Open Journal of Rheumatology and Autoimmune Diseases
Vol. 2 No. 2 (2012) , Article ID: 18963 , 7 pages DOI:10.4236/ojra.2012.22004
TNF-α Antagonist and Infection in Rheumatoid Arthritis
![]()
1Department of Epidemiology, Harvard School of Public Health, Boston, USA; 2Clinical Epidemiology Unit, Karolinska Institutet, Stockholm, Sweden; 3Department of Medicine, Division of Rheumatology, Immunology, and Allergy, Brigham and Women’s Hospital, Boston, USA.
Email: *julia.simard@post.harvard.edu
Received December 2nd, 2011; revised January 5th, 2012; accepted January 12th, 2012
Keywords: Inverse Probability Weighting; Anti-TNF; Infection; Rheumatoid Arthritis
ABSTRACT
Background: Anti-TNF treatment may increase infection risk, although this has been difficult to study because the timing of anti-TNF treatment is driven by disease activity, which may influence infection susceptibility leading to confounding that varies over time. We evaluated the association between anti-TNF initiation in rheumatoid arthritis (RA) patients on disease modifying anti-rheumatic drugs (DMARD) and infection using multiple approaches adjusting for time-varying confounding. Methods: 383 anti-TNF-naïve RA patients on ≥1 non-biologic-DMARD at enrollment from the Brigham and Women’s Rheumatoid Arthritis Sequential Study (BRASS) were followed up to two years. Pooled logistic regressions estimated the association between anti-TNF and infection by including time-varying covariates in the adjusted models and inverse probability treatment weighting (IPTW). Results: Adjustment for time-varying disease activity and other suspected confounders yielded non-statistically significant positive associations between antiTNF start and infection regardless of analytic approach (RRmvar_adj = 2.1, 95% CI: 0.8 - 5.8). Conclusions: Incorporating changing clinical status, and treatment indications and consequences, yielded consistently (though not significantly) elevated relative risks of infection associated with anti-TNF initiation. Due to limited statistical power, we cannot draw firm conclusions. However, we have illustrated multiple approaches adjusting for potential time-varying confounding in longitudinal studies and hope to replicate the approaches in larger studies.
1. Introduction
Until recently the treatment for rheumatoid arthritis (RA) centered on symptom management and medications to reduce inflammation, consisting of non-steroidal anti-inflammatory drugs, corticosteroids and non-biologic disease modifying anti-rheumatic drugs (DMARDs) such as hydroxychloroquine and methotrexate (MTX). Biologic agents, such as tumor necrosis factor alpha antagonists (anti-TNFs), were introduced in 1999 initially targeting patients with more severe disease and, in whom, the risks of infection with use of immunosuppressive drugs is of concern.
Bacterial and mycobacterial infections have been associated with TNF-α suppression [1-3], but some studies found that patients with RA also have an elevated incidence of infections [4-8]. Disease activity and measures of severity have been associated with higher risk of infection [9], even before the biologics era [4]. Furthermore, common immunosuppresive treatments [3,10-14] have also been associated with infection [15-17]. Therefore, it is unclear whether the observed risk of infection in RA is a product of disease-related activity, pharmacologically-induced immuno suppression, common co-morbid conditions, or a combination of these factors.
Early on, anti-TNF therapies were reserved for patients with active disease, poorly controlled by non-biologic DMARD therapy. This treated population was placed on anti-TNF treatment to manage increased levels of disease activity, both factors associated with infection. By focusing on prevalent non-biologic DMARD users at enrollment, our goal was to investigate the clinicallyrelevant question of what is the risk of infection associated with introducing an anti-TNF treatment to patients with RA on background non-biologic DMARDs at study start? In addition to this, we wanted to demonstrate alternate analytic approaches to account for the potential time-varying effects of changes in treatment indication and consequences of treatment such as changing disease activity. We, therefore, evaluated the effect of anti-TNF use on the occurrence of infection in a prospective cohort study of patients with confirmed RA in the Brigham and Women’s RA Sequential Study (BRASS). The hypothesis was that the addition of anti-TNF treatment to patients treated with non-biologic-DMARDs would increase the risk of infection in subjects with RA.
2. Patients and Methods
2.1. Study Population
BRASS is an ongoing prospective cohort of patients with RA started in September 2003. Subjects had to be current confirmed RA patients treated at the Brigham and Women’s Hospital in Boston, MA, English-speaking, at least 18 years of age, and have no diagnosis of lupus or psoriatic arthritis. Subjects provided signed, informed consent. Nearly 1000 patients with RA were recruited by May 2007. We restricted the study population to antiTNF-naïve subjects who reported current non-biologic DMARD treatment at enrollment, did not have an infection in the prior month, and were followed for at least six months leaving 383 BRASS participants.
2.2. Data Collection
Subjects completed structured questionnaires every six months that included a disease activity instrument, the RA Disease Activity Index (RADAI) [18] and were examined by study physicians annually (Figure 1). At enrollment and annually, blood and urine were collected and data were collected on demographic and lifestyle factors, current and past medication use, general health, function, and pain symptoms. Rheumatoid factor (RF) was measured by an immunoturbidimetric technique on the Cobas Integra 700 analyzer (Roche Diagnostics—Indianapolis, IN). Anti-cyclic citrullinated peptide antibody titer (CCP) was measured by a second generation anti-CCP ELISA assay (Inova Diagnostics, Inc.—San Diego, CA). From the patient questionnaires, medication use, co-morbid conditions, adverse events, use of complementary and alternative medicines, disease exacerbations, diet, health care utilization, and quality of life were also collected. Subjects could seek medical care from their rheumatologists and other health care providers outside of the
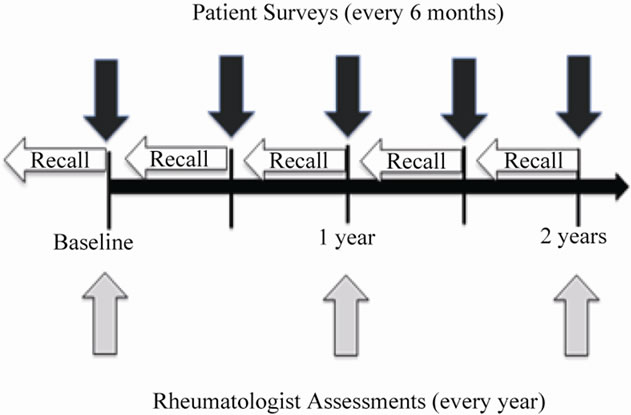
Figure 1. Structure of follow-up and data collection
BRASS follow-up protocol and the electronic inpatient and outpatient records were available for review for patients seen within the Partners HealthCare network.
2.3. Anti-TNF Treatment
Medication use including anti-TNF treatment was assessed at baseline and at 6, 12, 18 and 24 months. Physicians reported medication changes and reasons for changes. Subjects provided details about current medication use (dose, frequency, and duration), including etanercept, adalimumab and infliximab. There was excellent agreement between self-reported medication use and medical records in a sample of approximately 10% of the full BRASS cohort (κ = 0.8 to 0.95) [19]. For the present analysis exposure was defined as initiation of an antiTNF drug between enrollment and the 18 month survey. Subjects were considered exposed when their first antiTNF treatment was reported during follow-up and allowed to vary over time; to account for prolonged exposure we also evaluated the effect of current exposure while simultaneously adjusting for exposure in the previous survey period. During follow-up, however, nearly all patients remained on therapy once initiated. In a sensitivity analysis, we assumed once a subject was exposed, they were always exposed.
2.4. Documented Infection
Infections were reported by rheumatologists and subjects by survey (Figure 1). Study physicians annually reported data on infections and drug-related toxicities occurring since the previous assessment. Questionnaire items asked about infections requiring antibiotics or intravenous therapy, as well as any infections as reason for change in therapy. Subjects were asked to self-report infections every six months during follow-up; they were specifically asked about infections requiring antibiotics. Any report of infection during the follow-up was verified by medical record review using a previously published algorithm that incorporated serologic evidence, antimicrobial treatment, and clinical and radiologic findings [20,21].
2.5. Other Covariates
At enrollment, we collected data on age, sex, education (college graduate vs not), race/ethnicity, co-morbidities (diabetes, alcoholism/drug abuse, COPD), cigarette smoking, and disease duration. RA-related autoantibodies were measured at baseline to assess seropositivity, which was defined as either RF > 15 or CCP > 20. Baseline presence of extra-articular manifestations was reported by each patient’s treating rheumatologist and included pericarditis, pulmonary nodule and neuropathy, among others.
Time-varying factors included disease activity, assessed by RADAI scores collected every 6 months [18]; high disease activity was defined as RADAI ≥ 4.9. Use of concomitant medications (corticosteroids, MTX, and other DMARDs) and frequency of physician visits was collected at each survey (baseline and during follow-up). Results from laboratory tests were collected from the electronic medical records on white blood cell count (WBC), albumin, and hematocrit for any time during participation in BRASS. Abnormal serology was defined as WBC > 10 × 109 cells per liter, albumin < 3.7 U/L, or hematocrit < 36%.
2.6. Follow-Up
Subjects were followed until first documented infection, loss to follow-up, two years of follow-up, or end of study period (May 2007), whichever came first. The structured survey and data collection resulted in interval censored data. Subjects initiating anti-TNF therapy between their first (baseline) and second (6 months) survey periods reported exposure on the 6 month survey and were therefore classified as having exposure start at month 6 (or the second survey period). Infection outcomes were included from the next survey period to ensure temporality.
2.7. Statistical Analysis
Cohort characteristics were summarized using descriptive statistics by initiation of anti-TNF treatment during follow-up and by whether an infection was documented during follow-up. These groups were compared using t-tests, chi-square tests and Fisher’s exact test as appropriate. Pooled logistic regression models were used to estimate the relative risk (odds ratios) of infection associated with use of anti-TNF exposure. Subjects were followed to the end of each survey period and classified as having an event or not at that time. Covariates in the multivariable-adjusted models were selected a priori based on their hypothesized relationship to anti-TNF initiation, other exposures and risk of infection. In multivariable-adjusted models, we included time-invariant confounders measured at baseline including sex, age, education, race/ethnicity, co-morbid conditions, disease duration and seropositivity. We modeled disease activity, concomitant medications, and physician visit frequency as time-varying covariates. Logistic regression models were used to calculate each subject’s probability of remaining uncensored as a function of age, sex, disease duration, race/ethnicity, seropositivity, disease activity, MTX treatment, doctor visit frequency, and extra-articular manifestations, and conducted analyses incorporating inverse probability of censoring weights (IPCW) to account for possible loss to follow-up related to disease status [22].
Finally, inverse probability treatment weighting (IPTW) of pooled logistic regression models with robust variance to estimate 95% CI, was conducted to estimate the OR of infection [23]. We used standard methods to construct the IPW by logistic regression to include disease activity and other suspected determinants of treatment status (i.e. the time-varying confounders included in the unweighted, adjusted pooled logistic models). Time-invariant and baseline covariates were also included in the models to construct weights. Stabilized weights were calculated and their distribution examined to check for outliers and influential weights. We used simple diagnostics to identify overt violations to the positivity assumption such as examining contingency tables for zero cells and looking at the distribution of propensity of treatment and the above calculated weights. To account for potential informative censoring, IPCW were also incorporated into the stabilized weights [23].
3. Results
Of 383 BRASS subjects taking non-biologic DMARDs at baseline, 66 (17%) initiated anti-TNF treatment during follow-up; initiators were more likely to be female, younger, and have been treated with corticosteroids (Table 1). There were 56 subjects with at least one documented infection during follow-up. Seven subjects had documented infections after initiating anti-TNF therapy and the remaining 49 infections were among those had not initiated anti-TNF treatment. Women, corticosteroid users, those with extra-articular manifestations, and higher disease activity at enrollment had a higher incidence of documented infection (Table 2).
In the simplest models accounting for follow-up time, recent anti-TNF exposure was positively but not significantly associated with infection (RR = 2.3, 95% CI = 0.9 to 5.9) adjusting for anti-TNF exposure during the previous survey period as well. Results were similar in multivariable adjusted models adjusting for age, sex, extraarticular manifestations, corticosteroids and seropositivity (RR = 2.3, 95% CI: 0.9 to 6.0). Further adjustment for education, disease duration at baseline, race, smoking status at baseline, baseline disease activity, high MDHAQ, and presence of co-morbid conditions with potential contraindications for therapy did not materially alter the results, nor did using propensity score adjustment for multivariable adjustment. When adjusting for changing disease activity and treatment status, i.e. the time-varying confounders, the results were similar (RR = 2.1, 95% CI: 0.8, 5.8) (Table 3). When we applied IPCW to the above pooled logistic regression models, effect estimates and 95% CI were consistent with those obtained from IPTW models.
Most of the infections among the anti-TNF initiators occurred early; six of the seven anti-TNF exposed events
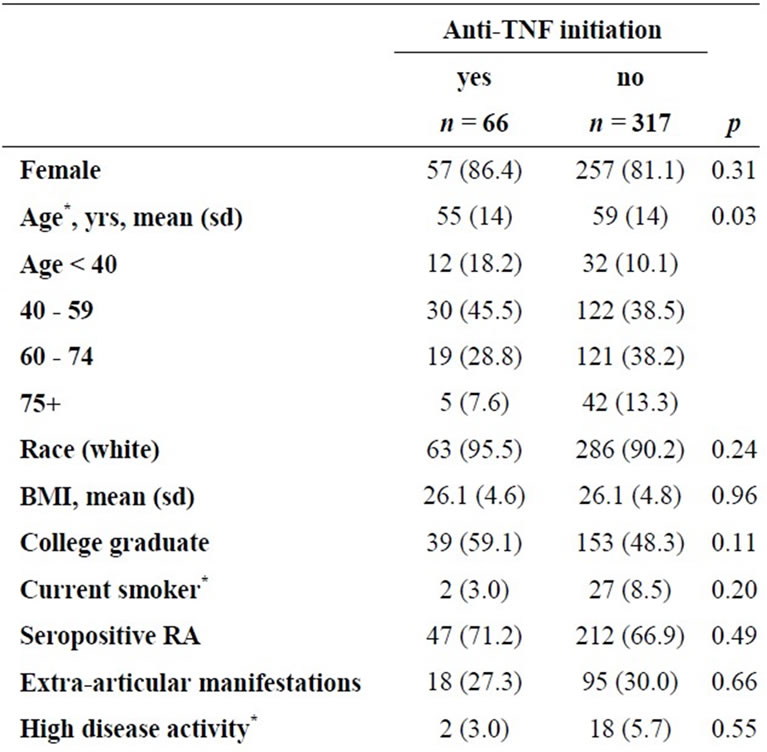
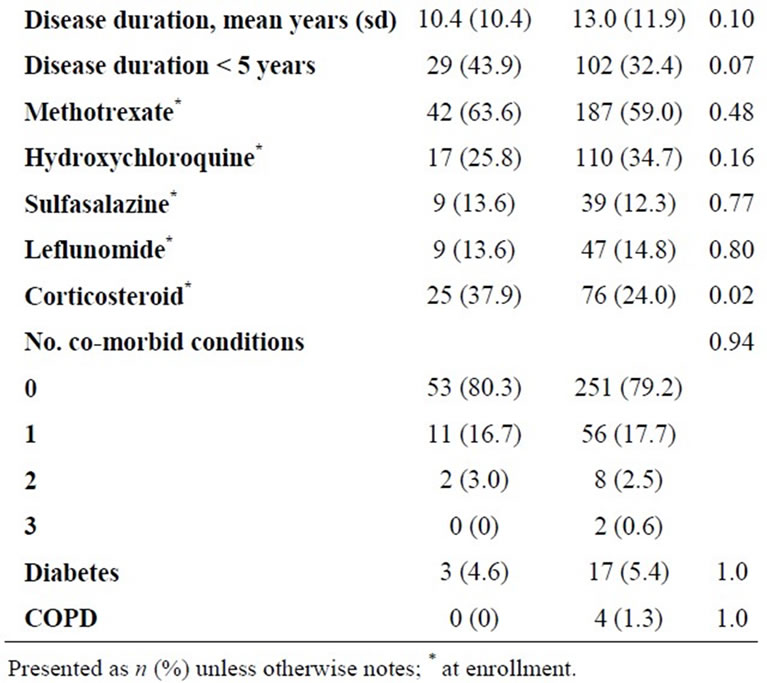
Table 1. Characteristics of BRASS participants by TNF-α treatment initiation in the first 18 months of follow-up (restricted to TNF-naïve, prevalent DMARD users).
occurred in the first 6 month period following anti-TNF treatment initiation.
4. Discussion
Using different analytic techniques to explore the association between anti-TNF treatment and documented infection in subjects with RA, we found that initiating anti-TNF therapy in subjects on non-biologic DMARDs at enrollment may increase the risk of infection although we did not have sufficient statistical power in this study with only seven infections occurring after anti-TNF initiation. However, the magnitude of our observed associations between anti-TNF exposure and infection were
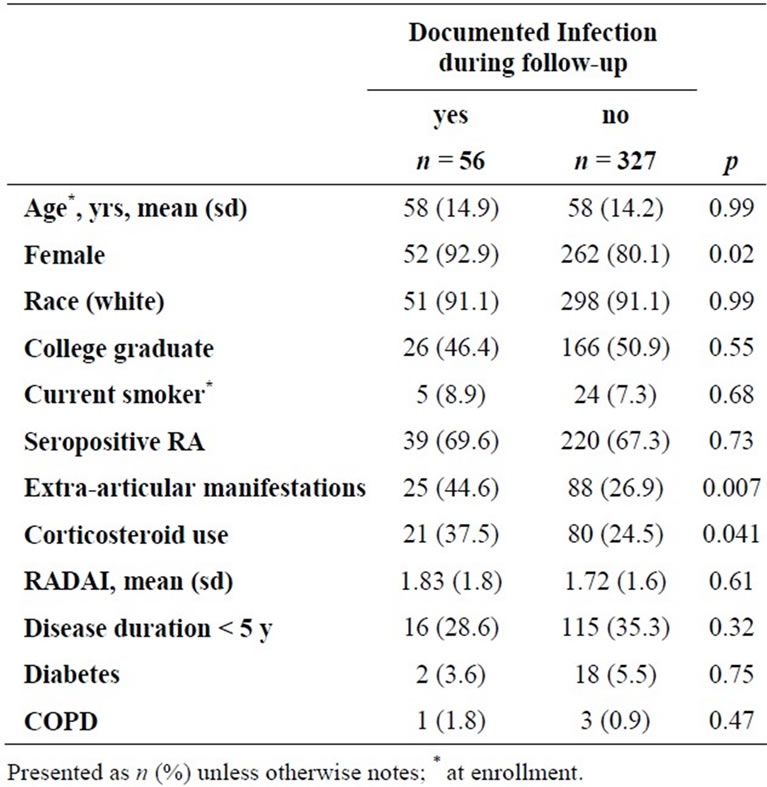
Table 2. Clinical predictors of documented infection during follow-up of 383 patients with rheumatoid arthritis.
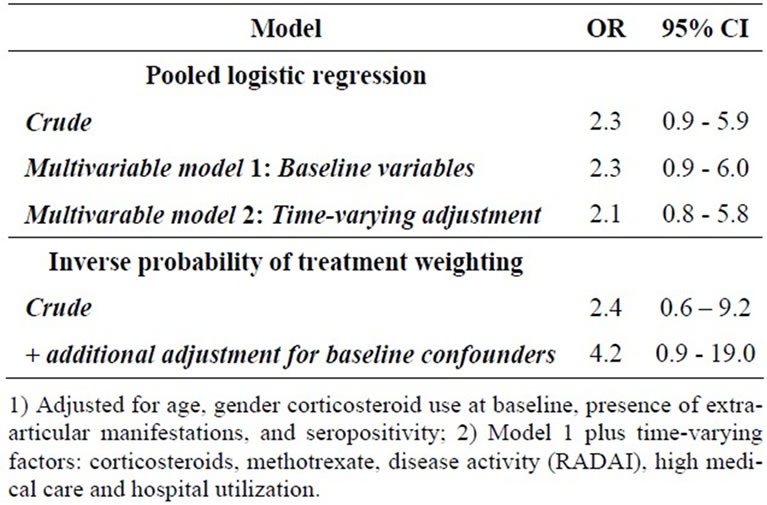
Table 3. Estimated effect of anti-TNF therapy on infection in RA under multiple modeling scenarios.
consistent with some previously published results [24,25]. As we demonstrated in our analyses, the highest risk of infection associated with anti-TNF initiation was observed in the period following initiation, which suggests a higher risk is in the early period following treatment start [8,26].
Over the past decade numerous investigators have studied the link between infection and anti-TNF medications [25] but not all studies have agreed. Differences in patient populations, outcome ascertainment and validation, follow-up, and study design may explain the differences between the studies. One ongoing discussion in this area is the prevalent vs. incident user design [26,27]. Briefly, the debate focuses on whether it is appropriate to compare a new user of a therapy (incident user) to a current or ongoing user (prevalent user) because those most susceptible to the adverse reactions or outcomes of interest may no longer be included in the prevalent pool because they discontinued the medication. Those susceptible to the outcomes associated with treatment initiation, such as infection, may be depleted from the study population and those remaining exposed (prevalent users) have survived without the outcome.
The present study evaluates start of anti-TNF therapy (i.e. incident user) compared to no anti-TNF exposure, in a study population that was restricted to anti-TNF-naïve patients with RA on at least one non-biologic DMARD therapy at start of follow-up. Thus, our study addresses the clinical question: what is the risk of infection associated with initiation of anti-TNF therapy in patients on background non-biologic DMARDs? We believe that this research question is clinically relevant because prescription of anti-TNF therapy, since its introduction is common for patients with RA following non-biologic DMARDs [28].
A number of other studies have made the comparison between initiators of an anti-TNF therapy and prevalent users of non-biologic DMARDs or specifically, methotrexate [24,29]. Unlike some of these studies, we did not require that the non-initiators maintain their nonbiologic DMARD therapy for an extended period of time. Among both the anti-TNF initiators and non-initiators, longitudinal changes in methotrexate and corticosteroid treatment were modeled as time-varying confounders. Similar to analysis from the National Data Bank for Rheumatic Diseases, users of a treatment were compared to non-users, conditional on other therapies [30].
Several studies before the introduction of anti-TNF medications in RA management have reported conflicting results regarding the risk of infections in RA patients. Higher infection incidence rates were found in one study of RA patients compared to subjects free of RA, suggesting that either RA or its management might be associated with infections [4,6,31]. These, and other similar findings, highlighted the need to consider possible bias due to changes in susceptibility to infection related to disease activity that may lead to changes in treatment choice over time. In other studies [32,33] results from employing alternative modeling strategies have yielded significantly different results, both statistically and clinically. Choi and colleagues found a dramatically reduced mortality associated with methotrexate in RA using IPTW [32]. In this study we showed that using IPTW to adjust for time-varying confounders did not alter the results as compared to the conventional modeling approach where these potential confounders were included as time-varying covariates in the model. Whether residual confounding, power limitations, unmeasured confounders, or limited time-varying confounding in the data explain this is unknown. When the baseline confounders were added to the IPTW model to further reduce potential residual confounding, the magnitude of the association increased but was not statistically significant.
Conventional regression models with time-varying covariates in the models do not fully account for timevarying confounding; ignoring confounders may result in bias from residual confounding, but statistical adjustment may lead to other biases [23,32,34]. Inverse probability weighting tries to circumvent this bias by reweighting the observed population to answer our counterfactual question: “What would happen if everyone in our study population were treated as compared to if everyone were not treated?”. The unweighted approach evaluated asks instead whether those who were treated were more likely to have an infection compared to those who were untreated. While the difference is subtle, it has led to different results in previous studies [33]. Similarly, the primary regression analysis here evaluated the risk of infection in those exposed to anti-TNF treatment versus those who did not receive treatment. Using the IPTW approach, we answered the question: “What is the risk of infection if everyone in our study population had been exposed to anti-TNF treatment versus if everyone had not?”. The statistical method employed changes the scientific question answered, as does how the study population is defined and what comparator is used [26]. Therefore, special attention should be paid when interpreting the results of the present study into the context of what has been published.
Our study has some limitations. Despite the plethora of clinical data and patient-reported measures, we had limited follow-up of a maximum of two years for each subject, during which time only 66 patients started anti-TNF treatment, and only seven of whom also had an infection. Also, we may have missed infections treated outside of the network by another physician for which we had no documentation. We found no overt violation of the positivity assumption for the IPW method, however due to the sparsity of the data, results must be interpreted cautiously. As such we had limited statistical power and were unable to explore possible effect modification, subtypes of infection or type of anti-TNF, or investigate seropositive and seronegative RA separately. Protocol required only annual examination by physicians and semiannual reporting by the participants, possibly resulting in residual confounding by a number of potential confounders. Further we relied upon self-report of treatment, for exposure and concomitant therapies, which was previously shown to be reliable [19].
Despite these limitations, our study explores alternative methods to address potential bias due to time-varying confounding. Incorporating changing clinical status and strong indications for treatments, and also the consequences of treatment, we found generally consistent results using a variety of statistical approaches. This study sought to illustrate the use of these methods to address the common analytic problem of time-varying confounding in a context familiar to clinical researchers. Although we are limited from drawing any significant conclusions from this underpowered study, we have illustrated the use of alternative statistical models that may be of use in the presence of time-varying confounding in longitudinal studies and hope to replicate the approaches herein in larger, longer term studies.
5. Acknowledgements
We would like to extend a sincere thanks to the staff, clinicians, and participants of BRASS. In addition, we thank Dr. Daniel H. Solomon for his critical feedback on study design and analysis.
6. Statement of Funding
JFS was supported by the Arthritis Foundation Doctoral Dissertation Award and the NIH training grant, T32- A1007535-07: Ruth L. Kirschstein National Research Service Award (NRSA) Institutional Research Training Grants (T32) Epidemiology of Infectious Disease and Biodefense Training Program. Additional support includes NIH grants AR0524, AR47782, AR049880 for EWK. NAS receives additional grant funding from AMGEN and Abbott pharmaceuticals. BRASS is funded by Biogen Idec and Crescendo Biosciences.
REFERENCES
- V. P. Mohan, C. A. Scanga, K. Yu, H. M. Scott, K. E. Tanaka, E. Tsang, et al., “Effects of Tumor Necrosis Factor Alpha on Host Immune Response in Chronic Persistent Tuberculosis: Possible Role for Limiting Pathology,” Infection and Immunity, Vol. 69, No. 3, 2001, pp. 1847- 1855. doi:10.1128/IAI.69.3.1847-1855.2001
- M. Nucci and K. A. Marr, “Emerging Fungal Diseases,” Clinical Infectious Diseases, Vol. 41, No. 4, 2005, pp. 521- 526. doi:10.1086/432060
- N. Wig, R. Handa, P. Aggarwal and J. P. Wali, “Rheumatoid Arthritis—Current Trends in Management,” Indian Journal of Medical Sciences, Vol. 51, No. 8, 1997, pp. 255-264.
- M. F. Doran, C. S. Crowson, G. R. Pond, W. M. O’Fallon and S. E. Gabriel, “Predictors of Infection in Rheumatoid Arthritis,” Arthritis and Rheumatism, Vol. 46, No. 9, 2002, pp. 2294-2300. doi:10.1002/art.10529
- M. F. Doran, C. S. Crowson, G. R. Pond, W. M. O’Fallon and S. E. Gabriel, “Frequency of Infection in Patients with Rheumatoid Arthritis Compared with Controls: A Population-Based Study,” Arthritis and Rheumatism, Vol. 46, No. 9, 2002, pp. 2287-2293. doi:10.1002/art.10524
- G. A. Van Albada-Kuipers, J. Linthorst, E. A. Peeters, F. C. Breedveld, B. A. Dijkmans, J. Hermans, et al., “Frequency of Infection among Patients with Rheumatoid Arthritis versus Patients with Osteoarthritis or Soft Tissue Rheumatism,” Arthritis and Rheumatism, Vol. 31, No. 5, 1988, pp. 667-671. doi:10.1002/art.1780310513
- J. P. Vandenbroucke, R. Kaaks, H. A. Valkenburg, J. W. Boersma, A. Cats, J. J. Festen, et al., “Frequency of Infections among Rheumatoid Arthritis Patients, before and after Disease Onset,” Arthritis and Rheumatism, Vol. 30, No. 7, 1987, pp. 810-813. doi:10.1002/art.1780300711
- E. C. Keystone, “Does Anti-Tumor Necrosis Factor Alpha Therapy Affect Risk of Serious Infection and Cancer in Patients with Rheumatoid Arthritis?: A Review of Longterm Data,” The Journal of Rheumatology, Vol. 38, No. 8, 2011, pp. 1552-1562. doi:10.3899/jrheum.100995
- K. Au, G. Reed, J. R. Curtis, J. M. Kremer, J. D. Greenberg, V. Strand, et al., “Extended Report: High Disease Activity Is Associated with an Increased Risk of Infection in Patients with Rheumatoid Arthritis,” Annals of the Rheumatic Diseases, Vol. 70, No. 5, 2011, pp. 785-791. doi:10.1136/ard.2010.128637
- M. E. Weinblatt and A. L. Maier, “Treatment of Rheumatoid Arthritis,” Arthritis and Rheumatism, Vol. 2, No. 3, 1989, pp. A23-A32. doi:10.1002/anr.1790020311
- M. E. Weinblatt and A. L. Maier, “Disease-Modifying Agents and Experimental Treatments of Rheumatoid Arthritis,” Clinical Orthopaedics and Related Research, Vol. 265, 1991, pp. 103-115.
- G. Stucki and T. Langenegger, “Management of Rheumatoid Arthritis,” Current Opinion in Rheumatology, Vol. 9, No. 3, 1997, pp. 229-235. doi:10.1097/00002281-199705000-00009
- S. Anuradha and N. P. Singh, “Recent Trends in the Management of Rheumatoid Arthritis,” The Journal of the Indian Medical Association, Vol. 96, No. 11, 1998, pp. 345-348.
- P. Lamprecht, “TNF-α Inhibitors in Systemic Vasculitides and Connective Tissue Diseases,” Autoimmunity Reviews, Vol. 4, No. 1, 2005, pp. 28-34. doi:10.1016/j.autrev.2004.06.001
- L. A. Witty, F. Steiner, M. Curfman, D. Webb and L. J. Wheat, “Disseminated Histoplasmosis in Patients Receiving Low-Dose Methotrexate Therapy for Psoriasis,” Archives of Dermatology, Vol. 128, No. 1, 1992, pp. 91- 93. doi:10.1001/archderm.1992.01680110101015
- G. P. LeMense and S. A. Sahn, “Opportunistic Infection during Treatment with Low Dose Methotrexate,” American Journal of Respiratory and Critical Care Medicine, Vol. 150, No. 1, 1994, pp. 258-260.
- K. S. Kanik and J. M. Cash, “Does Methotrexate Increase the Risk of Infection or Malignancy?” Rheumatic Disease Clinics of North America, Vol. 23, No. 4, 1997, pp. 955- 967. doi:10.1016/S0889-857X(05)70368-9
- G. Stucki, M. H. Liang, S. Stucki, P. Bruhlmann and B. A. Michel, “A Self-Administered Rheumatoid Arthritis Disease Activity Index (RADAI) for Epidemiologic Research. Psychometric Properties and Correlation with Parameters of Disease Activity,” Arthritis and Rheumatism, Vol. 38, No. 6, 1995, pp. 795-798. doi:10.1002/art.1780380612
- D. H. Solomon, M. Stedman, A. Licari, M. E. Weinblatt, N. Maher and N. Shadick, “Agreement between Patient Report and Medical Record Review for Medications Used for Rheumatoid Arthritis: The Accuracy of Self-Reported Medication Information in Patient Registries,” Arthritis and Rheumatism, Vol. 57, No. 2, 2007, pp. 234-239. doi:10.1002/art.22549
- S. Schneeweiss, A. Robicsek, R. Scranton, D. Zuckerman and D. H. Solomon, “Veteran’s Affairs Hospital Discharge Databases Coded Serious Bacterial Infections Accurately,” Journal of Clinical Epidemiology, Vol. 60, No. 4, 2007, pp. 397-409. doi:10.1016/j.jclinepi.2006.07.011
- J. F. Simard, M. L. Stoll, N. A. Shadick, E. W. Karlson and D. H. Solomon, “Validity of Self-Report of Infections in a Longitudinal Cohort of Patients with Rheumatoid Arthritis Differs by Source of Report and Infection Severity,” Journal of Clinical Epidemiology, Vol. 63, No. 12, 2010, pp. 1358-1362. doi:10.1016/j.jclinepi.2010.01.014
- J. M. Robins and D. M. Finkelstein, “Correcting for Noncompliance and Dependent Censoring in an AIDS Clinical Trial with Inverse Probability of Censoring Weighted, (IPCW) Log-Rank Tests,” Biometrics, Vol. 56, No. 3, 2000, pp. 779-788. doi:10.1111/j.0006-341X.2000.00779.x
- M. A. Hernan, B. A. Brumback and J. M. Robins, “Estimating the Causal Effect of Zidovudine on CD4 Count with a Marginal Structural Model for Repeated Measures,” Statistics in Medicine, Vol. 21, No. 12, 2002, pp. 1689-1709. doi:10.1002/sim.1144
- J. R. Curtis, N. Patkar, A. Xie, C Martin, J. J. Allison, M. Saag, et al., “Risk of Serious Bacterial Infections among Rheumatoid Arthritis Patients Exposed to Tumor Necrosis Factor Alpha Antagonists,” Arthritis and Rheumatism, Vol. 56, No. 4, 2007, pp. 1125-1133. doi:10.1002/art.22504
- T. Bongartz, A. J. Sutton, M. J. Sweeting, I. Buchan, E. L. Matteson and V. Montori, “Anti-TNF Antibody Therapy in Rheumatoid Arthritis and the Risk of Serious Infections and Malignancies: Systematic Review and MetaAnalysis of Rare Harmful Effects in Randomized Controlled Trials,” The Journal of the American Medical Association, Vol. 295, No. 19, 2006, pp. 2275-2285. doi:10.1001/jama.295.19.2275
- D. H. Solomon, M. Lunt and S. Schneeweiss, “The Risk of Infection Associated with Tumor Necrosis Factor Alpha Antagonists: Making Sense of Epidemiologic Evidence,” Arthritis and Rheumatism, Vol. 58, No. 4, 2008, pp. 919-928. doi:10.1002/art.23396
- W. G. Dixon, L. Carmona, A. Finckh, M. L. Hetland, T. K. Kvien, R. Landewe, et al., “EULAR Points to Consider When Establishing, Analysing and Reporting Safety Data of Biologics Registers in Rheumatology,” Annals of Rheumatic Diseases, Vol. 69, No. 9, 2010, pp. 1596-1602. doi:10.1136/ard.2009.125526
- P. P. Sfikakis, “The First Decade of Biologic TNF Antagonists in Clinical Practice: Lessons Learned, Unresolved Issues and Future Directions,” Current Directions in Autoimmunity, Vol. 11, 2010, pp. 180-210. doi:10.1159/000289205
- W. G. Dixon, K. Watson, M. Lunt, K. L. Hyrich, A. J. Silman and D. P. Symmons, “Rates of Serious Infection, Including Site-Specific and Bacterial Intracellular Infection, in Rheumatoid Arthritis Patients Receiving AntiTumor Necrosis Factor Therapy: Results from the British Society for Rheumatology Biologics Register,” Arthritis and Rheumatism, Vol. 54, No. 8, 2006, pp. 2368-2376. doi:10.1002/art.21978
- F. Wolfe, L. Caplan and K. Michaud, “Treatment for Rheumatoid Arthritis and the Risk of Hospitalization for Pneumonia: Associations with Prednisone, Disease-Modifying Antirheumatic Drugs, and Anti-Tumor Necrosis Factor Therapy,” Arthritis and Rheumatism, Vol. 54, No. 2, 2006, pp. 628-634. doi:10.1002/art.21568
- M. J. Van Der Veen, A. Van Der Heide, A. A. Kruize and J. W. Bijlsma, “Infection Rate and Use of Antibiotics in Patients with Rheumatoid Arthritis Treated with Methotrexate,” Annals of the Rheumatic Diseases, Vol. 53, No. 4, 1994, pp. 224-228. doi:10.1136/ard.53.4.224
- H. K. Choi, M. A. Hernan, J. D. Seeger, J. M. Robins and F. Wolfe, “Methotrexate and Mortality in Patients with Rheumatoid Arthritis: A Prospective Study,” The Lancet, Vol. 359, No. 9313, 2002, pp. 1173-1177. doi:10.1016/S0140-6736(02)08213-2
- T. Kurth, A. M. Walker, R. J. Glynn, K. A. Chan, J. M. Gaziano, K. Berger, et al., “Results of Multivariable Logistic Regression, Propensity Matching, Propensity Adjustment, and Propensity-Based Weighting under Conditions of Nonuniform Effect,” American Journal of Epidemiology, Vol. 163, No. 3, 2006, pp. 262-270. doi:10.1093/aje/kwj047
- M. A. Hernan, B. Brumback and J. M. Robins, “Marginal Structural Models to Estimate the Causal Effect of Zidovudine on the Survival of HIV-Positive Men,” Epidemiology, Vol. 11, No. 5, 2000, pp. 561-570. doi:10.1097/00001648-200009000-00012
NOTES
*Corresponding author.

