Open Journal of Endocrine and Metabolic Diseases
Vol.4 No.3(2014), Article ID:43974,9 pages DOI:10.4236/ojemd.2014.43007
Short Report: Association of IL-15 with Peripheral and Hepatic Insulin Sensitivity in Healthy Middle-Aged Men
Yuichiro Nishida1, Kumpei Tokuyama2, Shoichiro Nagasaka3, Akira Kiyonaga4, Yasuki Higaki4, Megumi Hara1, Keitaro Tanaka1, Hiroaki Tanaka4
1Department of Preventive Medicine, Faculty of Medicine, Saga University, Saga, Japan
2Laboratory of Biochemistry of Exercise and Nutrition, Institute of Health and Sport Sciences, University of Tsukuba, Ibaraki, Japan
3Division of Endocrinology and Metabolism, Department of Medicine, Jichi Medical University, Tochigi, Japan
4Laboratory of Exercise Physiology, Faculty of Health and Sports Science, Fukuoka University, Fukuoka, Japan
Email: ynishida@cc.saga-u.ac.jp
Copyright © 2014 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/


Received 13 February 2014; revised 1 March 2014; accepted 8 March 2014
ABSTRACT
Rodent study suggests that interleukin (IL)-15 administration enhances insulin sensitivity. Although it is well known that circulating levels of typical inflammatory markers (C-reactive protein [CRP] and IL-6) are positively associated with homeostasis model assessment-insulin resistance (HOMA-IR), there are no studies investigating the associations of other inflammatory markers including IL-15 with peripheral/hepatic insulin sensitivity in humans. The current study aimed to examine the relationship between the levels of adiopokines or inflammatory cytokines and insulin sensitivity in 8 healthy middle-aged men. Circulating levels of 10 insulin sensitizing adipokines or inflammatory cytokines (total adiponectin [APN], high molecular weight adiponectin [HMW-APN], IL-4, IL-5, IL-6, IL-8, IL-15, interferon [IFN]-γ, tumor necrosis factor [TNF]-α, and TNF-β) were measured. A stable-labeled frequently sampled intravenous glucose tolerance test was performed to assess peripheral (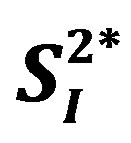 ) and hepatic (
) and hepatic (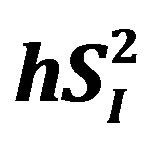 ) insulin sensitivity estimated by 2-compartment minimal model. The levels of 3 inflammatory cytokines (IL-4, IL-6, and IL-15) were significantly and inversely correlated with either
) insulin sensitivity estimated by 2-compartment minimal model. The levels of 3 inflammatory cytokines (IL-4, IL-6, and IL-15) were significantly and inversely correlated with either 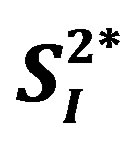 and
and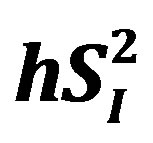 . The association between IL-15 and either
. The association between IL-15 and either 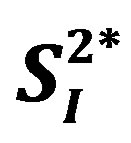 or
or 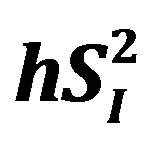 was significant even after adjusting for age and percent body fat (p < 0.01). The current study showed a possible inverse association between serum IL-15 level and peripheral/hepatic insulin sensitivity in healthy middle-aged males, indsependent of percent body fat; this association in humans warrants further study.
was significant even after adjusting for age and percent body fat (p < 0.01). The current study showed a possible inverse association between serum IL-15 level and peripheral/hepatic insulin sensitivity in healthy middle-aged males, indsependent of percent body fat; this association in humans warrants further study.
Keywords:Inflammatory Cytokine; Interleukin; Adipokine; Insulin Sensitivity

1. Introduction
Low-grade chronic systemic inflammation plays an important role in the pathogenesis of obesity and type 2 diabetes [1] [2] . Inflammatory cytokines such as interleukin (IL)-6 and tumor necrosis factor (TNF)-α induce insulin resistance and suppress molecules involved in insulin signaling [2] . Intravenous administration of TNF-α impairs peripheral insulin sensitivity assessed by euglycemic hyperinsulinemic clamp in healthy humans [3] [4] . Consistently, epidemiological studies have shown that higher levels of serum IL-6 and C-reactive protein (CRP) were associated with the increased risk of type 2 diabetes [5] [6] .
Studies have reported a positive relationship between the circulating levels of representative inflammatory markers (e.g., CRP, IL-6, and TNF-α) and homeostasis model assessment-insulin resistance (HOMA-IR) [7] [8] . Recently, other cytokines such as IL-4, IL-8, and IL-15 have emerged as risk factors for adverse health. Inflammatory cytokines such as IL-4, IL-8, and IL-15 are involved in the process of atherogenesis [9] -[11] . For instance, an epidemiological study showed that serum IL-15 concentration in hypertension patients with severe organ damage (88.7 pg/mL) was significantly higher than that in those with no organ damage (55.2 pg/mL) [12] . On the other hand, rodent studies suggest that IL-15 has beneficial effects in the regulation of glucose metabolism. It has been demonstrated that IL-15 administration increased insulin sensitivity in obese mice [13] . There is, however, no information on the associations between the levels of inflammatory cytokines such as IL-4, IL-8, and IL-15 and insulin sensitivity in humans.
A stable-labeled 2-compartment minimal model can provide indices of peripheral insulin sensitivity (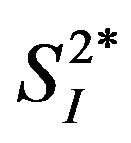 ), as well as glucose effectiveness (
), as well as glucose effectiveness (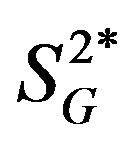 ) (i.e., ability of glucose to stimulate its own uptake), insulin secretion, and endogenous glucose production (EGP) in patients and healthy individuals [14] -[16] . Additionally, this model enabled us to assess hepatic insulin sensitivity (i.e., the ability of insulin to inhibit EGP,
) (i.e., ability of glucose to stimulate its own uptake), insulin secretion, and endogenous glucose production (EGP) in patients and healthy individuals [14] -[16] . Additionally, this model enabled us to assess hepatic insulin sensitivity (i.e., the ability of insulin to inhibit EGP,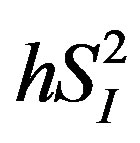 ) and hepatic glucose effectiveness (i.e., the ability of glucose to inhibit EGP,
) and hepatic glucose effectiveness (i.e., the ability of glucose to inhibit EGP,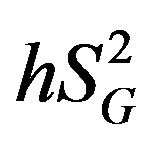 ) [17] . Therefore, we examined the relationships between the circulating levels of 10 adipokines or cytokines including IL-15 and peripheral/hepatic insulin sensitivity indices (
) [17] . Therefore, we examined the relationships between the circulating levels of 10 adipokines or cytokines including IL-15 and peripheral/hepatic insulin sensitivity indices (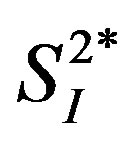 and
and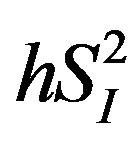 ) assessed by a stable-labeled 2-compartment minimal model in healthy middle-aged men.
) assessed by a stable-labeled 2-compartment minimal model in healthy middle-aged men.
2. Materials and Methods
Subjects: The study subjects were 8 healthy middle-aged men with normal glucose tolerance. We included healthy participants with a wide range of insulin sensitivity to examine the associations between serum cytokines and insulin sensitivity indices. Of these 8 subjects, 4 men were sedentary, defined as not engaged in any habitual exercise for at least 2 years, and 4 were runners, defined as those who run 79 ± 27 km/week. None of the subjects were taking any medications or supplements at the time of study enrollment. Before beginning the study, the nature, purpose, and the risks of the study were explained to all subjects, and written informed consent was obtained. The protocol was approved by the local ethical committee of the Jichi Medical University and was conducted in accordance with the Helsinki Declaration.
Anthropometric indices, oral glucose tolerance test, and stable-labeled frequently sampled intravenous glucose tolerance test: Body mass index (BMI) was determined by dividing body weight in kilograms by the square of height in meters. Each subject’s percent body fat was measured by hydrostatic weighing and was estimated based on the hydrostatic density with a correction for the residual lung volume [18] . An oral glucose tolerance test (OGTT; 75 g glucose) was performed at least 7 days prior to the frequently sampled intravenous glucose tolerance tests (FSIGTTs) to confirm normal glucose tolerance in all subjects. Stable-labeled FSIGTTs were performed, as previously described [14] -[16] . Briefly, after overnight fasting, glucose (300 mg/kg body weight) (isotopically labeled with [6, 6-2H2] glucose [Aldrich, Milwaukee, WI]) was administered in the contralateral antecubital vein within 1 min. Regular insulin (Humalin; Shionogi, Osaka, Japan) was infused (20 mU/kg) into the antecubital vein from 20 to 25 min after the glucose bolus. Blood samples for glucose and insulin were frequently obtained up to 180 min. On the day before undergoing the FSIGTT, all subjects were provided with an evening meal consisting of ≥140 g carbohydrate, ≥30 g fat, and ≥33 g protein.
Deuterated glucose was analyzed as a penta-acetate derivative, and 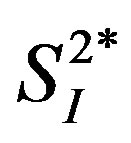 and
and 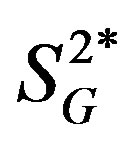 specific to glucose uptake, and EGP were estimated by a 2-compartment minimal model [14] -[16] . Hepatic insulin sensitivity (i.e., the ability of insulin to enhance glucose suppression of EGP,
specific to glucose uptake, and EGP were estimated by a 2-compartment minimal model [14] -[16] . Hepatic insulin sensitivity (i.e., the ability of insulin to enhance glucose suppression of EGP,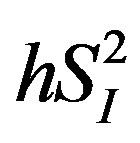 ) and hepatic glucose effectiveness (i.e., the ability of glucose to inhibit EGP,
) and hepatic glucose effectiveness (i.e., the ability of glucose to inhibit EGP,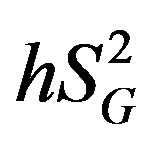 ) were also analyzed as previously described [17] . To assess endogenous insulin secretion, insulin area above the basal level between 0 and 20 min after the administration of glucose was calculated using the conventional trapezoid rule. The glucose disappearance constant (KG) was calculated as the slope of the least squares regression line related to the natural logarithm of the glucose concentration to the time from samples drawn between 10 and 19 min. The HOMA-IR was calculated as the product of the fasting serum insulin concentration (µU/mL) and fasting plasma glucose concentration (mg/dL) divided by 405 [19] .
) were also analyzed as previously described [17] . To assess endogenous insulin secretion, insulin area above the basal level between 0 and 20 min after the administration of glucose was calculated using the conventional trapezoid rule. The glucose disappearance constant (KG) was calculated as the slope of the least squares regression line related to the natural logarithm of the glucose concentration to the time from samples drawn between 10 and 19 min. The HOMA-IR was calculated as the product of the fasting serum insulin concentration (µU/mL) and fasting plasma glucose concentration (mg/dL) divided by 405 [19] .
Measurement of serum cytokine: Fasting levels of serum total-APN and HMW-APN were measured by enzyme-linked immunosorbent assay (ELISA) (Human adiponectin ELISA kit, Human HMW-adiponectin ELISA kit, Otsuka Pharmaceutical Company, Tokushima, Japan). Both intraand inter-assay coefficients of variation for total-APN and HMW-APN were <15%. Fasting levels of serum IL-4, IL-5, IL-6, IL-8, IL-15, IFN- γ, TNF-α, and TNF-β were measured by multiplex sandwich ELISA kit (Q-Plex™ Human Cytokine, Quansys Bioscience Inc., Utah, USA), according to the manufacturer’s instructions. Chemiluminescence was quantified with a Lumino-Image Analyzer LAS-3000 System (Fujifilm Inc., Tokyo). Lower detectable limits for IL-4, IL-6, IL-8, IL-15, and TNF-α were 0.39, 0.93, 0.24, 0.60, and 1.37 pg/mL, respectively. A good agreement between signal intensity and cytokine level was observed within the assay range (r2 ≥ 0.99). Intraand inter-assay coefficients of variation were 10.6% and 17.4% for IL-4, respectively; 7.6% and 18.2% for IL-5, respectively; 6.5% and 11.7% for IL-6, respectively; 4.1% and 20.5% for IL-8, respectively; 5.4% and 14.7% for IL-15, respectively; 8.9% and 15.9% for IFN-γ, respectively; 6.1% and 18.8% for TNF-α, respectively; and 4.3% and 14.4% for TNF-β, respectively. All cytokines were measured 3 times, in duplicate, and the average of the 3 measurements was used for data analyses. Free fatty acid (FFA) levels were measured by standard method.
Statistical analysis: Values are shown as the mean ± SD. The significance of the correlation between variables was assessed by the Pearson correlation coefficient. Multiple regression analyses with adjustment for age and percent body fat were performed to examine if cytokine levels (independent variables) could be associated with insulin sensitivity indices (dependent variables), independent of percent body fat. A p value of < 0.05 was considered statistically significant. All analyses were performed using SAS software (version 9.3 for Windows, SAS Institute, Cary, NC, USA).
3. Results
General characteristics of the study subjects are shown in Table 1. The percent body fat tended to be positively correlated with IL-4 (r = 0.68, p = 0.06), and it was significantly and positively correlated with IL-6 and IL-15 (Figure 1). Likewise, BMI was positively correlated with the levels of IL-4 and IL-6 (IL-4: r = 0.84, p < 0.01; IL-6: r = 0.80, p < 0.05; IL-15: r = 0.65, p = 0.08). Insignificant correlations were observed between either percent body fat or BMI and the other 7 cytokines (total-APN, HMW-APN, IL-5, IL-8, IFN-γ, TNF-α, and TNF-β) (data not shown). Three cytokines (IL-4, IL-6, and IL-15) were inversely correlated with 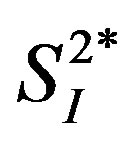 (Figure 2), while correlations between these cytokines and insulin secretion, insulin-independent glucose uptake (
(Figure 2), while correlations between these cytokines and insulin secretion, insulin-independent glucose uptake (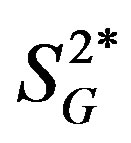 ), or basal EGP were not statistically significant (data not shown). Additionally, the 3 cytokines (IL-4, IL-6, and IL-15) showed significant inverse correlations with
), or basal EGP were not statistically significant (data not shown). Additionally, the 3 cytokines (IL-4, IL-6, and IL-15) showed significant inverse correlations with 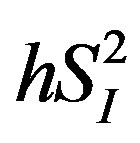 (Figure 3), whereas among the 3 cytokines but only IL-6 was significantly and inversely correlated with
(Figure 3), whereas among the 3 cytokines but only IL-6 was significantly and inversely correlated with 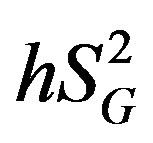 (r = −0.73, p < 0.05).
(r = −0.73, p < 0.05).
When the analyses were performed separately for sedentary men (n = 4) and runners (n = 4), the correlation between percent body fat and IL-15 became non-significant in sedentary men (r = −0.71, p = 0.29) and runners (r = 0.86, p = 0.14). On the other hand, the relationship between IL-15 and  tended to be significant in sedentary men (r = −0.93, p = 0.07) and remained significant in runners (r = −0.995, p < 0.01). Additionally, the correlation between IL-15 and hSI2 remained significant in sedentary men (r = −0.97, p < 0.05) and runners (r = −0.993, p < 0.01).
tended to be significant in sedentary men (r = −0.93, p = 0.07) and remained significant in runners (r = −0.995, p < 0.01). Additionally, the correlation between IL-15 and hSI2 remained significant in sedentary men (r = −0.97, p < 0.05) and runners (r = −0.993, p < 0.01).
In the multiple regression analysis using data from all subjects (n = 8), the associations between either IL-4 or IL-6 and 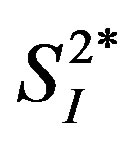 became insignificant, whereas that between IL-15 and
became insignificant, whereas that between IL-15 and 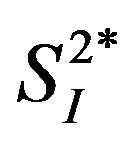 remained statistically significant after the adjustment for age and percent body fat (Table 2). The inverse relationship between either IL-4 or IL-15 and
remained statistically significant after the adjustment for age and percent body fat (Table 2). The inverse relationship between either IL-4 or IL-15 and 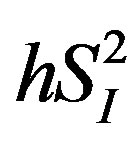 were also significant even after adjusting for age and percent body fat (Table 2). Additionally, among
were also significant even after adjusting for age and percent body fat (Table 2). Additionally, among
 (a)
(a) (b)
(b) (c)
(c)
Figure 1. Correlations between percent body fat and the levels of 3 inflammatory cytokines (IL-4, IL-6, and IL-15) in healthy middle-aged individuals.
 (a)
(a) (b)
(b) (c)
(c)
Figure 2. Relationships between the levels of 3 inflammatory cytokines (IL-4, IL-6, and IL-15) and 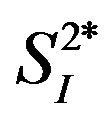 in healthy middle-aged men.
in healthy middle-aged men.
 (a)
(a) (b)
(b) (c)
(c)
Figure 3. Associations between the levels of 3 inflammatory cytokines (IL-4, IL-6, and IL-15) and 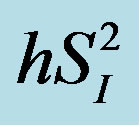 in healthy middle-aged males.
in healthy middle-aged males.
cytokines that were measured, only IL-6 was significantly and positively correlated with HOMA-IR (r = 0.71, p < 0.05), but this association became insignificant after adjusting for age and percent body fat.
4. Discussion
In the current study, we observed inverse associations between the circulating level of IL-15 and either peri
Table 1. General characteristics of the study subjects.
Values are mean ± SD. BMI, body mass index; IL, interleukin; IFN, interferon; TNF, tumor necrosis factor; APN, adiponectin; HMW-APN, high molecular weight-adiponectin; HOMA-IR, homeostasis model assessment-insulin resistance; FFA, free fatty acid; KG, glucose disappearance constant;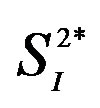 , peripheral insulin sensitivity;
, peripheral insulin sensitivity;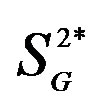 , peripheral glucose effectiveness; EGP, endogenous glucose production;
, peripheral glucose effectiveness; EGP, endogenous glucose production;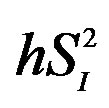 , hepatic insulin sensitivity;
, hepatic insulin sensitivity;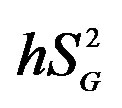 , hepatic glucose effectiveness. aPlasma glucose concentration in 2-h oral glucose tolerance test.
, hepatic glucose effectiveness. aPlasma glucose concentration in 2-h oral glucose tolerance test.
Table 2. Multiple regression analyses with 3 serum cytokines as predictors for peripheral/hepatic insulin sensitivity.
Adjusted for age and percent body fat. IL, interleukin;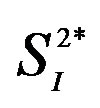 , peripheral insulin sensitivity;
, peripheral insulin sensitivity;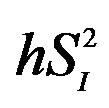 , hepatic insulin sensitivity.
, hepatic insulin sensitivity.
pheral (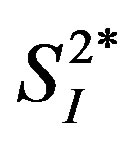 ) or hepatic (
) or hepatic (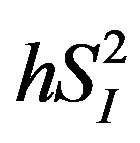 ) insulin sensitivity in healthy middle-aged men, independent of percent body fat. When data were analyzed in sedentary men (n = 4) as a more homogeneous group, a similar pattern of results regarding these inverse relationships as those analyzed in all subjects (n = 8) were obtained; this corroborates the current results. This is an unexpected finding, since IL-15 administration has been linked to higher insulin sensitivity IL-15 administration has been linked to higher insulin sensitivity in obese mice [13] . To our knowledge, there is no previous study that simultaneously assessed IL-15 and insulin sensitivity in humans.
) insulin sensitivity in healthy middle-aged men, independent of percent body fat. When data were analyzed in sedentary men (n = 4) as a more homogeneous group, a similar pattern of results regarding these inverse relationships as those analyzed in all subjects (n = 8) were obtained; this corroborates the current results. This is an unexpected finding, since IL-15 administration has been linked to higher insulin sensitivity IL-15 administration has been linked to higher insulin sensitivity in obese mice [13] . To our knowledge, there is no previous study that simultaneously assessed IL-15 and insulin sensitivity in humans.
There is a previous study showing that the serum IL-15 concentration in the exerciseand diet-induced weight-loss group was decreased by 33% in obese individuals [20] . In another study, the circulating level of IL- 15 in the physical exercise intervention group decreased by 2.8% in older adults, although this decrease was not statistically significant [21] . In this previous study, physical exercise intervention significantly reduced the serum level IL-15 by 10% in older participants with a high baseline IL-15 [21] . Additionally, it is well known that insulin sensitivity is increased with moderate exercise training [16] [22] , and the current results suggest that lower IL-15 could be associated with higher peripheral/hepatic insulin sensitivity. Our cross-sectional study is limited by the fact that we are not able to analyze the association between the longitudinal changes in IL-15 and insulin sensitivity before and after the intervention. Thus, we can only speculate that the decreased levels of circulating IL-15 may be associated with improved insulin sensitivity after diet and/or exercise program in humans.
The biological mechanisms underlying the association of IL-15 with insulin sensitivity are largely unclear, but there are a few possible explanations for the association. It has been shown that IL-15 induces nuclear factor-κB (NF-κB) activation in human neutrophils [23] . Since hepatic activation of NF-κB induces local and systemic insulin resistance [24] , IL-15 might act directly on insulin-targeted organs such as skeletal muscle and liver to worsen insulin sensitivity, assuming that IL-15 induces NF-κB activation in insulin-sensitive organs. There is also a possibility that IL-15 exerts its adverse effect on insulin sensitivity through regulating other factors, such as FFA and IL-6. IL-15 stimulates lipolysis in primary pig adipocytes [25] . Prolonged increases in FFA, which is produced as a result of lipolysis (in company with glycerol), impair insulin action to promote glucose transport in skeletal muscle [26] . Thus, FFA might act as a mediator between IL-15 and peripheral insulin sensitivity. In this connection, higher IL-15 tended to be correlated with higher FFA in the current subjects (r = 0.53, p = 0.17). Additionally, a previous study showed that high IL-15 concentrations enhanced IL-6 production in cell culture [27] . Consistently, higher IL-15 was correlated with higher IL-6 in the current subjects (r = 0.80, p < 0.05). Since IL-6 induces insulin resistance [1] [2] [28] , IL-6 could be a possible mediator involved in the mechanisms underlying the inverse association between IL-15 and insulin sensitivity.
On the other hand, the possibility that IL-4 plays a role in regulation of serum IL-15 level cannot be denied. A previous study demonstrated that IL-15 mRNA expression was induced in human blood-derived dendritic cells during culture in granulocyte-macrophage colony-stimulating factor and IL-4 [29] . In this previous study, the induction of IL-15 gene expression was followed by a moderate increase of IL-15 protein in the culture supernatants [29] . Also, higher IL-4 was associated with higher IL-15 in the present study (r = 0.76, p < 0.05).
Rodent study suggests that IL-15 takes part in reducing the mass of adipose tissue. IL-15 administration for 7 days decreased white adipose tissue by approximately 30% in adult rats [30] . Consistently, Nielsen et al. [31] showed an inverse association between plasma IL-15 and percent fat mass was also observed in humans. On the other hand, the current study observed a positive relationship between percent body fat and IL-15 concentration in healthy middle-aged men who are sedentary or runners. When analyzing with data from 4 sedentary men, the correlation between percent fat and IL-15 became non-significant, as described in the result section. Thus, we are not able to exclude the possibility that the significant correlation observed in all subjects (n = 8) could be due to heterogeneity of the current participants (i.e., sedentary men and runners). Further studies are needed to determine the association between IL-15 and body fat in rodents and humans.
This study had several limitations. First, sample size of this study was very small. We were unable to increase the number of subjects due to an insufficiency of serum samples to measure cytokines. Despite the small sample size, we were able to confirm previously reported association of IL-6 with either body fat or insulin sensitivity [7] [32] by using most precise methods to measure percent body fat, serum cytokine levels, and peripheral/hepatic insulin sensitivity. This confirmation validates current methods and results. However, it is evident that further research is needed to examine if similar relationships are observed in a larger sample of subjects with varying degree of obesity, since the current participants only include men with mild obesity (percent body fat of 24% at the most). Also, additional risk factors potentially affecting insulin sensitivity in healthy humans (e.g., family history of lifestyle-related diseases, blood pressure, and lipid profiles) were not examined. Although we considered percent body fat to be the most important factor influencing insulin sensitivity, incomplete adjustment for other potential confounding variables might have influenced the results. Moreover, causality cannot be judged by this type of study. Longitudinal studies are highly warranted to investigate the association between the chronological changes in IL-15 and insulin sensitivity. Detailed mechanisms for associations between IL-15 and peripheral/hepatic insulin sensitivity remain to be elucidated.
5. Conclusion
The current study showed a possible inverse association between serum IL-15 level and peripheral/hepatic insulin sensitivity in healthy middle-aged men, independent of percent body fat; this association in humans warrants further study.
Disclosure Statement
The authors declare that they have no conflict of interest.
Acknowledgements
This work was supported by grants from the Japanese Ministry of Education, Culture, Sports, Science, and Technology (10770574, 11480005, 12671123, 19200049: Strategic Research Infrastructure), the Global FU Program funded by Fukuoka University, Japan Diabetes Foundation, the Institute for Adult Disease Asahi Life Foundation, Jichi Medical School Young Investigator Award.
References
- Pickup, J.C. (2004) Inflammation and Activated Innate Immunity in the Pathogenesis of Type 2 Diabetes. Diabetes Care, 27, 813-823. http://dx.doi.org/10.2337/diacare.27.3.813
- Hotamisligil, G.S. (2006) Inflammation and Metabolic Disorders. Nature, 444, 860-867. http://dx.doi.org/10.1038/nature05485
- Krogh-Madsen, R., Plomgaard, P., Moller, K., Mittendorfer, B. and Pedersen, B.K. (2006) Influence of TNF-α and IL-6 Infusions on Insulin Sensitivity and Expression of IL-18 in Humans. American Journal of Physiology: Endocrinology and Metabolism, 291, E108-114. http://dx.doi.org/10.1152/ajpendo.00471.2005
- Saghizadeh, M., Ong, J.M., Garvey, W.T., Henry, R.R. and Kern, P.A. (1996) The Expression of TNFα by Human Muscle. Relationship to Insulin Resistance. Journal of Clinical Investigation, 97, 1111-1116. http://dx.doi.org/10.1172/JCI118504
- Schmidt, M.I., Duncan, B.B., Sharrett, A.R., Lindberg, G., Savage, P.J., Offenbacher, S., Azambuja, M.I., Tracy, R.P. and Heiss, G. (1999) Markers of Inflammation and Prediction of Diabetes Mellitus in Adults (Atherosclerosis Risk in Communities Study): A Cohort Study. Lancet, 353, 1649-1652. http://dx.doi.org/10.1016/S0140-6736(99)01046-6
- Pradhan, A.D., Manson, J.E., Rifai, N., Buring, J.E. and Ridker, P.M. (2001) C-Reactive Protein, Interleukin 6, and Risk of Developing Type 2 Diabetes Mellitus. JAMA, 286, 327-334. http://dx.doi.org/10.1001/jama.286.3.327
- Marques-Vidal, P., Bastardot, F., von Kanel, R., Paccaud, F., Preisig, M., Waeber, G. and Vollenweider, P. (2013) Association between Circulating Cytokine Levels, Diabetes and Insulin Resistance in a Population-Based Sample (CoLaus study). Clinical Endocrinology, 78, 232-241. http://dx.doi.org/10.1111/j.1365-2265.2012.04384.x
- Abbatecola, A.M., Ferrucci, L., Grella, R., Bandinelli, S., Bonafe, M., Barbieri, M., Corsi, A.M., Lauretani, F., Franceschi, C. and Paolisso, G. (2004) Diverse Effect of Inflammatory Markers on Insulin Resistance and Insulin-Resistance Syndrome in the Elderly. Journal of the American Geriatrics Society, 52, 399-404. http://dx.doi.org/10.1111/j.1532-5415.2004.52112.x
- Lee, Y.W., Kuhn, H., Hennig, B., Neish, A.S. and Toborek, M. (2001) IL-4-Induced Oxidative Stress Upregulates VCAM-1 Gene Expression in Human Endothelial Cells. Journal of Molecular and Cellular Cardiology, 33, 83-94. http://dx.doi.org/10.1006/jmcc.2000.1278
- Gerszten, R.E., Garcia-Zepeda, E.A., Lim, Y.C., Yoshida, M., Ding, H.A., Gimbrone Jr., M.A., Luster, A.D., Luscinskas, F.W. and Rosenzweig, A. (1999) MCP-1 and IL-8 Trigger Firm Adhesion of Monocytes to Vascular Endothelium under Flow Conditions. Nature, 398, 718-723. http://dx.doi.org/10.1038/19546
- Houtkamp, M.A., van Der Wal, A.C., de Boer, O.J., van Der Loos, C.M., de Boer, P.A., Moorman, A.F. and Becker, A.E. (2001) Interleukin-15 Expression in Atherosclerotic Plaques: An Alternative Pathway for T-Cell Activation in Atherosclerosis? Arteriosclerosis, Thrombosis, and Vascular Biology, 21, 1208-1213. http://dx.doi.org/10.1161/hq0701.092162
- Kaibe, M., Ohishi, M., Ito, N., Yuan, M., Takagi, T., Terai, M., Tatara, Y., Komai, N., Rakugi, H. and Ogihara, T. (2005) Serum Interleukin-15 Concentration in Patients with Essential Hypertension. American Journal of Hypertension, 18, 1019-1025. http://dx.doi.org/10.1016/j.amjhyper.2005.02.014
- Barra, N.G., Chew, M.V., Holloway, A.C. and Ashkar, A.A. (2012) Interleukin-15 Treatment Improves Glucose Homeostasis and Insulin Sensitivity in Obese Mice. Diabetes, Obesity and Metabolism, 14, 190-193. http://dx.doi.org/10.1111/j.1463-1326.2011.01495.x
- Nagasaka, S., Tokuyama, K., Kusaka, I., Hayashi, H., Rokkaku, K., Nakamura, T., Kawakami, A., Higashiyama, M., Ishikawa, S. and Saito, T. (1999) Endogenous Glucose Production and Glucose Effectiveness in Type 2 Diabetic Subjects Derived from Stable-Labeled Minimal Model Approach. Diabetes, 48, 1054-1060. http://dx.doi.org/10.2337/diabetes.48.5.1054
- Nishida, Y., Tokuyama, K., Nagasaka, S., Higaki, Y., Fujimi, K., Kiyonaga, A., Shindo, M., Kusaka, I., Nakamura, T., Ishikawa, S.E., Saito, T., Nakamura, O., Sato, Y. and Tanaka, H. (2002) SG, SI, and EGP of Exercise-Trained Middle-Aged Men Estimated by a Two-Compartment Labeled Minimal Model. American Journal of Physiology: Endocrinology and Metabolism, 283, E809-E816.
- Nishida, Y., Tokuyama, K., Nagasaka, S., Higaki, Y., Shirai, Y., Kiyonaga, A., Shindo, M., Kusaka, I., Nakamura, T., Ishibashi, S. and Tanaka, H. (2004) Effect of Moderate Exercise Training on Peripheral Glucose Effectiveness, Insulin Sensitivity, and Endogenous Glucose Production in Healthy Humans Estimated by a Two-Compartment-Labeled Minimal Model. Diabetes, 53, 315-320. http://dx.doi.org/10.2337/diabetes.53.2.315
- Tokuyama, K., Nagasaka, S., Mori, S., Takahashi, N., Kusaka, I., Kiyonaga, A., Tanaka, H., Shindo, M. and Ishibashi, S. (2009) Hepatic Insulin Sensitivity Assessed by Integrated Model of Hepatic and Peripheral Glucose Regulation. Diabetes Technology & Therapeutics, 11, 487-492. http://dx.doi.org/10.1089/dia.2009.0011
- Goldman, R.F. and Buskirk, E.R. (1961) A Method for Underwater Weighing and the Determination of Body Density. In: Brozekand, J. and Hershel, A., Eds., Techniques for Measuring Body Composition, National Research Council, Washington DC, 78-89.
- Matthews, D.R., Hosker, J.P., Rudenski, A.S., Naylor, B.A., Treacher, D.F. and Turner, R.C. (1985) Homeostasis Model Assessment: Insulin Resistance and Beta-Cell Function from Fasting Plasma Glucose and Insulin Concentrations in Man. Diabetologia, 28, 412-419. http://dx.doi.org/10.1007/BF00280883
- Christiansen, T., Paulsen, S.K., Bruun, J.M., Pedersen, S.B. and Richelsen, B. (2010) Exercise Training Versus Diet-Induced Weight-Loss on Metabolic Risk Factors and Inflammatory Markers in Obese Subjects: A 12-Week Randomized Intervention Study. American Journal of Physiology: Endocrinology and Metabolism, 298, E824-E831. http://dx.doi.org/10.1152/ajpendo.00574.2009
- Beavers, K.M., Hsu, F.C., Isom, S., Kritchevsky, S.B., Church, T., Goodpaster, B., Pahor, M. and Nicklas, B.J. (2010) Long-Term Physical Activity and Inflammatory Biomarkers in Older Adults. Medicine & Science in Sports & Exercise, 42, 2189-2196. http://dx.doi.org/10.1249/MSS.0b013e3181e3ac80
- Nishida, Y., Higaki, Y., Tokuyama, K., Fujimi, K., Kiyonaga, A., Shindo, M., Sato, Y. and Tanaka, H. (2001) Effect of Mild Exercise Training on Glucose Effectiveness in Healthy Men. Diabetes Care, 24, 1008-1013. http://dx.doi.org/10.2337/diacare.24.6.1008
- McDonald, P.P., Russo, M.P., Ferrini, S. and Cassatella, M.A. (1998) Interleukin-15 (IL-15) Induces NF-κB Activation and IL-8 Production in Human Neutrophils. Blood, 92, 4828-4835.
- Cai, D., Yuan, M., Frantz, D.F., Melendez, P.A., Hansen, L., Lee, J. and Shoelson, S.E. (2005) Local and Systemic Insulin Resistance Resulting from Hepatic Activation of IKK-β and NF-κB. Nature Medicine, 11, 183-190. http://dx.doi.org/10.1038/nm1166
- Ajuwon, K.M. and Spurlock, M.E. (2004) Direct Regulation of Lipolysis by Interleukin-15 in Primary Pig Adipocytes. American Journal of Physiology: Regulatory, Integrative and Comparative Physiology, 287, R608-R611. http://dx.doi.org/10.1152/ajpregu.00192.2004
- Hawkins, M., Barzilai, N., Liu, R., Hu, M., Chen, W. and Rossetti, L. (1997) Role of the Glucosamine Pathway in Fat-Induced Insulin Resistance. Journal of Clinical Investigation, 99, 2173-2182. http://dx.doi.org/10.1172/JCI119390
- Alleva, D.G., Kaser, S.B., Monroy, M.A., Fenton, M.J. and Beller, D.I. (1997) IL-15 Functions as a Potent Autocrine Regulator of Macrophage Proinflammatory Cytokine Production: Evidence for Differential Receptor Subunit Utilization Associated with Stimulation or Inhibition. Journal of Immunology, 159, 2941-2951.
- Senn, J.J., Klover, P.J., Nowak, I.A. and Mooney, R.A. (2002) Interleukin-6 Induces Cellular Insulin Resistance in Hepatocytes. Diabetes, 51, 3391-3399. http://dx.doi.org/10.2337/diabetes.51.12.3391
- Jonuleit, H., Wiedemann, K., Muller, G., Degwert, J., Hoppe, U., Knop, J. and Enk, A.H. (1997) Induction of IL-15 Messenger RNA and Protein in Human Blood-Derived Dendritic Cells: A Role for IL-15 in Attraction of T Cells. Journal of Immunology, 158, 2610-2615.
- Carbo, N., Lopez-Soriano, J., Costelli, P., Alvarez, B., Busquets, S., Baccino, F.M., Quinn, L.S., Lopez-Soriano, F.J. and Argiles, J.M. (2001) Interleukin-15 Mediates Reciprocal Regulation of Adipose and Muscle Mass: A Potential Role in Body Weight Control. Biochimica et Biophysica Acta, 1526, 17-24. http://dx.doi.org/10.1016/S0304-4165(00)00188-4
- Nielsen, A.R., Hojman, P., Erikstrup, C., Fischer, C.P., Plomgaard, P., Mounier, R., Mortensen, O.H., Broholm, C., Taudorf, S., Krogh-Madsen, R., Lindegaard, B., Petersen, A.M., Gehl, J. and Pedersen, B.K. (2008) Association between Interleukin-15 and Obesity: Interleukin-15 as a Potential Regulator of Fat Mass. The Journal of Clinical Endocrinology & Metabolism, 93, 4486-4493. http://dx.doi.org/10.1210/jc.2007-2561
- Fernandez-Real, J.M., Vayreda, M., Richart, C., Gutierrez, C., Broch, M., Vendrell, J. and Ricart, W. (2001) Circulating Interleukin 6 Levels, Blood Pressure, and Insulin Sensitivity in Apparently Healthy Men and Women. The Journal of Clinical Endocrinology & Metabolism, 86, 1154-1159. http://dx.doi.org/10.1210/jcem.86.3.7305


