of Abbreviation
Acid Phosphatase—ACP Alkaline Phosphatase—ALP Chemical Dispensing Unit—CDU Cytokine-Induced Neutrophil Chemoattractant-1—CINC-1 Interleukin-1β—IL-1β
Osteoarthritis—OA
Osteoporosis—OSP Tartrate Resistant Acid Phosphatase—TRAP Tumor necrosis factor α—TNF-α
Viviparous bengalensis—VB Viviparous bengalensis extract—VBE
NOTES
*Conflict of interest statement: We declare that there is no conflict of interest among the authors.
#Corresponding author.

