Advances in Sexual Medicine
Vol.3 No.4(2013), Article ID:37412,7 pages DOI:10.4236/asm.2013.34013
Ameliorative Effects of Syzygium aromaticum Essential Oil on Fertility in Male Rats Exposed to Manganese
1Department of Biology, Faculty of Sciences and Technology, Moulay Tahar University of Saida, Saida, Algeria
2Department of Biology, Faculty of Sciences, Djillali Liabes University of Sidi-Bel-Abbes, Sidi-Bel-Abbes, Algeria
3Research Laboratory of Environment and Health (RLEH), Faculty of Medicine, University Hospital Complex (UHC) of Sidi-Bel-Abbes, Sidi-Bel-Abbes, Algeria
4Pathology Laboratory, University Hospital Complex of Sidi-Bel-Abbes, Sidi-Bel-Abbes, Algeria
Email: farouk.boudou@yahoo.fr
Copyright © 2013 Farouk Boudou et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received July 15, 2013; revised August 15, 2013; accepted August 22, 2013
Keywords: Fertility; Sterility; Manganese; Essential Oil; Syzygium aromaticum
ABSTRACT
Introduction: Several substances were likely to cause the decline in male fertility which could go up to the complete sterility. The aim of this study was to assess the effects of Syzygium aromaticum essential oil on fertility in male rats exposed to manganese. Materials and Methods: Twenty four male rats, 6 months old, were divided into 2 groups; 12 rats in one group received, by oral route, a water containing manganese chloride tetrahydrate (MnCl2∙4H2O) is at the dose of 4.79 mg∙ml−1 for 12 weeks. The group of control male rats received a distilled water in the same conditions. After a period of experimentation, each group was divided into two subgroups of 6 rats which received daily, by intraperitoneal way, a 0.1 mg/kg body weight, of Syzygium aromaticum essential oil. Results: After a chronic exposure, microscopic examination of the testes showed degeneration of the seminiferous tubules and the gremlin cell. Seminal parameters indicated a decrease in the sperm levels (21.3 × 106 cells/ml) and a rise of morphological abnormalities (66.1%). However, intraperitoneal administration of essential oil extracted from the flower buds of Syzygium aromaticum, during 3 weeks, to the rats previously intoxicated with Mn, showed a significant rising of sperm concentration (61.2 × 106 cells/ml) and a reduction of morphological abnormalities (10.8%). These changes were associated with a significant regeneration of seminiferous tubules and interstitial cells. Conclusion: This study revealed an ameliorative effect of essential oil Syzygium aromaticum in testicular tissue and the sperm quality.
1. Introduction
Manganese (Mn) is one of the most abundant elements and is widely distributed in soil, air, water, and food [1]. It is an essential element for humans in small quantities, playing many roles for normal mammalian physiology [2]. Several enzyme systems have been reported to interact with or depend on Mn2+ for their optimal catalytic or regulatory function [3]. It has been reported that a deficiency in intake of manganese can retard growth, impair fertility and cause birth defects [4]. Conversely, industrial use of Mn and Mn containing compounds (for example, in the production of paint pigments, dry cell batteries, glass and ceramics as well as mining of Mn ores and welding of mild steel) may expose workers to excessive amounts of this chemical [5].
However, high dose of Mn seems to cause serious neurotoxicity, immunotoxicity and developmental toxicity, particularly in male [6]. It is also known that Chronic exposure to this metal can cause alterations in development as well as reproductive dysfunction [7]. In human occupational exposure to Mn2+ decreased libido and impotency, and may result in lowered sperm count and semen quality [8,9]. It was reported that chronic administration of MnCl2 at low doses to female rats resulted in increased serum levels of puberty-related hormones such as luteinizing hormone (LH), follicle-stimulating hormone (FSH), and estradiol, and advanced the time of vaginal opening [10].
The use of medicinal plants is a great contribution to treat primary medical problems. A variety of plants are claimed to have fertility regulating properties and a few have been tested for such effect [11-13]. The flower bud of Syzygium aromaticum of the Myrtaceae family, commonly known as clove, is one of these plants. It is a small or average size tree, terrestrial which can reach 8 - 12 m in height, native to Indonesia. Syzygium aromaticum is a well known food flavor for exotic food preparations and a popular remedy for dental disorders, respiratory disorders, headache and soar throat in traditional medicines of Australia, and Asian countries [14,15]. In addition to its antimicrobial, antifungal and antiviral properties, clove oil possesses anti-inflammatory and anesthetic properties [16]. Because of its antiseptic and antibiotic properties, clove is frequently used to treat toothache and as an ingredient in popular toothpastes and mouth fresheners in India [17]. In Asian countries, the Syzygium aromaticum (clove) is well known for its aphrodisiac property, and used to treat male sexual disorders [18].
After a bibliographic record, our work tends to assess the effects of Syzygium aromaticum essential oil on fertility in male rats exposed to manganese.
2. Materials and Methods
2.1. Preparation of Plant Material
The essential oil (EO) of clove bud dried fruits from Syzygium aromaticum was obtained by a conventional hydro-distillation process with an excellent yield (10.45%). However, in order to prepare the solution for intra-peritoneal injection the clove bud oil was suspended in double distilled water along with a few drops of Tween 80 to prepare solution of the required dose (0.1mL/kg) [19].
2.2. Biological Material
Experiments were carried out on 24 “Wistar” forty adult male, aged of 6 months and weighing 280 ± 10 g. The animals were housed in room with a 12/12-hour light/ dark cycle, at 22 ± 2˚C and had access to ad libitum water and food (15% protéins).
2.3. Experimental Protocol
The rats were distributed into two groups of twelve rats. The first group is the control group (T) receiving distilled water and the second group (M) is the lot of rats exposed to manganese, receiving oral manganese chloride tetrahydrate (MnCl2∙4H2O) at the dose of 4.79 mg Mn.l1 [20]. After twelve weeks of experimentation, the animals were divided into four groups (6 rats per groups) in separate cages. From the group (T) was obtained the group (T-EO) which is the control group receiving essential oil by intra-peritoneal route (IP). And from the groups (M) was obtained the group (M-EO) which is the lot treated with essential oil for 21 days with a daily intake of 0.1 mg/kg body weight.
2.4. Fertility Test
Just before the end of the experiment the animal of different groups were housed with female rats of the same age in order to test their fertility. After a period of seven days of cohabitation, the females have been separated from the males. The number of females gravid and the birth rate is unregistering.
2.5. Sacrifice of Animals and Collection of Biological Samples
At the end of the experiment, the animals were sacrificed in the morning after fasting for 12 hours, by IP injection with a solution of chloral (C2H3C13O2) to 10%. After incision of the abdomen, blood is collected by cardiac puncture in heparin tubes for biochemical analysis. The testicles are carefully removed, rinsed with cold saline, dried and weighed. Then they were fixed in formalin, to be studied by histological techniques.
2.6. The Effect of Manganese on the Testicles
Histological study was performed according to standard techniques, after fixation in fixative (formalin 1/10), paraffin embedding and staining with hematoxylin-eosin.
2.7. Spermatozoa Count
Testis and epididymis each group of rats cut with scissors and homogenized 10 to 20 ml of 0.9% NaCl containing 0.05% Triton X-100. The homogenate are placed in the refrigerator at 4˚C for one hour. 400 ml of each homogenate of rat are diluted in 0.9% NaCl containing 0.05% Triton X-100 and 500 ml of Trypan blue to 4%. The resulting mixture, 10 μl of sample is placed on a Thoma cell [21].
2.8. Sperm Morphology
A drop of spermatozoal suspension was mounted between the slide and the cover slide. Each sample was examined at 40× magnification. At least 200 spermatozoa were observed for the calculation of percentage of the total numbers of spermatozoa. The percentage of abnormal sperm morphology was calculated from the following formula:
% = (Abnormal sperm/total sperm count) × 100 Abnormal sperm morphology the sperm cells were categorised based on the presence of one or more abnormal features such as tail defects (short, irregular, coiled or multiple tails); neck and middle piece defects (distended, irregular, bent middle piece, abnormally thin middle piece); and head defects (round head, small or large size, double or detached head) [22].
2.9. Statistical Analysis
The mean ± SEM values were calculated for each group to determine the significance of intergroup difference. Each parameter was analyzed separately using two ways analysis of variance (ANOVA). To find the difference between the groups Student “t” test was used. p values <0.05 were considered to be significant.
3. Results
3.1. Effect of Manganese on the Fertility of Male Rats
The fertility test done can highlight the impact of manganese poisoning on the fertility of male rats. The results in Table 1 indicate the existence of a dose effect relationship between dose and fertility in rats. We note later that females coupled with intoxicated males have a reduced pregnancy rate 33.3%, compared to females mated with control rats (75%).
3.2. Body Weight and Organ Weights
A statistically significant change in the body weight difference and organs weight of the expérimental animals was showed. However, the results in Table 2 show a significant diminution body weight and Testis weights of manganese-exposed groups. Compared to the control group of rats (T and T-EO) and compared to the group of rats treated with essential oil of clove Mn-EO.
3.3. Semen Parameters
Semen parameters in all the treatment groups (Groups Mn and Mn-EO) decreased when compared with the control group (Groups T and T-EO) Table 3. Only a minimal decrease was observed in the percentage sperm concentration and in the percentage of morphologically abnormal spermatozoa of the groupe traited with essentiel oil (Mn-EO) compared to group of rat exposed only to manganese (Mn). The percentage of abnormal spermatozoa was markedly increased in the animals exposed to daily concetration of manganese chloride (66.17 ± 2.93) compared with animals that traited with daily concetration of essentiel oil during 21 days (57.17 ± 1.47) and the control animals (10.83 ± 1.30) given distilled water and those who reveived only essentiell oil of clove (13.92 ± 0.96).
3.4. Histological Study
Histological study in the testicles reveals normal architecture in the control animals (T and T-EO). Figures 1(a), (b) showed that seminiferous tubules were richly popu-
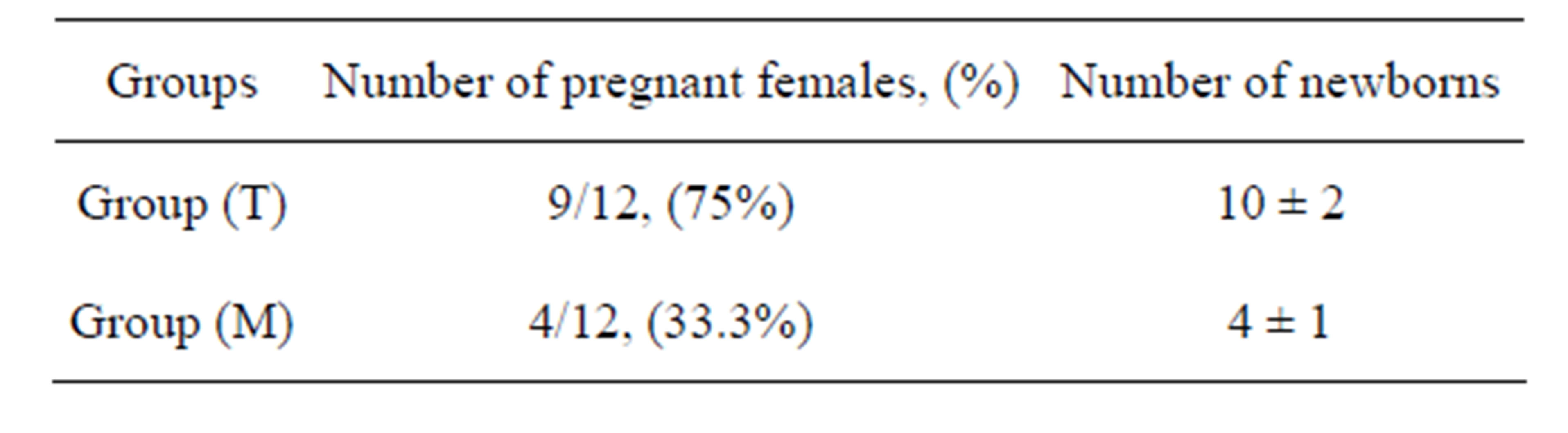
Table 1. Effect of manganese on the fertility of male rats.
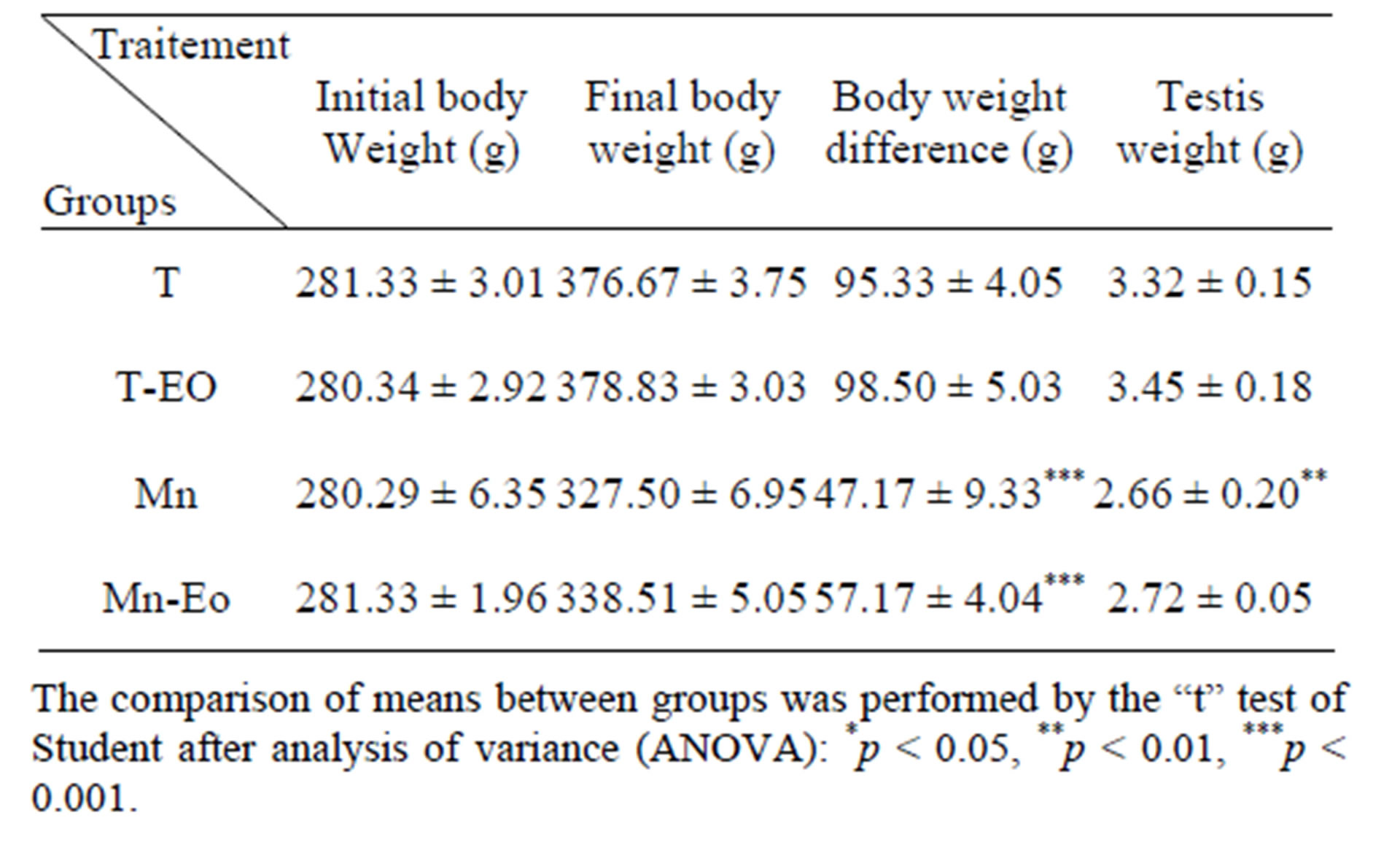
Table 2. Body weight and organ weights of the different experimental groups.
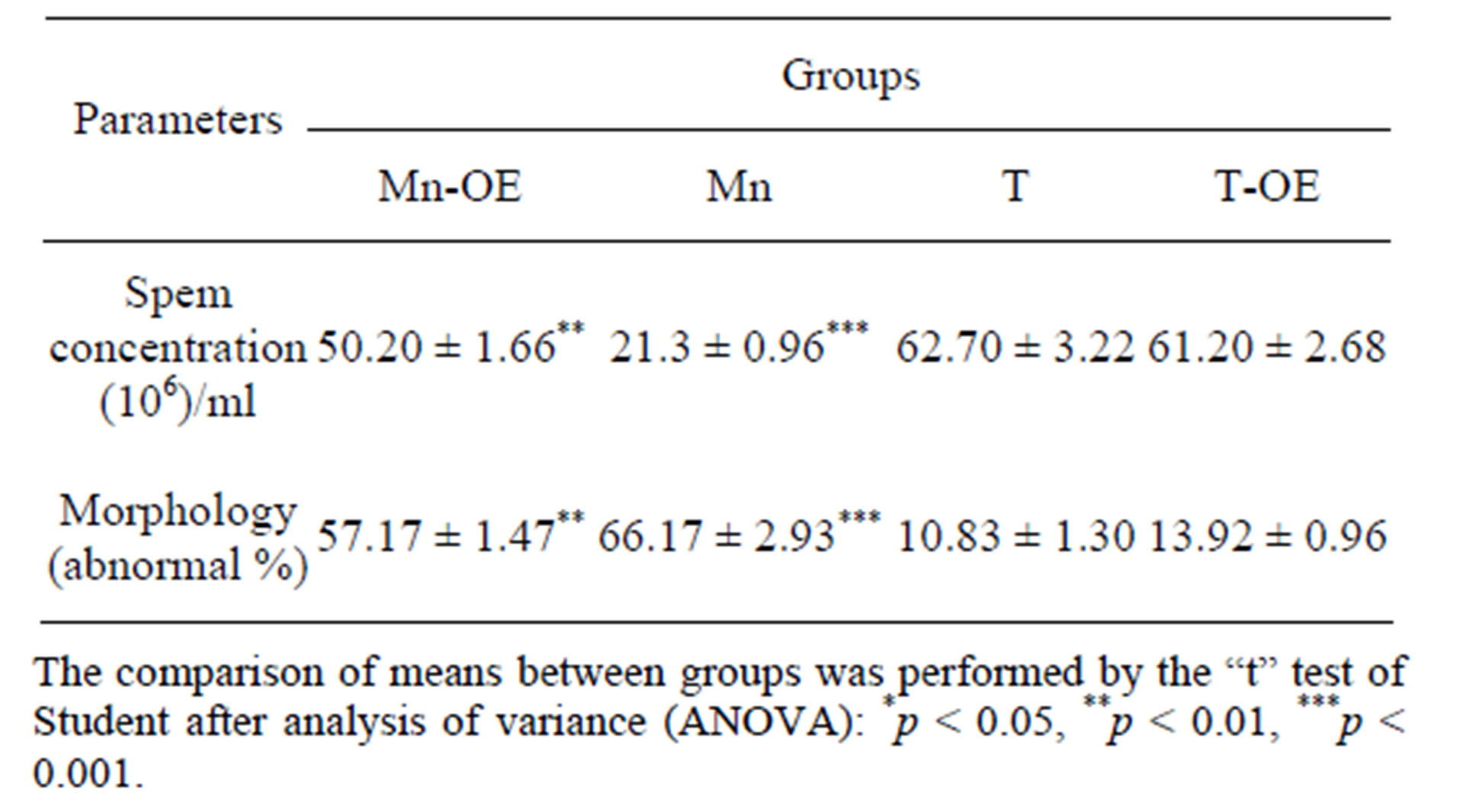
Table 3. Semen parameters of the different experimental groups.
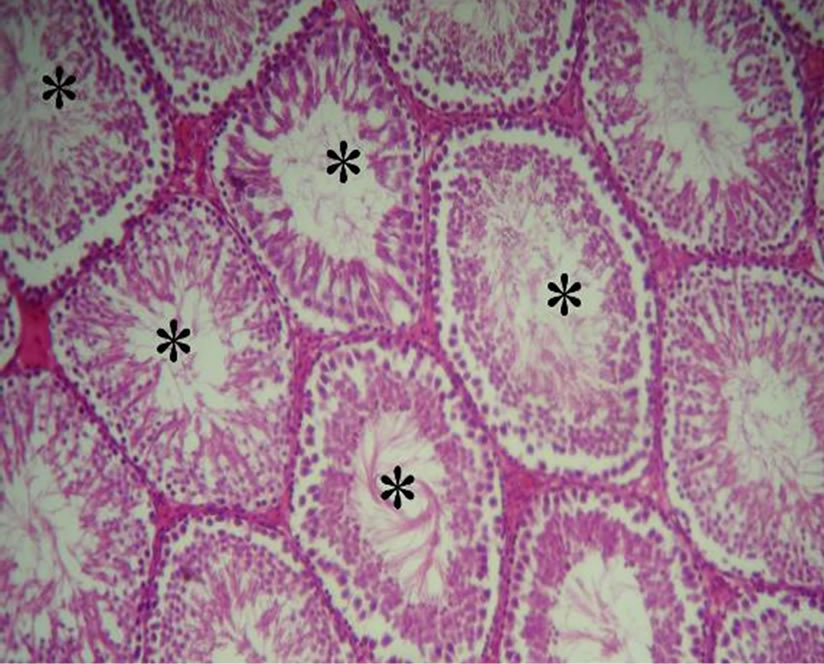
lated and gave healthy appearance. All the cells of the spermatogenic series such as spermatogonia, spermatocyte, spermatids and spermatozoa, even sertoli cells could be identified in the tubules. Lumen could easily be delineated in almost all the tubules and majority of them were occupied by mature spermatozoïdes. While the analysis of histological sections of the testes of manganese poisoning group (M) Figure 1(c) shows that these stages are affected. Among the disturbances reported: degeneration of the seminiferous tubules, a total absence of sperm and/or a low sperm count, with large interstitial spaces and lack of cells Lydig around basement membranes. However administration of essential oil S.aromaticum, to rats previously poisoned by manganese chloride Figure 1(d), shows a significant regeneration of the majority of seminiferous tubules and interstitial cells, with a good development of the spermatogenesis indicated by lumens of seminiferous tubes filled with sperm.
 (a)
(a)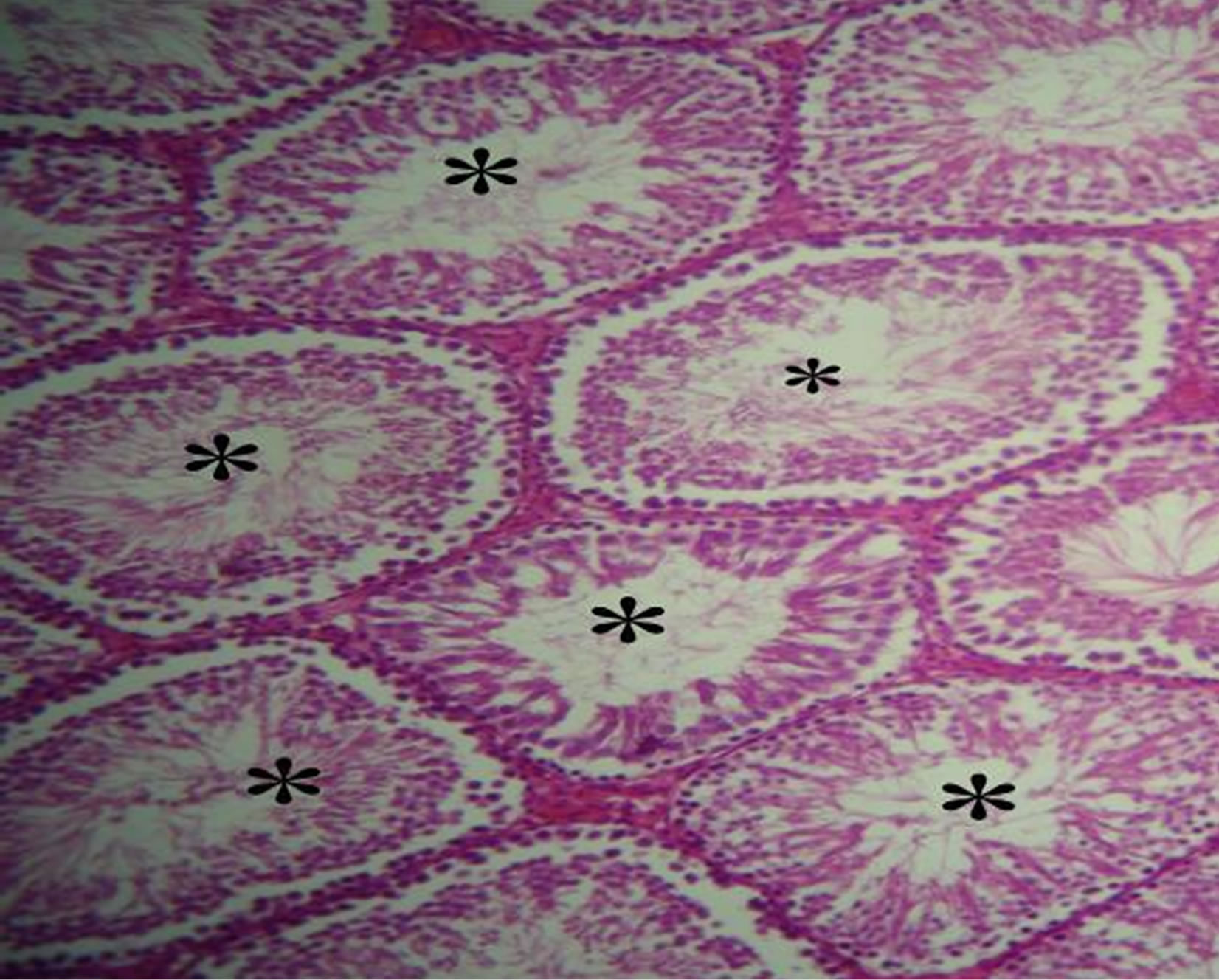 (b)
(b)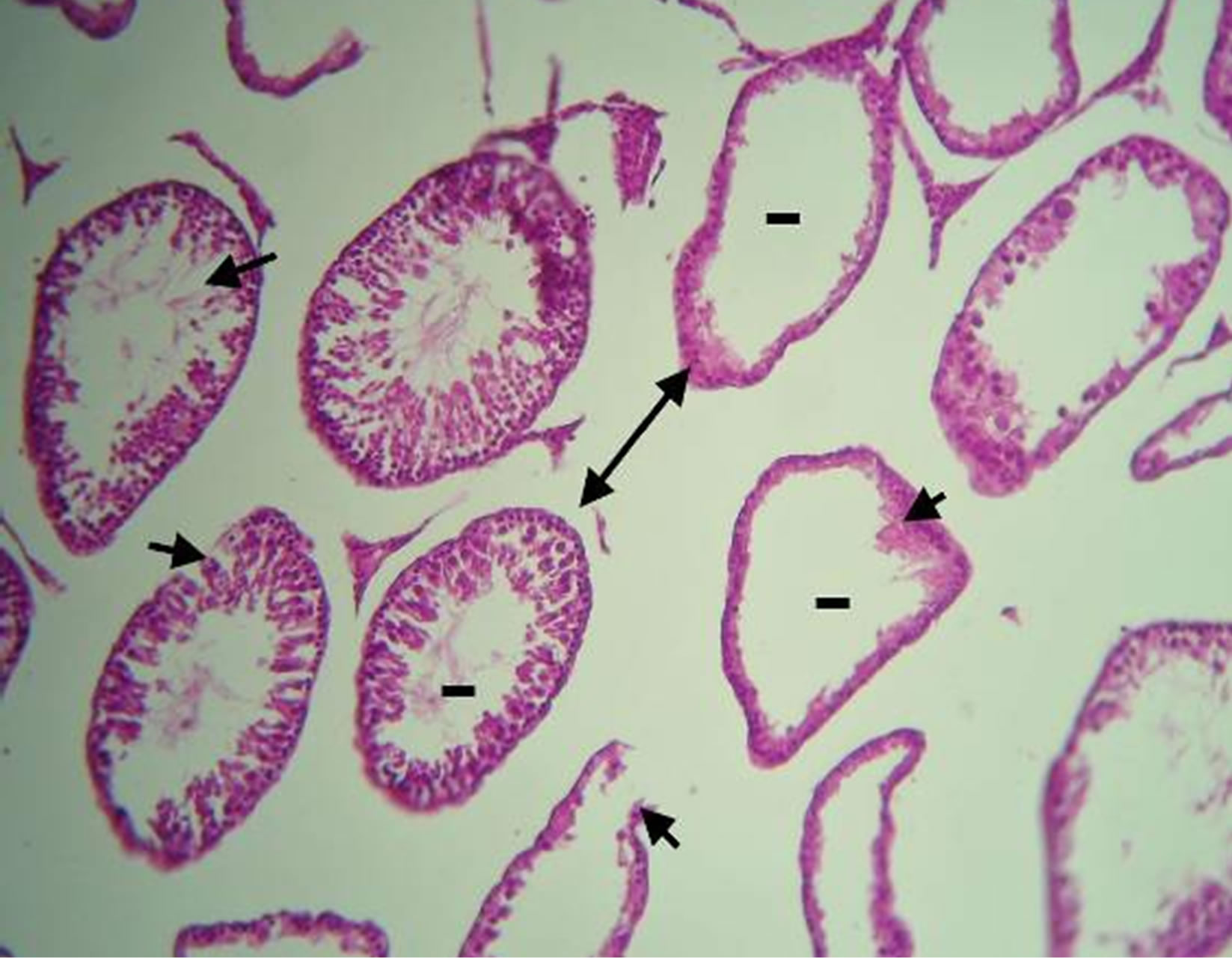 (c)
(c)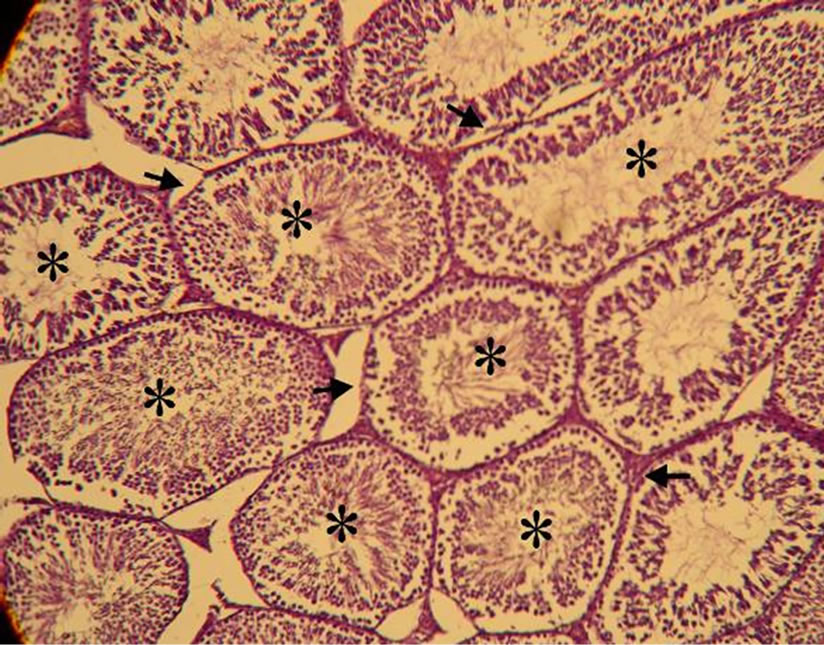 (d)
(d)
Figure 1. (a)-(d) are Haematoxylin stained sections of rats testis. ×40. (a) Normal architecture and seminiferous tubes filled with sperm (*) in the control group (T) (animals received distilled water). (b) Group (T-EO) received only essentiel oil, showing normal appearance of seminiferous tubules and seminiferous tubes filled with sperm (*). (c) Group (M) expose to manganese (4.79 mg Mn /ml during 12 weeks) testis showing degeneration of the seminiferous tubules (arrow), a total absence of sperm and/or a low sperm count (-), with large interstitial spaces and lack of cells Lydig (arrow double sense). (d) Group (Mn-EO) Previously expose to manganese and treated with essential oil of S.aromaticum (0.1 mg OE/KG BW) testis showing regeneration of the majority of seminiferous tubules (arrow) and interstitial cells, with a good development of the spermatogenesis and lumens of seminiferous tubes filled with sperm (*).
4. Discussion
Manganese is one of the most dangerous occupational and environmental toxins. It accumulates in the human organism mainly in brain, liver and kidneys, where it causes functional changes [23]. Manganese exposure induced a significant decrease in weight of testis in rats exposed to Mn compared with control rats, which is consistent with the work undertaken by Ajibade et al. [24], indicating a significant reduction in organ weights of rats exposed to oral concentrations of 5, 15 to 25 mg∙Mn/kg.
However crosses between males exposed to manganese chloride and control male rats with healthy females, showed a decrease of implantation sites per litter and pregnancy rates and the number of births. These results are consistent with those made by Elbetieha et al. [25], which show that the manganese induced in mice decreased fertility in males and decreased number of implantations in the uterus. Domenec et al. [26], indicate that exposure of mice to manganese concentration of 8 and 16 mg/kg/day. Causes Foetotoxicity, consisting mainly of a reduction in fetal weight and an increased incidence of morphological abnormalities.
Otherwise, in our results, decrease in the number of pregnant females is caused by the inability of a male to fertilized, because of various defects in sperm morphology and/or decrease in the numbers of sperm. Confirmed by Barber et al. [27], who reported that MnSO4 adversely affected semen quality index and sperm viability in broiler breeder semen in vitro.
In addition, we also suggest that the increased of abnormal sperm in intoxicated rats may be related to the effect of manganese on the Sertoli cells, given their important role in the differentiation of sperm and spermatogenesis. The high percentage of anomalies can be explained by the cytotoxicity of manganese during spermiogenesis (transformation of the spermatid into sperm) during which form the head, the middle part and the flagellum.
To confirm this suggestion, histological techniques were used. The results clearly indicate a testicular damage characterized by remarkable disturbances at the seminiferous tubules where the different stages of spermatogenesis were affected. This is confirmed by Ponnapakkam et al .[28], who shown that subcutaneous administration of Mn chloride for a period of three weeks cause testicular damage in Sprague-Dawley rats. They reported that Mn causes degeneration of the seminiferous epithelium, a decrease of number of spermatids and spermatocytes in the seminiferous tubules. The results of the study showed that the male reproductive system is a target of Mn exposure. However, the achievement of Sertoli cells may also be explained by the disruption of the functioning of Leydig cells, which have a direct role in the regulation of spermatogenesis by producing hormones specifically testosterone. Indeed our observations of histological sections have indicated low activity and a decrease in interstitial cells (composed of cells Lydig). This is consistent with the work of Jana et al. [29], who suggest that decreased serum testosterone is due to inhibition of testicular steroidogenic enzymes such as delta 5,3 beta-hydroxysteroid dehydrogenase (Δ5, 3β-HSD) and 17 beta-hydroxysteroid dehydrogenase (17β-HSD) responsible for the synthesis of testosterone. In another hand, in vitro studies conducted by Cheng et al. [30], on the Leydig cells, indicate that exposure to manganese from 2 to 4 hours disrupts steroidogenesis in Leydig cell by decreasing the expression of StAR protein (steroidogenic acute regulatory protein), while exposition 24 to 48 hours cause of adverse effects on both StAR protein and P450 as well as the enzymatic activity of 3b-HSD.
After administration of the essential oil Syzygium aromaticum by intraperitoneal route to rats previously intoxicated by Manganese, we noticed an improvement in weight of rats treated with essential oil. This weight regain recorded could be due to the presence of terpenoid compounds that act by stimulating glucose transport into cells. Given that changes in sugar and the hormone insulin in the blood are related to appetite, hunger and various food needs, particularly the need for carbohydrates. By controlling the levels of these parameters in the blood, so it is an effective complement to correct weight loss in animals [31].
The administration of essential oil S. aromaticum, in rats previously poisoned by manganese chloride, helped save a significant regeneration of the majority of seminiferous tubules and interstitial cells, with a smoothly spermatogenesis resulted in lights seminiferous tubules filled with spermatozoa, which can be explained by the therapeutic effect of HE of clove, widely used as a treatment for male sexual dysfunction in Asian countries. These results are consistent with those made by Mishra et al. [32] reported that the essential oil of clove administration because of 15 mg/kg body weight to mice for 35 days, indicating an increase of enzyme activity (Δ5, 3β-HSD and 17β-HSD) and the rate of blood testosterone. This explains the success of spermatogenesis and spermiogenesis. Shodehinde et Ganiyu Oboh. [33], indicate that plants rich in polyphenols case of eugenol, is therefore a powerful antioxidant that inhibits lipid peroxidation prevents damage caused by oxidative stress in the testes.
To conclude, this study revealed the ameliorative effect of the essential oil of Syzygium aromaticum in testicular tissue after the deleterious effect caused by manganese on male gonads.
REFERENCES
- Li, et al., “Effects of Manganese on Routine Semen Quality Parameters,” BMC Public Health, Vol. 12, 2012, p. 919.
- S. I. Kim, Y. S. Jang, S. H. Han, M. J. Choi, E. H. Go, Y.-P. Cheon, J. S. Lee and S.-H. Lee, “Effect of Manganese Exposure on the Reproductive Organs in Immature Female Rats,” Development and Reproduction, Vol. 16, No. 4, 2012, pp. 295-300.
- T. E. Gunter, C. E. Gavin, M. Aschner and K. K. Gunter, “Speciation of Manganese in Cells and Mitochondria: A Search for the Proximal Cause of Manganese Neurotoxicity,” Neurotoxicology, Vol. 27, No. 5, 2006, pp. 765-776. http://dx.doi.org/10.1016/j.neuro.2006.05.002
- C. L. Keen, S. Zidenberg-Cherr and B. Lönnerdal, “Nutritional and Toxicological Aspects of Manganese Intake: An Overview,” In: W. Mertz, C. O. Aternathy and S. S. Olin, Eds., Risk Assessment of Essential Elements, International Life Sciences Institute, Washington DC, 1994, pp. 221-235.
- A. Elbetieha, H. Bataineh, H. Darmani and M. H. Al-Hamood, “Effects of Long-Term Exposure to Manganese Chloride on Fertility of Male and Female Mice,” Toxicology Letters, Vol. 119, No. 3, 2001, pp. 193-201. http://dx.doi.org/10.1016/S0378-4274(00)00312-X
- B. Michalke, S. Halbach and V. Nischwitz, “Speciation and Toxicological Relevance of Manganese in Humans,” Journal of Environmental Monitoring, Vol. 9, No. 7, 2007, pp. 650-656. http://dx.doi.org/10.1039/b704173j
- J. W. Laskey, J. F. Rehnberg and J. F. Hein, “Effects of Chronic Manganese Exposure on Selected Reproductive Parameters,” Journal of Toxicology and Environmental Health, Vol. 9, No. 4, 1982, pp. 677-687. http://dx.doi.org/10.1080/15287398209530195
- A. M. Emara, S. H. el-Ghawabi, O. I. Madkour and G. H. el-Samra, “Chronic Manganese Poisoning in the Dry Battery Industry,” British Journal of Industrial Medicine, Vol. 28, No. 1, 1971, pp. 78-82.
- N. H. Hjollund, J. P. Bonde, T. K. Jensen, E. Ernst, T. B. Henriksen, H. A. Kolstad, A. Giwercman, N. E. Skakkebaek and J. Olsen, “Semen Quality and Sex Hormones with Reference to Metal Welding,” Reproductive Toxicology, Vol. 12, No. 2, 1998, pp. 91-95. http://dx.doi.org/10.1016/S0890-6238(97)00156-1
- M. Pine, B. Lee, R. Dearth, J. K. Hiney and W. L. Dees, “Manganese Acts Centrally to Stimulate Luteinizing Hormone Secretion: A Potential Influence on Female Pubertal Development,” Toxicological Sciences, Vol. 85, No. 2, 2005, pp. 880-885. http://dx.doi.org/10.1093/toxsci/kfi134
- P. B. Telefo, P. F. Moundipa and F. M. Tchouanguep, “Oestrogenicity and Effect on Hepatic Metabolism of the Aqueous Extract of the Leaf Mixture of Aloe buettneri, Dicliptera verticillata, Hibiscus macranthus and Justicia insularis,” Fitoterapia, Vol. 73, No. 6, 2002, pp. 472-478. http://dx.doi.org/10.1016/S0367-326X(02)00177-6
- M. Ganguly, M. Borthakur, N. Devi and R. Mahanta, “Antifertility Activity of the Methanolic Leaf Extract of Cissampelos pareira in Female Albino Mice,” Journal of Ethnopharmacology, Vol. 111, No. 3, 2007, pp. 688-691. http://dx.doi.org/10.1016/j.jep.2007.01.023
- W. Cherdshewasart, Y. Kitsamai and S. Malaivijitnond, “Evaluation of the Estrogenic Activity of the Wild Pueraria mirifica by Vaginal Cornification Assay,” Journal of Reproduction and Development, Vol. 53, No. 2, 2007, pp. 385-393. http://dx.doi.org/10.1262/jrd.18065
- M. Domaracky, P. Rehak, S. Juhas and J. Koppel, “Effects of Selected Plant Essential Oils on the Growth and Development of Mouse Preimplantation Embryos in Vivo,” Physiological Research, Vol. 56, No. 1, 2007, pp. 97-104.
- A. Gurib-Fakim, “Medicinal Plants: Traditions of Yesterday and Drugs of Tomorrow,” Molecular Aspects of Medicine, Vol. 27, No. 1, 2006, pp. 1-93. http://dx.doi.org/10.1016/j.mam.2005.07.008
- K. Chaieb, H. Hajlaoui, T. Zmantar, A. B. Kahla-Nakbi, M. Rouabhia, K. Mahdouani and A. Bakhrouf, “The Chemical Composition and Biological Activity of Clove Essential Oil, Eugenia caryophyllata (Syzigium aromaticum L. Myrtaceae): A Short Review,” Phytotherapy Research, Vol. 21, No. 6, 2007, pp. 501-506. http://dx.doi.org/10.1002/ptr.2124
- S. Banerjee, C. K. Panda and S. Das, “Clove (Syzygium aromaticum L), a Potential Chemopreventive Agent for Lung Cancer,” Carcinogenesis, Vol. 27, No. 8, 2006, pp. 1645-1654. http://dx.doi.org/10.1093/carcin/bgi372
- Tajuddin, S. Ahmad, A. Latif and I. A. Qasmi, “Aphrodisiac Activity of 50% Ethanolic Extract of Myristica fragrans Houtt (nutmeg) and Syzygium aromaticum (L.) Merr. & Perry. (Clove) in Male Mice: A Comperative Study,” BMC Complementary and Alternative Medicine, Vol. 3, 2003, p. 6. http://dx.doi.org/10.1186/1472-6882-3-6
- S. Halder, A. K. Mehta, R. Kar, M. Mustafa, P. K. Mediratta and K. K. Sharma “Clove Oil Reverses Learning and Memory Deficits in Scopolamine-Treated Mice,” Planta Medica, Vol. 77, No. 8, 2011, pp. 830-834. http://dx.doi.org/10.1055/s-0030-1250605
- R. M. Molina, S. Phattanarudee, J. Kim, K. Thompson, M. Wessling-Resnick, T. J. Maher and J. D. Brain, “Ingestion of Mn and Pb by Rats during and after Pregnancy Alters Iron Metabolism and Behavior in Offspring,” NeuroToxicology, Vol. 32, No. 4, 2011, pp. 413-422. http://dx.doi.org/10.1016/j.neuro.2011.03.010
- O. Shinshi, “Lack of Spermatotoxic Effects of Methyl and Ethyl Esters of p-Hydroxybenzoic Acid in Rats,” Food and Chemical Toxicology, Vol. 42, No. 11, 2004, pp. 1854-1859.
- I. M. Al-Ani, I. N. Al-Khfaji, M. B. Fakhrildin, H. H. Mangalo and S. R. Al-Obaidi, “The Effect of Lead Exposure of Mice during Pregnancy on the Morphology of Epididymal and Testicular Spermatozoa of Their Offspring,” The International Medical Journal, Vol. 8, No. 1, 2009.
- M. S. Celik, K. G. Veysi, Akpolat, M. Z. Akdag, M. Nazıroglu, R. Gul-Guven, M. Y. Celik and S. Erdogan, “Extremely Low-Frequency Magnetic Field Induces Manganese Accumulation in Brain, Kidney and Liver of Rats,” Toxicology and Industrial Health, 2013.
- A. J. Ajibade, P. B. Fakunle, O. O. Fatoba and O. T. Olayemi, “Some Effects of Manganese Dichloride Administration on the Body Weight, Purkinje Cell Population, Brain, and Cerebellar Weights of Adult Wistar Rats,” Journal of Neuroscience and Behavioral Health, Vol. 3, No. 7, 2011, pp. 87-90.
- A. Elbetieha, H. Bataineh, H. Darmani and M. H. Al-Hamood. “Effects of Long-Term Exposure to Manganese Chloride on Fertility of Male and Female Mice,” Toxicology Letters, Vol. 119, No. 3, 2001, pp. 193-201. http://dx.doi.org/10.1016/S0378-4274(00)00312-X
- D. J. Sánchez, J. L. Domingo, J. M. Llobet and C. L. Keen, “Maternal and Developmental Toxicity of Manganese in the Mouse,” Toxicology Letters, Vol. 69, No. 1, 1993, pp. 45-52.
- S. J. Barber, H. M. Parker and C. D. McDaniel, “Broiler Breeder Semen Quality as Affected by Trace Minerals in Vitro,” Poultry Science, Vol. 84, No. 1, 2005, pp. 100- 105.
- T. P. Ponnapakkam, G. H. Sam and M. B. Iszard, “Histopathological Changes in the Testis of the Sprague Dawley Rat Following Orally Administered Manganese,” Bulletin of Environmental Contamination & Toxicology, Vol. 71, No. 6, 2003, pp. 1151-1157.
- K. Jana, S. Jana and P. K. Samanta, “Effects of Chronic Exposure to Sodium Arsenite on Hypothalamic PituitaryTesticular Activities in Adult Rats: Possible Anestrogenic Mode of Action,” Reproductive Biology and Endocrinology, Vol. 4, 2006, p. 9. http://dx.doi.org/10.1186/1477-7827-4-9
- J. Cheng, J. L. Fu and Z. C. Zhou, “The Inhibitory Effects of Manganese on Steroidogenesis in Rat Primary Leydig Cells by Disrupting Steroidogenic Acute Regulatory (StAR) pr Tein Expression,” Toxicology, Vol. 187, No. 2-3, 2003, pp. 139-148. http://dx.doi.org/10.1016/S0300-483X(03)00063-5
- A. Judþentienë and D. Mockutë, “Chemical Composition of Essential Oils of Artemisia absinthium L. (Wormwood) Growing Wild in Vilnius,” CHEMIJA, Vol. 15, No. 4, 2004, pp. 64-68.
- R. K. Mishra and S. K. Singh, “Safety Assessment of Syzygium aromaticum flower Bud (Clove) Extract with Respect to Testicular Function in Mice,” Food and Chemical Toxicology, Vol. 46, No. 10, 2008, pp. 3333-3338. http://dx.doi.org/10.1016/j.fct.2008.08.006
- Shodehinde1 and G. Oboh, “Antioxidant Properties of Aqueous Extracts of Unripe Musa paradisiaca on Sodium Nitroprusside Induced Lipid Peroxidation in Rat Pancreas in Vitro Sidiqat Adamson,” Asian Pacific Journal of Tropical Biomedicine, Vol. 3, No. 6, 2013, pp. 449-457. http://dx.doi.org/10.1016/S2221-1691(13)60095-7

