Natural Resources
Vol.4 No.2(2013), Article ID:32235,5 pages DOI:10.4236/nr.2013.42022
The Effects of Herbicide Treatment, Life History Stage, and Application Date on Cut and Uncut Teasel, Dipsacus laciniatus (Dipsacacae)
![]()
1Department of Biology, Illinois State University, Normal, USA; 2Prairie Engineers of Illinois, Lincoln, USA; 3Illinois Natural History Survey, Champaign, USA; 4Department of Biology, Millikin University, Decatur, USA; 5Center for Ecological Entomology, Illinois Natural History Survey, Champaign, USA.
Email: jparrish@millikin.edu
Copyright © 2013 Laura M. Zimmerman et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received January 23rd, 2013; revised April 7th, 2013; accepted April 22nd, 2013
Keywords: Dipsacus laciniatus; Management of Invasive Species; Herbicide Control; Cutleaf Teasel
ABSTRACT
Cutleaf teasel (Dipsacus laciniatus L.) is an invasive plant that is spreading through natural and disturbed areas. Teasel grows for two or more years as a rosette which stays green late in the growing season and begins growth earlier in spring than its native competitors. The purpose of this study was to find a time both seasonally and in cutleaf teasel’s life history when herbicides could be applied to decrease teasel with the least impact on the surrounding vegetation. We tested the effects of three different herbicides (glyphosate (Round-UpTM), triclopyr amine (GarlonTM), and clopyralid (LontrelTM)) on cut and uncut teasel at three different times of the year (July and October 2005, and April 2006) near Clinton Lake in Dewitt Co. Illinois. Photosynthetic measurements were taken before application to determine teasel’s susceptibility to the herbicides, and we harvested seed heads and rosettes in late October 2006. Results indicated teasel was photosynthetically active at all three application times. Cutting before herbicide application had no significant effect on the number of seeds produced or the dry weight of the rosettes. Herbicide treatment in April significantly reduced the amount of seeds produced, but there were no significant differences among the three herbicides. Clopyralid application in April significantly reduced rosette biomass, but none of the herbicides significantly affected rosette biomass at the other two times. Our studies suggest herbicide application early in the growing season may be beneficial in controlling the spread of teasel, and that mowing at the time of spraying will not increase effectiveness of the herbicide.
1. Introduction
The Dipsacus laciniatus, cutleaf teasel, is an invasive plant that can be found growing in a variety of places, such as by roadsides, in ditches, and natural areas. Teasel is a monocarpic perennial that forms a basal rosette from which a prickly, 2 m tall, flowering stalk arises [1,2]. Each plant develops multiple ovoid, spiny seed heads, each of which can produce over 2000 seeds [3,4]. These seeds have a 30% - 80% survival rate and can remain viable for about three years [5].
Cutleaf teasel is native to Eurasia and northern Africa and arrived in North America prior to the 1900’s [6]. In the past 20 - 30 years, cutleaf teasel has expanded its breeding grounds to natural areas in North America as a result of having few natural enemies [7]. Invasive species such as teasel pose a major threat to ecosystems and can out compete native species for resources, which, if not controlled, can result in the suppression or elimination of the native species [8]. Mowing is a common management tool for teasel; however this is generally costly and ineffective and can actually facilitate the dispersal of the teasel seeds [9,10].
A study of invasive species in a national park in Canada found that the key to controlling invasions is to limit the access of the invasive species to new areas while also stopping the removal of native biomass [11]. Therefore, new methods must be developed to not only control invasion by teasel, but also protect native species. Herbicides may be an effective solution, especially since teasel is photosynthetically active both earlier and later than surrounding plants [6,12]. Herbicides are absorbed when the plants are actively photosynthesizing, thus providing a window that may leave teasel, as a target species, vulnerable during its extended photosynthetic season [10]. In a previous study, the Illinois Department of Transportation successfully used triclopyr amine to kill weeds and brush without disturbing sensitive ornamentals [13]. Therefore, herbicides may be specific enough to control teasel without affecting surrounding vegetation, but timing is important.
A successful management plan requires that it be determined what herbicide causes the most damage to the teasel, when is the best application date, what stage of teasel’s life history needs to be targeted, and if cutting before herbicide application increases the effects. All of this information has to be balanced against the damage inflicted on native plant species. The purpose of this study was to test the effects of three different herbicides applied at three different times of the year on teasel at varying life stages to help develop the most effective control method while also protecting the surrounding species.
2. Methods
2.1. Sampling Design and Treatment Protocols
We conducted this study at Mascoutin Recreational Area in DeWitt County, Illinois. Thirty plots were established with approximately equal amounts of teasel. Each plot was 12 m × 1.5 m and was subdivided into eight subplots with a 1 m2 application area and a 0.5 m buffer between subplots. The harvest area was 0.5 m2 in the center of the application area.
We used a split plot design. The within-plot factors were herbicide used and whether vegetation was cut or uncut. The between plot factor was season of application. We randomly assigned each plot to one of three application times: summer, fall, and spring. Each subplot was randomly assigned to one of six herbicide treatment conditions (one of three herbicides and cut or uncut). Our controls consisted of one subplot cut with no herbicide application, and one subplot that was neither cut nor treated with herbicide. Therefore, there were 10 replications of each of eight herbicide and cutting treatments for each of the three application times.
Herbicides used were glyphosphate (Round-UpTM), triclopyr amine (GarlonTM), and clopyralid (LontrelTM). The herbicides were mixed with distilled water in the concentrations directed on the labels of the herbicides. Each herbicide was applied using a calibrated backpack sprayer to the appropriate plot in the application area for a total of ten seconds, calculated based on recommended application rates. Sprayed plants were well wetted, but not dripping. For the cut treatments, clippers and a weed eater were used to cut the teasel and surrounding vegetation to a height of approximately 10 cm (typical mower height) before herbicide application. Application dates were 1 July 2005 (summer), 18 October 2005 (fall), and 17 April 2006 (spring).
2.2. Harvest
We harvested all above ground biomass in late October and early November 2006. We separated the plants into bolting stems, rosettes, and non-target plants, and also counted the number of seedlings per plot. We estimated the number of seeds produced by each bolting head using the previously determined equation 22.201X + 14.407, where X is the length of the bolting head [14]. Rosettes and non-target plants were dried and then weighed.
2.3. Statistics
All data from the harvest were square root transformed and analyzed using SAS statistical package (SAS Institute, Inc.). Split plot ANOVAs were used to compare number of seeds per plot, number of seedlings, and biomass of rosettes and non-target plants for each treatment at different application times. All interactions were tested, and non-significant interactions were removed from the model. Tukey comparisons of least square means were conducted post hoc.
2.4. Determination of Photosynthetic Rates
Due to equipment malfunctions, two different machines were used to determine the photosynthetic rates: an LCA 4 machine (ADC: Analytical Development Company LTD) and a Qubit systems CO2 anaylzer (Model No. S151). The photosynthetic rates of ten large (>30 cm) and ten small (< 30 cm) rosettes were measured using the Qubit machine nine days before the October application. We also measured photosynthetic rates of ten large and ten small rosettes 25 days after the October application using the LCA 4. Photosynthetic rates were measured before the April application on 15 April 2006 and also after the application on 2 May 2006 using the LCA-4 machine. To compare readings from the two machines, we measured the photosynthetic rates on individuals of five different species of plants in the Millikin University greenhouse. We then used a regression equation to adjust the Qubit readings in order to compare them to the LCA readings. Photosynthetic rates were log transformed and compared using a two-way ANOVA. Tukey comparisons of least square means were conducted post hoc.
3. Results
3.1. Harvest
Control plots were not significantly different among the three application dates for the number of seeds per plot (F = 0.47, p = 0.63, df = 2), number of seedlings per plot (F = 2.17, p = 0.13, df = 2), or biomass of non-target plants (F = 0.85, p = 0.44, df = 2). The ANOVA model showed a significant difference in biomass of rosettes (F = 3.35, p = 0.05, df = 2), however Tukey post hoc comparison of least square means showed no significant differences between comparisons for the three application dates.
For number of seeds produced per plot, there was a significant interaction of application date and herbicide used (F = 4.33, p = 0.0004, df = 6; Figure 1). All three herbicides applied in April resulted in significantly fewer seeds per plot than for the April control plot (p < 0.0001 for each herbicide). Clopyralid (p = 0.02) and glyphosate (p = 0.03) applied in July also significantly reduced seeds per plot when compared to the July control plot. Cutting did not significantly affect the number of seeds per plot (F = 2.11, p = 0.15, df = 1).
For the number of seedlings per plot, there was a significant effect of application date (F = 8.51, p = 0.0004, df = 2; Figure 2) and herbicide used (F = 5.68, 0.001, df = 3). April plots (p = 0.02) and July plots (p = 0.0002) had significantly fewer seedlings than October plots. Glyphosate was the only herbicide that significantly reduced the number of seedlings when compared to the control plots over all application dates (p = 0.005). Cutting before application did not significantly affect the number of seedlings per plot (F = 0.62, p = 0.43, df = 1).
For the biomass of the rosettes, there was a significant effect of application date (F = 13.97, p < 0.0001, df = 2; Figure 3) and herbicide used (F = 4.91, p = 0.003, df = 3). April applications resulted in a significantly higher biomass of rosettes than July (p = 0.0009) and October applications (p < 0.0001). Applying glyphosate resulted in a significantly higher biomass of rosettes than the control plots over all application dates (p = 0.008). None of the herbicides significantly decreased the biomass of the rosettes. Cutting before application did not signifiantly affect biomass of rosettes (F = 0.04, p = 0.85, df = 1).
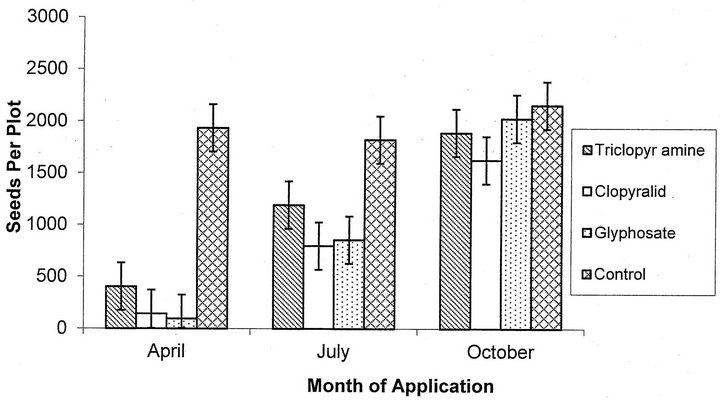
Figure 1. Mean estimated number of seeds per plot plus or minus SE for each herbicide for both cut and uncut treatments.
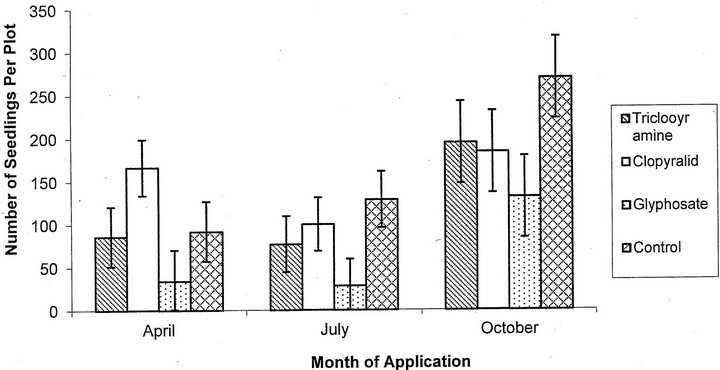
Figure 2. Mean number of seedlings per plot plus or minus the SE for each herbicide treatment including both cut and uncut treatments.
For the biomass of the non-target plants, there was a significant effect of application date (F = 5.38, p = 0.006, df = 2; Figure 4) and herbicide used (F = 3.21, p = 0.03, df = 3). All of the April applications resulted in a significantly lower biomass of non-target plants compared to July (p = 0.02) and October (p = 0.01). Glyphosate was the only herbicide that had a significantly lower biomass of non-target plants compared to the control plots (p = 0.04). Cutting before application did not significantly affect the biomass of non-target plants (F = 0.77, p = 0.38, df = 1).
3.2. Photosynthetic Rates
Comparison of measurements of the two photosynthetic machines resulted in a significant correlation between machines (r2 = 0.66, p = 0.004). There was a significant effect of date on photosynthetic rates of teasel (F = 63.56, p < 0.0001, df = 3; Figure 5). There was no significant difference in photosynthetic rates of large and small rosettes (F = 0.01, p = 0.91, df = 1).
4. Discussion
Invasive species have recently gained concern as a major challenge to conservation and management of natural ecosystems as they can reduce or displace native species, both plant and animal, and may even alter ecosystem function. Therefore, the control of non-native plants has become one of the most expensive and urgent tasks of managers of parks and preserves around the United States [15]. Teasel has become problematic in several states where natural areas are being threatened by its spread. Therefore, the purpose of this study was to find a time both seasonally and in teasel’s life history when herbicides could be applied to decrease teasel but not the surrounding vegetation.
Development of a management plan for the control of teasel will have to take into account how well established teasel is in a given area. If the plot is well established, glyphosate may be the most effective choice. It sig-
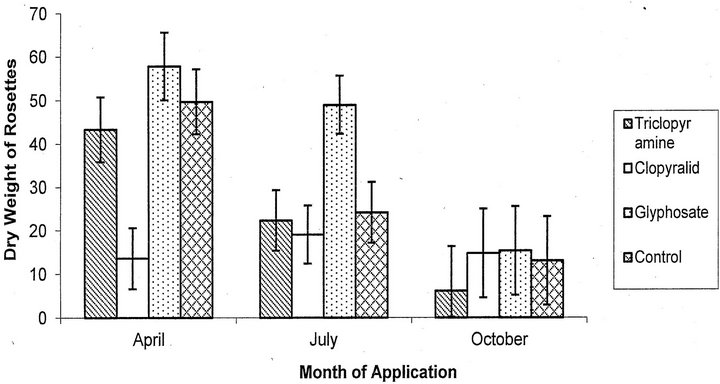
Figure 3. Mean dry weight of rosettes plus or minus the SE for each herbicide treatment including both cut and uncut treatments.
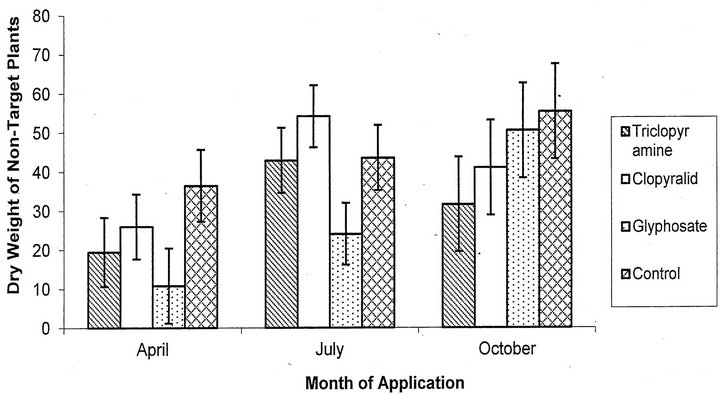
Figure 4. Mean dry weight of non-target plants plus or minus the SE for each herbicide treatment including both cut and uncut treatments.
nificantly reduced the number of seedlings compared to controls over all applicable dates and also significantly reduced the number of seeds when applied in July and April, as it did in the study by Bentivegna and Smeda [12]. However, it also significantly reduced the biomass of non-target plants and therefore may not be the best option in areas where teasel is beginning to invade. In situations where teasel is only beginning to be established clopyralid is a better option. It significantly reduced the number of seeds when applied in April and July, and while it did not significantly reduce the number of seedlings, the seed bank should not be well established yet. Clopyralid also had the advantage of not adversely affecting the dry weight of non-target plants. Triclopyr amine applied in April also reduced the numbers of seeds while not significantly affecting the non-target plants.
Rosettes appear to be the most difficult to control. None of the herbicide treatments significantly reduced the biomass of the rosettes. Glyphosate actually significantly increased the biomass. This may result from glyphosate’s adverse effect on non-target plants, allowing rosettes to grow larger with reduced competition. A similar effect was found for all herbicides applied in April with non-target plants reduced while biomass of the rosettes increased.
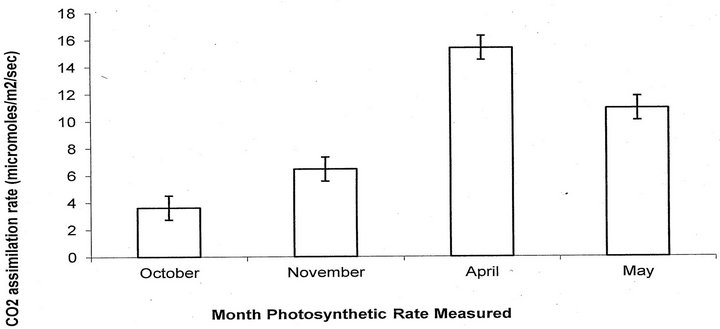
Figure 5. Mean photosynthetic rates plus or minus the SE for both large and small rosettes. All bars are significantly different from each other (P < 0.05).
Cutleaf teasel was photosynthetically active in the fall (although not as high as in spring and summer), suggesting that it might be vulnerable to herbicides longer than surrounding vegetation. However, in spite of vulnerability predicted by photosynthetic rates, herbicide application in October was ineffective on any life stage in this experiment.
Cutting before herbicide application did not alter the effects of herbicides on the number of seedlings, seeds, or the biomass of rosettes and non-target plants, and therefore it is not useful to mow before herbicide application. Previous studies have found that mowing may actually increase seed production and facilitate dispersal, and that the best time for mowing is in early July after bolting but before flowers develop [14]. A combination of herbicide application in April along with cutting D. laciniatus once in early July would further reduce the number of seeds produced and thus help reclaim natural areas.
Overall, our study highlights the difficulty of controlling invasive species. No herbicide was entirely successful in removing the various life stages of teasel. However, we provide a useful set of guidelines to consider when developing a management plan that over time will help control the spread of cutleaf teasel.
REFERENCES
- P. A. Werner, “Colonization Success of a ‘Biennial’ Plant Species: Experimental Field Studies of Species Cohabitation and Replacement,” Ecology, Vol. 58, No. 4, 1977, pp. 840-849. doi:10.2307/1936219
- K. C. Foote, “Central New York’s Rings of Teasel,” New York State Conservationist, Vol. 58, No. 1, 2003, p. 1. doi:10.1016/S0167-8809(97)00131-X
- O. D. Cheesman, “The Impact of Some Field Boundary Management Practices on the Development of Dipsacus fullonum L. Flowering Stems, and Implications for Conservation,” Agriculture Ecosystems and Environment, Vol. 68, No. 1-2, 1998, pp. 41-49.
- B. G. Rector, V. Harizanova, R. Sforza, T. Widmer and R. N. Wiedenmann, “Prospects for Successful Biological Control of Teasels, Dipsacus spp., a New Target in the United States,” Biological Control, Vol. 36, No. 1, 2006, pp. 1-14. doi:10.1016/j.biocontrol.2005.09.010
- D. J. Bentivegna and R. J. Smeda, “Cutleaf Teasel (Dipsacus laciniatus): Seed Development and Persistence,” Invasive Plant Science and Management, Vol. 4, No. 1, 2011, pp. 31-37. doi:10.1614/IPSM-D-10-00026.1
- M. K. Solecki, “Cut-leaved and Common Teasel (Dipsacus laciniatus L. and D. sylvestris Huds.): Profile of Two Invasive Aliens,” In: B. McKnight, Ed., Biological Pollution: The Control of Impact of Invasive Exotic Species,” Indiana Academy of Science, Indianapolis, 1993, pp. 85- 92.
- R. Sforza, “Candidates for the Biological Control of Teasel, Dipsacus spp.,” Proceedings of the XI International Symposium on Biological Control of Weeds, Vol. 27, Canberra, 2003, pp. 155-161.
- L. F. Huenneke and J. K. Thomson, “Potential Interference between a Threatened Endemic Thistle and an Invasive Nonnative Plant,” Conservation Biology, Vol. 9, No. 2, 1995, pp. 416-425. doi:10.1046/j.1523-1739.1995.9020416.x
- M. K. Solecki, “The Viability of Cut-Leaved Teasel (Dipsacus laciniatus L.) Seed Harvested from Flowering Stems: Management Implications,” Natural Areas Journal, Vol. 9, 1989, pp. 102-105.
- B. Glass, “Vegetation Management Guideline: Cut-Leaved Teasel (Dipsacus laciniatus L.) and Common Teasel (Dipsacus sylvestris Huds.),’” Natural Areas Journal, Vol. 11, 1991, pp. 213-214. doi:10.1890/04-0840
- M. A. De Gruchy, R. J. Reader and D. W. Larson. “Biomass, Productivity, and Dominance of Alien Plants: A Multihabitat Study in a National Park,” Ecology, Vol. 86, No. 5, 2005, pp. 1259-1266.
- D. J. Bentivegna and R. J. Smeda, “Chemical Management of Cutleaved Teasel (Dipsacus laciniatus) in Missouri,” Weed Technology, Vol. 22, No. 3, 2008, pp. 502- 506. doi:10.1614/WT-08-043.1
- P. Caylor, “Herbicides Help Illinois DOT Control Roadside Weeds,” American City & County, Vol. 113, No. 3, 1998, pp. 17-18.
- M. P. Dudley, J. A. D. Parrish, S. L. Post, D. G. Helm, and R. N. Wiedenmann, “The Effects of Fertilization and Time of Cutting on Regeneration and Seed Production of Dipsacus laciniatus (Dipsacacae),” Natural Areas Journal, Vol. 29, No. 2, 2009, pp. 140-145. doi:10.3375/043.029.0206
- R. Hobbs and L. F. Huenneke, “Disturbance, Diversity, and Invasion: Implications for Conservation,” Conservation Biology, Vol. 6, No. 3, 1992, pp. 324-337. doi:10.1046/j.1523-1739.1992.06030324.x

