Open Journal of Endocrine and Metabolic Diseases
Vol.4 No.6(2014), Article
ID:47322,9
pages
DOI:10.4236/ojemd.2014.46016
The Incidence and Alliance of Metabolic Syndrome with Cardiovascular Risk Markers among Kodavas
Deepti A. Lokanath1,2, Sharada A. Chandrashekariah1, D. Xaviour2, Jayashankar Rao2
1Department of Biochemistry, Yuvaraja’s College, Mysore, India
2Anthropological Survey of India, Southern Regional Centre, Bogadi North, Mysore, India
Email: deeptilokanath@gmail.com
Copyright © 2014 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/



Received 23 May 2014; revised 10 June 2014; accepted 17 June 2014
ABSTRACT
Background: Metabolic syndrome is the major cause for life threatening disorders such as cardiovascular diseases and type 2 diabetes. These disorders are associated with hyperuricemia and the number is growing among the urban population. Methods: A cross sectional study was done among Kodava population by conducting health camps in Mysore district. Metabolic syndrome was defined according to Joint Interim Statement criteria. Anthropometry was done and blood pressure readings were noted. Clinical markers like fasting glucose, triglyceride, high density lipoprotein, CVD markers and uric acid levels were analyzed. Results: The prevalence of metabolic syndrome was 60.77% and the utmost occurrence was in 41 - 60 age groups. Women were more affected than men (31.58%) and MetS became pronounced with advance of age. Biochemical levels of C-reactive protein, ApolipoproteinB/ApolipoproteinA1 ratio and uric acid were raised (P < 0.05) and the severity correlated with the number of components of metabolic syndrome. Conclusions: This study helped in identifying new subjects with metabolic syndrome wherein, abdominal obesity was the most common abnormality followed by elevated fasting glucose. Female subjects and subjects with increased waist circumference along with mid aged people are more susceptible to MetS which amplified their CVD risk factors and hyperuricemic conditions. Life style modifications and therapeutic approach are critical prerequisite. However, there is an urgent need for further health camps for the awareness, and prevention of MetS and its associated risk factors among Kodavas.
Keywords:ApoB/ApoA1 Ratio, CRP, Metabolic Syndrome, Abdominal Obesity, Homocysteine, Uric Acid

1. Introduction
Metabolic syndrome (MetS) is a compilation of multiple interrelated risk factors such as obesity, dyslipidemia and decreased glucose tolerance which can ultimately cause cardio vascular diseases (CVD) and type 2 diabetes (T2D) [1] . MetS also causes lipid rich plaques and premature coronary artery disease (CAD) and is said to be the major threat to cardiovascular mortality and morbidity [2] [3] . Studies have shown that prevalence of metabolic syndrome is found to be high among urban Indian population [4] .
Kodavas are inhabitants of Kodagu district, Karnataka, South India. They belong to agricultural community with martial traditions and among the population excessive intake of pork meat and alcohol is followed habitually [5] [6] . The dietary habits, migration, sedentary life style and urbanization form the contributing factors of metabolic syndrome among Kodavas. Till date there are no studies available about the magnitude of metabolic syndrome in this community and therefore there is an urgent need to assess this health issue amongst Kodavas. In recent time, people have migrated to other places in India and abroad and the present study was conducted on Kodava community living in Mysore City, Karnataka, India. This study investigates the prevalence and manifestations of metabolic syndrome and also checks their association with other cardiovascular risk markers such as C-reactive protein (CRP), ApolipoproteinB/ApolipoproteinA1 (ApoB/ApoA1 ratio), homocysteine and uric acid.
2. Methods
2.1. Study Design
This cross sectional study was carried out in Mysore city from June 2011 to January 2012, by conducting 10 health camps with the aid of community concentrated localities and their associations. In this study, 426 subjects aged between 25 and 85 participated but 8 subjects were excluded as they were non-Kodavas or women married to Kodava men. A total of 418 subjects were selected after fulfilling the inclusion criteria in the present study. The subjects were asked to fill-in personal details like name, age, marital status, spouse name, family name which are very essential when we are studying in this community. A standardized questionnaire related to their medical history, medications/treatment, diet, alcohol consumption, smoking status and physical activity were noted. All the subjects gave their written informed consent for participating in the study and this was approved by Institutional Ethical Committee (IOE) Anthropological Survey of India, Kolkata.
2.2. Anthropometric Measurements
Anthropometric measurements like height, weight, waist and hip circumference were measured. Height was measured using Holtain anthropometric scale and weight with light clothing and without shoes. Waist circumference was measured using a flexible inextensible tape placed horizontally at the midpoint between the lowest rib and the iliac crest and hip circumference (HC) at the widest circumference over the major trochanters with the subject standing erect and waist hip ratio (WHR) was calculated using these measurements. Body fat %, Body Mass Index (BMI), Basal Metabolic Rate (BMR) was measured using Omron fat monitor with the subject standing erect without shoes. Blood pressure was measured using mercury Sphygmomanometer in sitting position.
2.3. Sample Collection and Laboratory Analysis
After overnight fast, 10 ml of venous blood was collected in EDTA vacutainer, plasma was separated and tested for fasting plasma glucose, High Density lipoprotein-cholesterol (HDL-C), Triglyceride (TG), C-Reactive Protein (CRP) using ERBA kits, supplied by Transasia. Uric acid was measured by uricase PAP method using ERBA kits. ApolipoproteinA1 (ApoA1) and ApolipoproteinB (ApoB) were tested using Randox Kits by turbidometric immunoassay method in automated biochemical analyzer EM360 (Transasia, ERBA Mannheim, Germany). Homocysteine was estimated using Immulite kit in Immulite1000 by chemiluminescence technology.
MetS was defined as per Joint Interim Statement (JIS) of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; International Association for the Study of Obesity and also Consensus Statement for Diagnosis of Obesity, Abdominal Obesity and the MetS for Asian Indians [7] [8] . The criteria includes increased waist circumference (males ≥ 90 cm and females ≥ 80 cm), Hypertriglyceridemia (TG ≥ 150 mg/dl), low HDL (males < 40 mg/dl and females < 50 mg/dl), elevated blood pressure (systolic blood pressure ≥ 130 mmHg and/or diastolic blood pressure ≥ 85 mmHg or under drug treatment for hypertension) and elevated blood sugar (fasting blood sugar ≥ 100 mg/dl or under treatment for diabetes mellitus). Thus, any three of the above mentioned parameters will confirm the presence of MetS.
We referred the kit ranges for the cut off values of CRP (>1 mg/dl), ApoA1/ApoB ratio (>0.8 mg/dl), homocysteine (>15 µmol/l) and uric acid (>7.7 mg/dl men and >6.6 mg/dl women).
2.4. Statistical Analysis
For determining the sample size, creative research system survey software was used with confidence level of 95% and confidence interval of 4.74. With a population of 15,000 in Mysore district wherein the overall population of the community is 125,000 according to Karnataka unit of Bureau of economics and statistics (2011).
All statistical analysis was performed using SPSS version 12.0 software (SPSS, Chicago, IL, USA). Continuous data were presented as mean and categorical data expressed as numbers and percentages. Differences between continuous data were analyzed by independent student’s t-test and categorical data by chi square test. Comparisons between the groups based on MetS components were done by Analysis of variance for continuous data.
3. Results
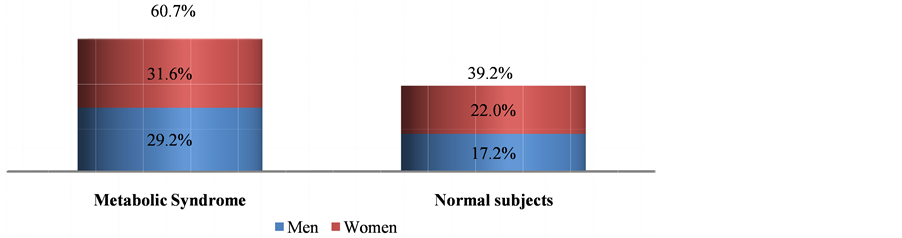
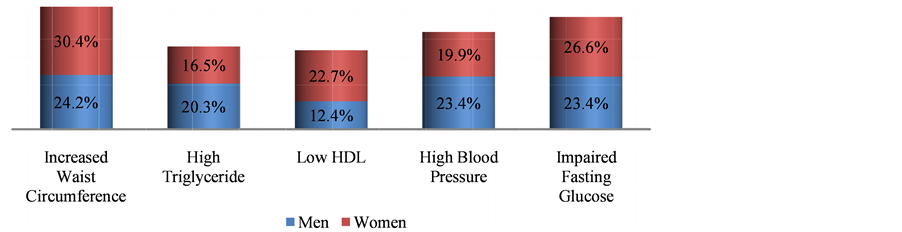
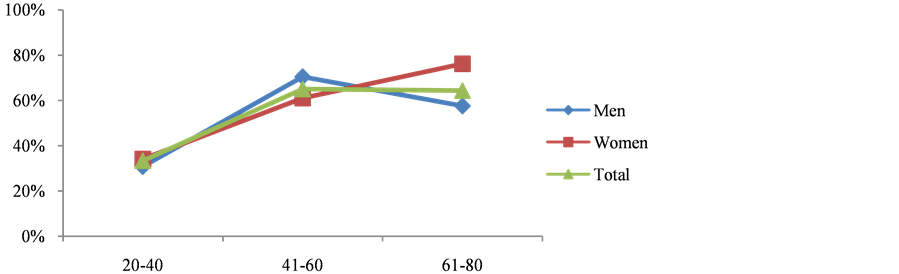
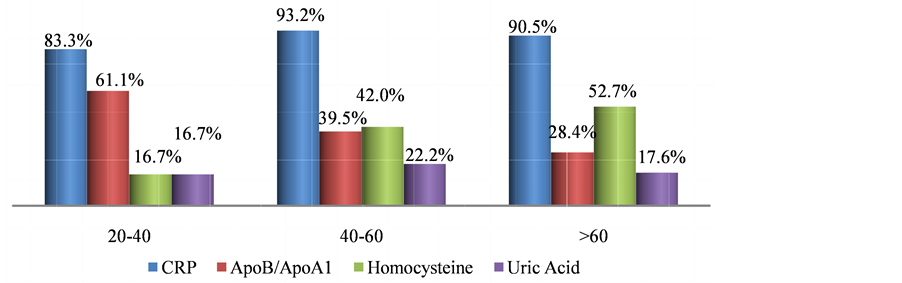
In this study, out of 418 subjects, 254 (60.77%) had MetS, there were significant differences in the anthropometric measurements like BMI, Body fat % and WHR among subjects with metabolic syndrome and normal subjects. There were noteworthy difference in blood pressure readings but not in alcohol intake and physical activity in Mets and non-Mets group (Table 1). Figure 1 shows the prevalence of MetS based on gender, women (31.58%) were more affected than men (29.19%). Figure 2 describes the manifestations of metabolic syndrome, Increased waist circumference (54.54%) and impaired glucose tolerance (50%) were the most common problem in MetS subjects. More over in women (22.73%) low HDL was highly prevalent than men. The prevalence of metabolic syndrome by age was explained in Figure 3. In the 41 - 60 age group both men and women displayed highest incidence. However with increase in age (>60 yrs) more women were affected than men. Table 2 shows significant differences in levels of CRP, ApoB/ApoA1 ratio and uric acid (P < 0.05) in MetS and normal subjects. Further there is a steady increase in the levels of CRP, ApoB/ApoA1 ratio and uric acid as the components Table 1. Baseline characteristics of subjects with and without metabolic syndrome. Figure 1. Prevelance of metabolic syndrome amongst men and women. Figure 2. Characterization of metabolic syndrome amongst men and women. P value by chi square test respectively: 0.00*, 0.05*, 0.00*, 0.02*, 0.433. Figure 3. Age wise distribution of metabolic syndrome. Table 2. Mean of CVD risk markers and uric acid level amongst metabolic syndrome and normal subjects. of MetS increased and their difference is significant, components refers to metabolic syndrome complications either high waist circumference, high triglyceride, low HDL, Hypertension or Elevated fasting glucose (Table 3). In a similar manner, there is rise in the levels of all CVD risk markers and uric acid in MetS patients of 41 - 60 age groups which is of great concern, except for homocysteine which is found to be high in >60 age group (Figure 4). Figure 4. Age-wise distribution of CVD markers and uric acid levels in metabolic syndrome subjects. Table 3. Metabolic components and their effect on mean of CVD risk markers and uric acid levels. *P value significant; 3—refers to presence of any 3 components of metabolic syndrome; 4—refers to presence of any 4 components of metabolic syndrome; 5—refers to presence of all components of metabolic syndrome. 3.1. Ascertainment of CVD In this study, 9 subjects reported previous history of cardiovascular problems like Myocardial infarction and had undergone angioplasty, it was known through the questionnaire, of these 7 (77.77%) were diagnosed with metabolic syndrome. 3.2. Prevalence of Hyperuricemia/Gout In the study, 17 subjects had previous history of hyperuricemia/gout and were on treatment, out of which 13 (76.47%) were newly diagnosed with metabolic syndrome. 4. Discussion Metabolic syndrome is known to increase the risk of cardiovascular diseases and type 2 diabetes, and its magnitude is reaching pandemic proportions worldwide. Other risk factors like obesity, dyslipidemia, hypertension, insulin resistance and hyperuricemia aggravate the problem. The present study shows the prevalence of metabolic syndrome and their association with non-traditional CVD risk markers and further substantiated with Uric Acid levels. Studies have shown that one third of the urban population suffer from metabolic syndrome [9] . Urbanization, sedentary life style and globalization of diet have led to high intake of non-traditional fast food which is the leading cause for metabolic syndrome. In our study, overall 60.77% of the subjects are suffering from metabolic syndrome and this is very high compared to 43.2% of MetS observed in Urban Eastern population and 41.1% found in urban Asian Indians [10] [11] . More women (31.58%) were affected and it was significantly (P < 0.001), higher compared to men (29.19%) which is similar to study conducted in semi urban area of Baloor (26.5% men and 31.5% of women) [12] and community study in Kolkata (48.2% in women and 16.3% in men) [13] . Even though the study areas are different in all the above cases more women are affected than men may be due to the changes in occupation, advent of new technologies for domestic use, transition in lifestyle and stress. Anthropometric indicator-abdominal obesity (54.54%) is the most persistent problem in the Kodava community and was higher in women, as seen in Chennai study, wherein 49.2% (38.5% of men and 58.3% of women) subjects had increased waist circumference [14] . This similarity may be due to lack of physical exercise and excess calorie intake which make them susceptible for metabolic syndrome. All the biochemical parameters were significantly high in MetS individuals showing dyslipidemia with decreased glucose tolerance according to the guidelines. In the present study, 36.84% showed hypertriglyceridemia, 35.17% had low HDL levels, while 43.3% were with hypertension and 50% had diabetes. Our result almost corroborates with the South Indian studies which showed high prevalence of metabolic syndrome wherein 31.4% had abdominal obesity, 45.6% showed hypertriglyceridemia, 65.5% had low HDL, 55.4% were hypertensive and 26.7% had high fasting plasma glucose [15] . In both the regions the prevalence was high due to exposure to modernization. Among the study population 209 subjects showed impaired fasting glucose levels out of which 36.84% were diabetic and were on hypoglycemic drugs and therefore 63.15% had the risk of acquiring diabetes which is alarming. These results are higher than Mumbai urban population studies wherein total of 40.15% had history of diabetes and 25% were at risk of acquiring [16] . Low HDL was more prevalent in female subjects, but it is very much lower compared to rural Indian cross sectional study wherein 65.7% of women had low HDL [17] . However triglyceride levels were high in male, comparable to western urban subjects. (32.1% in men and 28.6% in women) [18] Another risk factor hypertension was observed in 43.3% study subjects and men were significantly affected however it is lower compared to the study done on rural Wardha region (53.8%) [19] . Abdominal fat indicated as abdominal obesity (24.16%) associated with hypertension (23.44%) was more prevalent in male subjects and is comparable to urban north Indian men of industry study [20] . However in females abdominal obesity was associated with impaired fasting glucose. These results agree with Iranian study wherein WC was the better predictor of metabolic syndrome in women [21] . In our study prevalence of metabolic syndrome is seen in 65% of 41 - 60 age groups and decreased in men (>60) which is comparable to the study done on young, and aged nonagenarians [22] . Whereas in female prevalence of MetS increased with age, the gender variations may be due to lack of estrogen in post menopause women. This is very contradicting from the study done on Bangladeshi rural women (39.24%), but is similar to the German study wherein highest prevalence of metabolic syndrome was seen in 60 - 79 age groups [23] [24] . Studies have shown that metabolic syndrome is the immediate antecedents for cardio vascular diseases [25] . Metabolic syndrome is associated with two fold risk of CVD and subjects with MetS have 30% - 40% probability of acquiring CVD within 20 years depending on the components present [26] . Though the components of metabolic syndrome serve as conventional risk factors of CVD, there are several emerging non-traditional CVD markers like CRP, Lp(A), ApoB/ApoA1 ratio and homocysteine which are also associated with metabolic syndrome. This study report on Kodavas is a novel investigation as there is no previous history which associates and evaluates the magnitude of metabolic syndrome with nontraditional CVD risk markers on Indian community. Uric acid is the new entity which is gaining significance both in developing metabolic diseases as well as CVD [27] [28] . Hyperuricemia is an important marker associated with metabolic syndrome. In the present study mean of uric acid levels in subjects with MetS was 6.105 mg/dl and non-MetS was 5.238 mg/dl comparable to study done on obese metabolic Caucasians (MetS 6.6 mg/dl and non-MetS 6.1 mg/dl) [29] . A mild inflammation is associated with metabolic syndrome and it results in potential elevation of inflammatory markers like CRP. The components of the metabolic syndrome showed high correlation with CRP in the study done on American women [30] . The CRP levels in our study showed 1.82 mg/dl in subjects with metabolic syndrome and in normal subjects it was 1.78 mg/dl which can be compared with southern California community wherein 3.36 mg/l and 1.62 mg/l was observed in patients and normal subjects respectively [31] . Homocysteine has been recently discussed as a strong and independent prognosis factor for CVD [32] . In our study mean level of Homocysteine marginally increased in MetS (16.51 µmol/L) group compared to the NonMetS group (16.21 µmol/L) and was not significant (P > 0.431). Previous report on Mediterranean population showed significant difference among Mets and non-Mets individuals (Met S 12.0 µmol/l and non-MetS 11.9 µmol/l P = 0.829) [33] . The APO B/APO A1 ratio gives the exact fraction of atherogenic to anti atherogenic lipoprotein ratio which is an upgraded predictor of CVD than LDL and HDL levels. Several clinical studies have shown ApoB/ApoA1 ratio is an accurate risk factor for determining cardio vascular diseases [34] , and is related to the components of MetS in the USA population [35] . In our study there is a significant difference in the means of ApoB/ApoA1 ratio (0.772 in MetS and 0.627 in non-MetS P > 0.034) and comparable to the study done on Chinese population (0.80 in MetS and 0.54 in non-MetS P > 0.05) [36] . In our study age related distribution of CVD markers in subjects with MetS showed elevated levels of all markers in 41 - 60 age groups, which is intimidating. A high level of homocysteine was observed in 60 age group and above it might be due to lack of intake of diet rich in fruits and vegetables which are sources of vitamin B complex [37] . ApoB/ApoA1 ratio is high (61.11%) even in young subjects of 20 - 40 age group making them susceptible to CVD. In our study, we portray a steady increase in the mean of CVD markers as the components of MetS increased comparable to the study done on Chinese geriatric population wherein CVD mortality risk increased with 3, 4 and 5 components of metabolic syndrome [38] . The positive outcome of the study is many Kodava subjects were newly diagnosed with MetS. To obtain more accuracy in our results we have analyzed the cases and controls for CVD markers like CRP, Homocysteine, ApoB/ApoA1 ratio. In contrast most of the studies predict lipid disorders as the only factor for cardiovascular risk rather than testing specific markers to assess CVD. We were not able to diagnose subjects with cardiac disorders, only subjects with previous history were considered, as ECG (Electrocardiography) or angiogram was not feasible in health camps. A follow-up session is required to assess these metabolic syndrome patients after diet, lifestyle and therapeutic modification. 5. Conclusion Thus the study shows prevalence of metabolic syndrome which is high among the Kodava population. Middle aged, female individuals with increased waist circumference and impaired fasting glucose are more liable to metabolic syndrome. Cardiovascular risk markers such as ApoB/ApoA1 ratio, CRP and uric acid levels were high in subjects with metabolic syndrome. Therefore the subjects with metabolic complications have increased risk of hyperuricemia and CVD. The early diagnosis of metabolic syndrome in this population can be used as a precautionary measure to prevent CVD and other co-morbidities which demands strict control over dyslipidemia associated with diabetes. Lifestyle modifications and other therapeutic interventions will have beneficial effects. Acknowledgements The author Deepti A. Lokanath would like to thank Anthropological Survey of India, Kolkata for the financial support. Special thanks to area Associations and Kodava Samaj, Mysore for their support in conducting health camps. All the research scholars and lab technicians of AnSI for their support. References