World Journal of Cardiovascular Surgery
Vol.3 No.1(2013), Article ID:29183,8 pages DOI:10.4236/wjcs.2013.31003
Assessing Safety of CABG in the Era Post Primary PCI, an Outcome Analysis of STEMI Population*
1Bristol Heart Institute, Bristol Royal Infirmary, Bristol, UK
2Cardiothoracic Division, The James Cook University Hospital, Middlesbrough, UK
Email: #squreshi786@googlemail.com, mark.debelder@stees.nhs.uk, enoch.akowuah@nhs.net
Received January 23, 2013; revised February 21, 2013; accepted February 28, 2013
Keywords: Coronary Artery Bypass Grafting (CABG); Primary Percutaneous Coronary Intervention (PPCI); Infarct Related Artery (IRA); Intra Aortic Balloon Counterpulsation (IABP); ST-Elevation Myocardial Infarction (STEMI); Thrombolysis
ABSTRACT
Background: Primary PCI (PPCI) has replaced thrombolysis as the treatment of choice for STEMI. The effect of this change on outcomes of patients referred for subsequent CABG is unknown. Methods: All STEMI patients having thrombolysis or PPCI between 2000 and 2010 were identified. Of these, patients subsequently referred for isolated first time CABG form the cohort for this study. Results: 83 of 2476 (3.4%) patients from the PPCI cohort (median follow-up [FU] 3 years [range 6 m - 7.8 y]) and 49 of 528 (9.2%) from the thrombolysis cohort (median FU 9 y [range 1.5 - 10 y] were referred for subsequent CABG. In this referred group, initial reperfusion success (as defined) was: PPCI = 86%, lysis = 84%, p = 0.69. Surgical waiters with prior PPCI had less post infarct angina (1.2% vs. 25%, p < 0.01) and late re-infarction (6% vs. 20%, p = 0.034) prior to surgery. Timing of CABG was: <6 m (PPCI 82%, lysis 73%), 6 m-1 y (PPCI 8.4%, lysis: 9%), >1 y (PPCI 9.6%, lysis 18%).Other than an increased prevalence of diabetes in the thrombolysis group, there were no differences in demographic details or risk profile. There were no post-operative deaths, MIs or CVAs. There were no significant differences in post-op AF (28% vs. 22% p = 0.5), respiratory failure (8% vs. 18%, p = 0.08), renal failure (5% vs. 6%, p = 0.5) or re-openings (0% vs. 6%, p = 0.8). Mortality at 3 years was 2.4% in the PPCI cohort and 4% in the thrombolysis cohort. Overall mortality during follow-up for the PPCI group was 3.6% (n = 3) (median FU 3 years), and for the lysis group was 24.5% (n = 12) (median FU 9 years). Conclusions: In patients awaiting CABG after STEMI, PPCI reduces the risk of post-infarct angina and re-infarction prior to surgery, but early surgical results were equally favorable in both groups. Additional follow-up is needed in the PPCI cohort to determine whether there are any significantly different longer-term outcomes.
1. Introduction
The treatment of acute ST elevation myocardial infarcttion (STEMI) has evolved over recent decades [1]. In most parts of the UK, primary percutaneous coronary intervention (PPCI) has replaced thrombolysis as the treatment of choice. This is primarily because thrombolysis is associated with a 40% incidence of reperfusion failure [2]. On the contrary PPCI results in improved reperfusion of the infarct related artery (IRA) [3] and decreased re-occlusion, both of which translate into improved early and late outcomes after STEMI.
Irrespective of the treatment strategy used for STEMI, some patients require coronary artery bypass grafting (CABG) subsequently. The indication for CABG is usually one of: 1) failure of thrombolysis or PPCI; 2) concomitant coronary surgery at the time of other surgical procedures such as valve replacement or repair; or 3) symptomatic or perceived prognostic benefit in the setting of severe disease in the infarct-related or other coronary arteries or the presence of left main stem stenosis.
In this study we set out to examine the impact of the STEMI treatment strategy used on patients who subsequently undergo CABG.
2. Methods
Our hospital is a tertiary care referral centre for STEMI patients with a catchment population of approximately 1.5 million. All patients treated for STEMI in our institution from 2000 to 2010 form the cohort of this study. During the study period, there was a staged transition from thrombolysis to PPCI as the treatment of choice for STEMI with sequential roll-out to our referral districts. Patients undergoing thrombolysis or PPCI were identified from our unit’s data collection systems that enable our participation in the national databases of: 1) all patients admitted with STEMI (The Myocardial Ischaemia National Audit Project [4]); and 2) all patients treated by PCI (the British Cardiovascular Intervention Society [BCIS] database [5]).
Our inclusion criteria were to include all patients undergoing initial reperfusion with either thrombolysis or PPCI for index STEMI presentation who subsequently underwent isolated CABG in our hospital.
Patients were excluded if they had a previous MI treated with lysis or had prior PCI, had an MI while on the surgical waiting list, or needed surgery in addition to isolated CABG. We collected demographic data, details on thrombolysis and PPCI, post reperfusion results and finally post-operative variables including survival.
STEMI was diagnosed on the basis of standard clinical, electrocardiographic and biochemical criteria. Patients in either group received 300 mg of aspirin regardless of whether they were taking chronic aspirin therapy. Use of clopidogrel was routine following PPCI as per international guidelines [6] and was used selectively for the thrombolysis cohort, but especially after rescue PCI or for PCI following the acute phase; prasugrel and ticagrelor had not been introduced to local practice in the period reviewed. GPIIb/IIIa antagonists were routinely employed after PPCI unless the risk of bleeding was high. Both groups were treated with weight adjusted unfractionated heparin. Where relevant, clopidogrel was stopped for at least 5 days prior to surgery, unless deemed clinically inappropriate or where there was urgency for surgery that prevented the withdrawal period.
Successful reperfusion in thrombolysis group was defined as complete resolution of symptoms associated with >50% normalisation of ST segment changes. In the PPCI group this was defined as achieving TIMI-3 flow in the infarct-related vessel. Failed reperfusion in the lysis group was defined as <50% ST-segment resolution in the leads with the highest ST-segment elevation 60 - 90 min after lysis. In the PPCI group this was diagnosed if there was failure to achieve TIMI 3 flow at the end of procedure (TIMI 2 flow was defined as partial reperfusion). Early re-infarction was defined as re-infarction during the initial hospitalization while late re-infarctions were defined as infarction following discharge from hospital after the index event but prior to scheduled elective CABG.
For PPCI, bare metal stents were usually utilized for the infarct-related vessel if concomitant surgical disease was present, with a view to early withdrawal of clopidogrel before surgery. Use of an intra-aortic balloon pump (IABP) was mostly reserved for patients in cardiogenic shock.
Survival data were obtained from a combination of hospital notes, general practitioner enquiries and our hospital database (Clinical and Management Information System Version 17.1, ASCRIBE.UK) [7]. Approval from our ethical review board was waived, as this was a retrospective review of databases and case notes and did not require any intervention.
Statistical Analysis
Normally distributed continuous variables were compared using the student t-test, while categorical variables were compared using chi-square test or Fisher’s exact test if dichotomous. Subgroup analyses of outcomes were conducted using chi-square and ANOVA. Data that were not normally distributed when assessed using the Kolmogorov-Smirnov test were compared using the MannWhitney U test. Significance was established at p < 0.05. Survival curves were obtained using the Kaplan-Meier method. Overall survival was compared using Log-rank (Mantel-Cox) method. In addition, odds ratio is provided for 3-year survival. All analyses were carried out using SPSS 17.0 (SPSS Inc., Chicago, Ill, USA).
3. Results
49 out of a total of 528 (9.2%) STEMI patients (2000- 2004) treated with thrombolysis (Lysis group) and 83 out of a total of 2476 (3.4%) STEMI patients (2003-2010) treated with PPCI (PPCI group) were identified to have undergone isolated CABG at our centre. The baseline characteristics are shown in Table 1. Except for a higher prevalence of diabetes in the lysis group (p = 0.034), there were no other significant differences in the demographic variables.
Outcomes following treatment of STEMI are also shown in Table 1. The initial success of both strategies to reperfuse the infarct related vessel (as defined), were comparable (84% vs. 86%, p = 0.62), such that most patients undergoing surgery had reperfused. There was no difference in early complications following reperfusion, but there was a statistically significant increase in late complications with more post-infarct angina and re-infarction in the lysis group (24.5% vs. 1.2%, p = 0.0001 and 20% vs. 6%, p = 0.012) respectively.
Details of subsequent CABG are shown in Table 2. CABG occurred within 6 months of MI in most patients (lysis group: 73%, PPCI group: 82%). The mean logistic EuroSCORE for the lysis group was 5.6, and for the PPCI group was 6.3 (p = 0.078). The extent of surgical disease was similar in both groups. Most of the patients received 3 or 4 grafts. In patients who had prior PPCI, the infarct related artery was grafted in 72% (n = 60) of patients.
Post-operative outcome details are shown in Table 3.
Table 1. Demographic details and results of reperfusion.
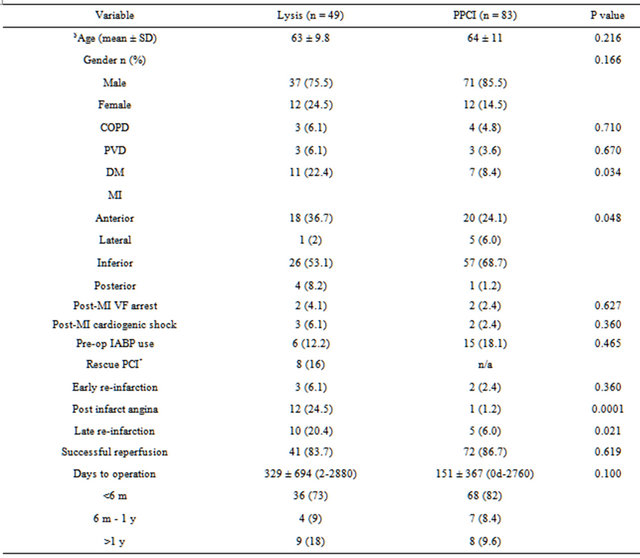
COPD: chronic obstructive pulmonary disease; PVD: peripheral vascular disease; DM: diabetes mellitus; MI: myocardial infarction; IABP: intra-aortic balloon counterpulsation; $numerical data described as mean ± standard deviation, categorical data described as number of patients (percentage); *patients who underwent salvage PCI after failed thrombolysis.
There were no post-operative myocardial infarctions or stroke. There was no statistically significant difference in the rates of post-operative atrial fibrillation, renal failure, respiratory complications, re-opening for bleeding or tamponade or wound infections. A further subgroup analysis showed prolonged ICU (p = 0.001) and hospital stay (p = 0.000) in patients operated within a week of undergoing PPCI prior to surgery (Table 4). Further analysis showed that this was true for patients who had undergone emergency surgery (Table 5). Subgroup analysis (Tables 4 and 5, did show significant differences in respiratory complications, wound infections, mortality in PPCI subgroup, however due to small number of patients it is difficult to draw any solid conclusions.
There were no in-hospital deaths. Median follow-up for PPCI group was 3 years, range (6 m - 7.8 y) and for thrombolysis, this was 9 years, range (1.5 - 10 y). 3 y mortality for the PPCI group was 2.4% (n = 2) and for the thrombolysis group 4% (n = 2) (Figures 1 and 2). The overall mortality for the PPCI group was 3.6% (n = 3) and for the thrombolysis group, with its longer follow-up period, this was 24.5% (n = 12).
4. Discussion
With widespread use of PCI, in both STEMI and acutecoronary syndrome patients, there is increasing realisation in the surgical community that early PCI can miti gate the negative effects of the infarct on ventricular function. We wanted to assess the impact of lysis or
Table 2. Operative details.
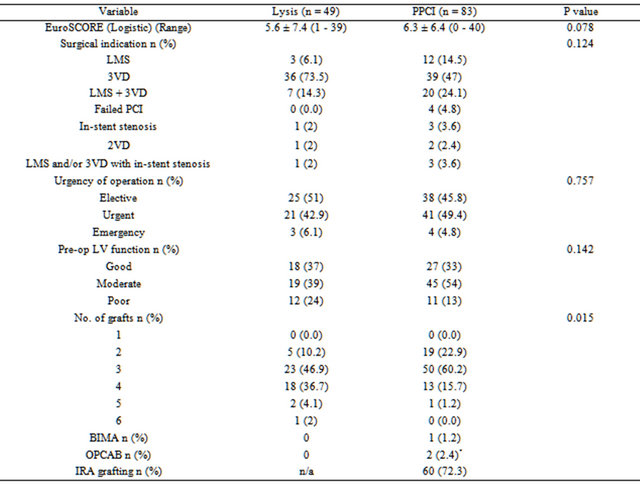
LMS: left main stem; 3VD: triple vessel disease; 2VD: double vessel disease; BIMA: bilateral internal mammary artery; OPCAB: off-pump coronary artery bypass; *1 patient was converted to on-pump surgery; IRA: infarct related artery; n/a: data not available.
Table 3. Operative outcomes.
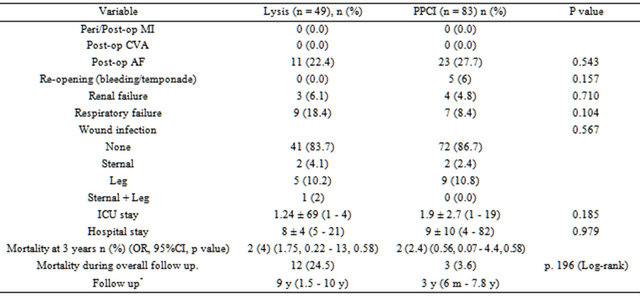
CVA: cerebrovascular accident; AF: atrial fibrillation; OR: odds ratio; CI: Confidence interval; *median follow up (range).
Table 4. Subgroup outcome analysis (initial intervention to CABG).
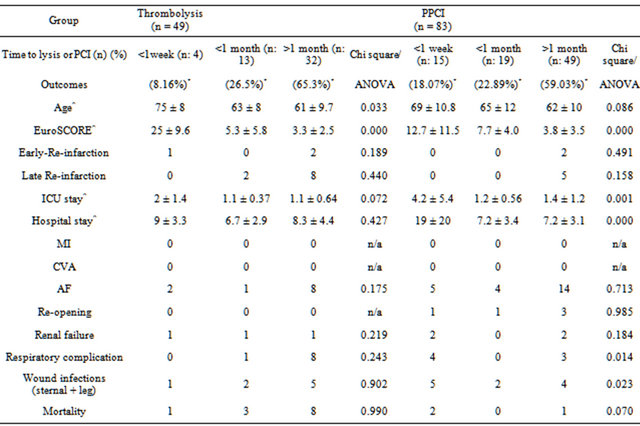
*p = 0.291; MI: myocardial infarction; CVA: cerebrovascular accident; AF: atrial fibrillation; ^mean ± SD; n/a: not applicable. Significant results shown in bold.
Table 5. Subgroup outcome (operative status).
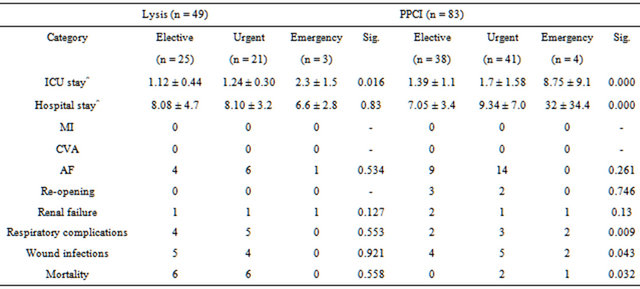
MI: myocardial infarction; CVA: cerebrovascular accident; AF: atrial fibrillation; ^mean ± SD. Significant results shown in bold.
PPCI for STEMI on subsequent CABG outcomes.
We observed significantly better immediate outcomes following PCI than thrombolysis in the interval prior to surgery. This is probably because of direct mechanical re-opening of IRA and establishment of TIMI-3 flows in vessels and also better preservation of ventricular func-
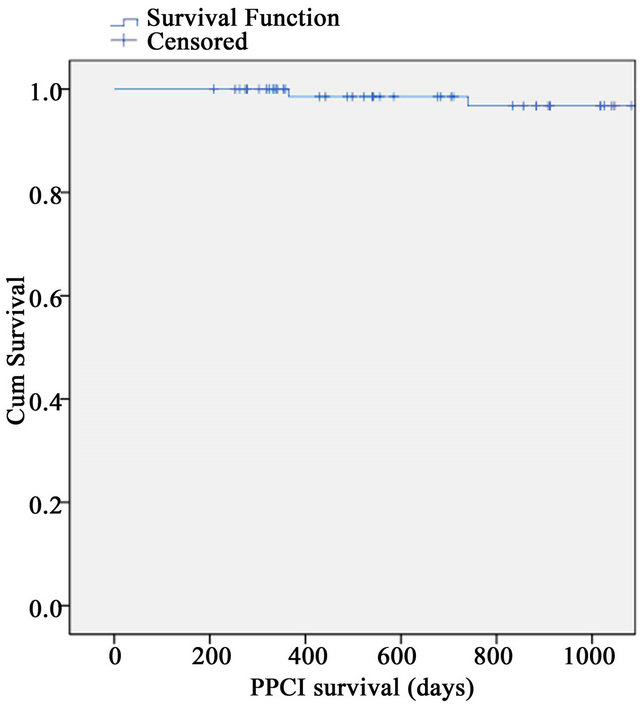
Figure 1. 3-year survival for PPCI group.
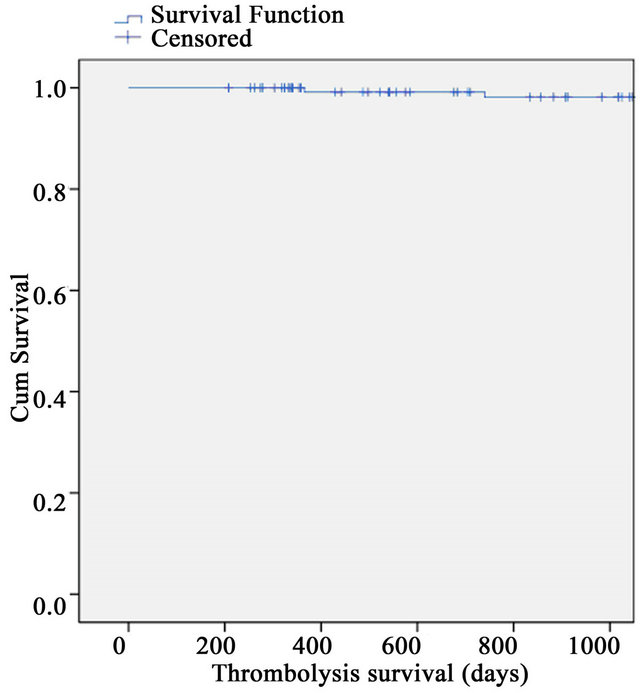
Figure 2. 3-year survival for thrombolysis group.
tion. Following successful thrombolysis, patients may be left with a tight residual stenosis and be more likely to suffer early/late re-infarction (6.1% vs. 2.4% [early] and 20.4% vs. 6% [late] in our study) and/or post infarct angina (24.5% vs. 1.2%) compared to those treated with PPCI. In spite of the above complications, hospital mortality following surgery in both reperfusion groups was 0% and survival rates at 3 years were very high in both groups. It is important to consider that a temporal bias in recovery time spans did exist due to transition of lysis strategy, yet time spans have not been statistically different (Table 1). A subgroup analysis of outcomes based on deliberate shorter time spans (less than 1 week, less then 1-month and beyond 1 month, decided based on the argument, that any differential outcome trends would be shown early rather than late), did show prolonged ICU and hospital stays in patients operated within a week of PPCI, however this could be explained based on a higher EuroSCORE’s in these patients (Table 4). In this subgroup analysis, most of the mortality in lysis group has been seen in patients operated beyond 1-month post lysis, but these differences were not significant. One must also consider the indication, which was to treat surgical disease to salvage myocardium. The outcomes may have been different in another subset of patients, i.e. patients with in-stent stenosis, presence of LV infarct, presence of concomitant coronary and valvular disease. Yet, the primary value of this study remains the observation of excellent safety of surgical revascularization post lysis or PPCI in this select subset of ischemic heart disease.
The extent of LV recovery prior to surgery or prior to discharge was not compared with immediate pre and post lysis functional decompensation for this study, yet a single point estimate was considered for risk scoring. It is then difficult to precisely comment on the impact of lysis strategy on LV recovery prior to surgery. However, the LV functions provided in Table 2 are pre-operative unless emergent, and were not significantly different in either category. This said, the strength of this study is clinical outcome analysis, demonstrating no in-hospital mortality and effectively all patients not only survived the surgical insult but were also discharged. Acknowledging that this study is not designed to assess the bias imposed by a prior PCI, and having witnessed these positive outcomes, we find it difficult to support the general notion that previous PCI should be included in risk profiling of pre-op patients [8] for this subset. There have been several large studies, which have attempted to determine the impact of PCI in general on the outcome of patients who subsequently undergo CABG. Some have suggested that a history of prior PCI adversely affect both early and late outcomes after CABG [9-14]. Others have found that prior PCI has no impact on outcomes after CABG [15- 18]. There are a number of possible mechanisms by which PCI may adversely affect subsequent surgical outcomes. PCI may limit the number of anastomoses performed during CABG by affecting the ability to graft more proximally on a vessel due to the presence of a stent. Secondly, there is some evidence that PCI particularly with a drug-eluting stent can affect coronary endothelial function in the rest of the artery [19-23].
For surgeons, the decision to graft a previously stented infarct-related artery is a matter of debate. An argument in favour of this practice is to protect against stent re-occlusion especially during the pro-thrombotic milieu of the early post-operative period. Anti-platelet agents might also be stopped prior to surgery, further increasing the risk of occlusion. However, there is a substantial body of evidence showing increased rates of graft attrition in the absence of significant flow limiting lesions especially when arterial conduits are used. In our series, 72% of patients in the PPCI group (Table 2) underwent grafting of the previously stented IRA, a subgroup analysis could not be done as the comparator was small. Whether bypassing a previously stented infarct-related artery imparts short and long-term surgical benefits, remains an intuitive question that the present study cannot answer. As this was not a priori outcome of interest, we are unable to provide specific angiographic data for its indications, i.e. presence of additional disease up or downstream of the culprit lesion, and we do not have a policy of undertaking post CABG angiograms on all patients. None the less, the policy in our unit has been that in the presence of flow limiting disease in a previously stented vessel, grafting would be carried out where ever a suitable site was available.
Left main stem disease was found to be involved in 38% of PPCI patients undergoing CABG. Although longterm results are still awaited, the scope for left main stem percutaneous intervention in STEMI will widen in light of the recent trials [24], which will no doubt impact on surgical practice. In present study though, multi-vessel disease was the main indication for surgical revascularization.
For majority, post-op long-term outlook was equally favourable, as most at the time of 6 weeks FU were free from any symptoms or complications. The survivors had no cardiac related re-admissions except one patient from PPCI group who remained with stable angina at the time of FU and had undergone revascularization for severe diffuse disease. Post-op he continued to smoke but was alive at the time of analysis (4 years post-op). In lysis group, 1 patient developed a symptomatic new lesion in native vessel 2 months post CABG, requiring balloon angioplasty. This patient was alive at the time of analysis. None of the deaths in PPCI group were cardiac related, however 1 death in lysis group was secondary to severe LV systolic dysfunction 7 years after CABG who had a moderate LV dysfunction at the time of surgery.
In our case, we have compared PPCI and thrombolysis groups who undergo isolated surgical revascularization and have found very good outcomes in both groups of patients. Despite moderately high EuroSCOREs, there was no 30-day mortality. For most patients CABG occurred within 6 months of PPCI, and the commonest indication was surgical disease untreated at the time of PPCI.
5. Limitations
This is a retrospective analysis and is therefore subject to bias in the way patients were selected for CABG following lysis or PPCI. Also, there was a gradual change in strategy for managing STEMI during the study period (such that thrombolysed patients during the transition period may have been different to thrombolysed patients when that was the primary reperfusion strategy, and patients undergoing PPCI in the thrombolysis period would have been a select group of patients with a contraindication to thrombolysis). However, our study was to determine whether the outcome following CABG would be affected by the initial reperfusion strategy.
Other limitations include wide time spans between initial reperfusion therapy and surgical revascularization, and the lack of specific data on the use of Clopidogrel, GPIIb/III inhibitors or stent type in patients undergoing PCI, and the use of arterial grafts and, where relevant, the indication for grafting the infarct-related artery. In addition, we have been unable to provide information on blood loss at the time of surgery.
6. Conclusion
In conclusion, when patients undergo isolated CABG after STEMI, PPCI reduces the risk of post-infarct angina and re-infarction prior to surgery, without having a negative impact on surgical outcomes. However, equivalent follow-up is needed in the PPCI cohort to determine whether there are any significant differences in longerterm outcomes.
7. Acknowledgments
The authors acknowledge cardiothoracic secretarial staff and the audit department at James Cook University Hospital, Middlesbrough, UK.
REFERENCES
- K. A. Eagle, B. K. Nallamothu, R. H. Mehta, C. B. Granger, P. G. Steg, F. Van de Werf, et al., “Trends in Acute Reperfusion Therapy for ST-Segment Elevation Myocardial Infarction from 1999 to 2006: We Are Getting Better but We Have Got a Long Way to Go,” European Heart Journal, Vol. 29, No. 5, 2008, pp. 609-617. doi:10.1093/eurheartj/ehn069
- C. P. Cannon, C. M. Gibson, C. H. McCabe, A. A. Adgey, M. J. Schweiger, R. F. Sequeira, et al., “TNK-Tissue Plasminogen Activator Compared with Front-Loaded Alteplase in Acute Myocardial Infarction: Results of the TIMI 10B Trial. Thrombolysis in Myocardial Infarction (TIMI) 10B Investigators,” Circulation, Vol. 98, No. 25, 1998, pp. 2805-2814. doi:10.1161/01.CIR.98.25.2805
- E. C. Keeley, J. A. Boura and C. L. Grines, “Primary Angioplasty versus Intravenous Thrombolytic Therapy for Acute Myocardial Infarction: A Quantitative Review of 23 Randomised Trials,” Lancet, Vol. 361, No. 9351, 2003, pp. 13-20. doi:10.1016/S0140-6736(03)12113-7
- The MINAP Steering Group, “Healthcare Quality Improvement Partnership,” Tenth Public Report, 2011. http://www.hqip.org.uk/assets/NCAPOP-Library/MINAP-public-report-2011.pdf
- British Cardiovascular Intervention Society, 2012. http://www.bcis.org.uk
- P. Kolh, W. Wijns, N. Danchin, C. Di Mario, V. Falk, T. Folliguet, et al., “Guidelines on Myocardial Revascularization,” European Journal of Cardio-Thoracic Surgery: Official Journal of the European Association for CardioThoracic Surgery, Vol. 38, No. S1, 2010, pp. S1-S52.
- ASCRIBE, 2012. http://www.ascribe.com
- N. Bonaros, D. Vill, D. Wiedemann, K. Fischler, G. Friedrich, O. Pachinger, et al., “Major Risk Stratification Models Do Not Predict Perioperative Outcome after Coronary Artery Bypass Grafting in Patients with Previous Percutaneous Intervention,” European Journal of Cardio-Thoracic Surgery: Official Journal of the European Association for Cardio-Thoracic Surgery, Vol. 39, No. 6, 2011, pp. e164-e169.
- S. Eifert, H. Mair, A. L. Boulesteix, E. Kilian, M. Adamczak, B. Reichart, et al., “Mid-Term Outcomes of Patients with PCI Prior to CABG in Comparison to Patients with Primary CABG,” Vascular Health and Risk Management, Vol. 6, 2010, pp. 495-501. doi:10.2147/VHRM.S8560
- P. Massoudy, M. Thielmann, N. Lehmann, A. Marr, G. Kleikamp, A. Maleszka, et al., “Impact of Prior Percutaneous Coronary Intervention on the Outcome of Coronary Artery Bypass Surgery: A Multicenter Analysis,” The Journal of Thoracic and Cardiovascular Surgery, Vol. 137, No. 4, 2009, pp. 840-845. doi:10.1016/j.jtcvs.2008.09.005
- N. Bonaros, D. Hennerbichler, G. Friedrich, A. Kocher, O. Pachinger, G. Laufer, et al., “Increased Mortality and Perioperative Complications in Patients with Previous Elective Percutaneous Coronary Interventions Undergoing Coronary Artery Bypass Surgery,” The Journal of Thoracic and Cardiovascular Surgery, Vol. 137, No. 4, 2009, pp. 846-852. doi:10.1016/j.jtcvs.2008.09.041
- A. T. Gurbuz, A. Sasmazel, H. Cui, A. A. Zia and A. Aytac, “Previous Percutaneous Coronary Intervention May Increase Symptom Recurrence and Adverse Cardiac Events Following Surgical Revascularization,” Anadolu Kardiyoloji Dergisi: The Anatolian Journal of Cardiology, Vol. 6, No. 2, 2006, pp. 148-152.
- S. Chocron, R. Baillot, J. L. Rouleau, W. J. Warnica, P. Block, D. Johnstone, et al., “Impact of Previous Percutaneous Transluminal Coronary Angioplasty and/or Stenting Revascularization on Outcomes after Surgical Revascularization: Insights from the Imagine Study,” European Heart Journal, Vol. 29, No. 5, 2008, pp. 673-679. doi:10.1093/eurheartj/ehn026
- C. Rao, L. Stanbridge Rde, J. Chikwe, J. Pepper, P. Skapinakis, O. Aziz, et al., “Does Previous Percutaneous Coronary Stenting Compromise the Long-Term Efficacy of Subsequent Coronary Artery Bypass Surgery? A Microsimulation Study,” The Annals of Thoracic Surgery, Vol. 85, No. 2, 2008, pp. 501-507. doi:10.1016/j.athoracsur.2007.09.036
- A. Boening, B. Niemann, A. Wiedemann, P. Roth, R. H. Bodeker, C. Scheibelhut, et al., “Coronary Stenting before Coronary Artery Bypass Graft Surgery in Diabetic Patients Does Not Increase the Perioperative Risk of Surgery,” The Journal of Thoracic and Cardiovascular Surgery, Vol. 142, No. 2, 2011, pp. e53-e57. doi:10.1016/j.jtcvs.2011.04.018
- J. M. van den Brule, L. Noyez and F. W. Verheugt, “Risk of Coronary Surgery for Hospital and Early Morbidity and Mortality after Initially Successful Percutaneous Intervention,” Interactive Cardiovascular and Thoracic Surgery, Vol. 4, No. 2, 2005, pp. 96-100. doi:10.1510/icvts.2004.093104
- C. H. Yap, B. P. Yan, E. Akowuah, D. T. Dinh, J. A. Smith, G. C. Shardey, et al., “Does Prior Percutaneous Coronary Intervention Adversely Affect Early and MidTerm Survival after Coronary Artery Surgery?” JACC Cardiovascular Interventions, Vol. 2, No. 8, 2009, pp. 758-764. doi:10.1016/j.jcin.2009.04.018
- T. Fukui, S. Manabe, T. Shimokawa and S. Takanashi, “The Influence of Previous Percutaneous Coronary Intervention in Patients Undergoing Off-Pump Coronary Artery Bypass Grafting,” Annals of Thoracic and Cardiovascular Surgery: Official Journal of the Association of Thoracic and Cardiovascular Surgeons of Asia, Vol. 16, No. 2, 2010, pp. 99-104.
- P. S. Munk, N. Butt and A. I. Larsen, “Endothelial Dysfunction Predicts Clinical Restenosis after Percutaneous Coronary Intervention,” Scandinavian Cardiovascular Journal, Vol. 45, No. 3, 2011, pp. 139-145. doi:10.3109/14017431.2011.564646
- G. Patti, V. Pasceri, R. Melfi, C. Goffredo, M. Chello, A. D’Ambrosio, et al., “Impaired Flow-Mediated Dilation and Risk of Restenosis in Patients Undergoing Coronary Stent Implantation,” Circulation, Vol. 111, No. 1, 2005, pp. 70- 75. doi:10.1161/01.CIR.0000151308.06673.D2
- C. V. Serrano Jr., J. A. Ramires, M. Venturinelli, S. Arie, E. D’Amico, J. L. Zweier, et al., “Coronary Angioplasty Results in Leukocyte and Platelet Activation with Adhesion Molecule Expression. Evidence of Inflammatory Responses in Coronary Angioplasty,” Journal of the American College of Cardiology, Vol. 29, No. 6, 1997, pp. 1276- 1283. doi:10.1016/S0735-1097(97)00070-3
- M. Joner, A. V. Finn, A. Farb, E. K. Mont, F. D. Kolodgie, E. Ladich, et al., “Pathology of Drug-Eluting Stents in Humans: Delayed Healing and Late Thrombotic Risk,” Journal of the American College of Cardiology, Vol. 48, No. 1, 2006, pp. 193-202. doi:10.1016/j.jacc.2006.03.042
- S. Fuke, K. Maekawa, K. Kawamoto, H. Saito, T. Sato, T. Hioka, et al., “Impaired Endothelial Vasomotor Function after Sirolimus-Eluting Stent Implantation,” Circulation Journal: Official Journal of the Japanese Circulation Society, Vol. 71, No. 2, 2007, pp. 220-225.
- A. P. Kappetein, T. E. Feldman, M. J. Mack, M. C. Morice, D. R. Holmes, E. Ståhle, K. D. Dawkins, F. W. Mohr, P. W. Serruys and A. Colombo, “Comparison of Coronary Bypass Surgery with Drug-Eluting Stenting for the Treatment of Left Main and/or Three-Vessel Disease: 3-Year Follow-Up of the SYNTAX Trial,” European Heart Journal, Vol. 32, No. 17, 2011, pp. 2125-2134. doi:10.1093/eurheartj/ehr213
NOTES
*These authors contribute equally to this work. Competing Interests: None.
#Corresponding author.

